- *Corresponding Author:
- R. B. Patel
Graduate School of Pharmacy, Gujarat Technological University, GTU Gandhinagar Campus, Gujarat 382028, India
E-mail: ravipatel.qa@gmail.com
| Date of Received | 15 November 2021 |
| Date of Revision | 11 May 2022 |
| Date of Acceptance | 06 July 2023 |
| Indian J Pharm Sci 2023;85(4):903-911 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A simple, rapid and novel reverse phase-high performance liquid chromatography method was developed for quantification of molnupiravir in its capsule dosage form which is recently approved for phase III clinical trials in moderate coronavirus disease patients in India. The chromatographic separation of Molnupiravir was achieved on reverse phase-high performance liquid chromatography using Eclipse Plus C18 (150×4.6 mm, 5 µ) column with buffer (pH 4.5) and methanol (70:30 v/v) as mobile phase. Method was validated in accordance with recommendations of International Council for Harmonisation Q2 (R1) guidelines. The linearity of the method was found to be excellent over the concentration range of 49.80-149.40 µg/ml. The mean of the coefficient of determinations (r2, n=3) was found to be 0.9999. The precision values (percentage relative standard deviation) and overall percentage recovery was found to be acceptable. The proposed method effectively separated the drug from its degradation products. Hence, it can be used as a stability-indicating assay method for the routine analysis of molnupiravir in pharmaceutical formulations.
Keywords
Severe acute respiratory syndrome coronavirus 2, molnupiravir, Reverse phase-high performance liquid chromatography, Coronavirus disease-19, antiviral, validation
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pathogen, which is a single-stranded Ribonucleic Acid (RNA) virus, causes Coronavirus Disease (COVID-19)[1]. COVID-19 is associated with a heavy disease burden and can severely impact several organ systems, including the lungs, kidneys, liver, muscles, and nervous system[2]. With the global spread of SARS-CoV-2 beginning in early 2020, significant efforts have been made in both vaccine research for the prevention of COVID-19, as well as in the identification and utilization of novel or repurposed therapeutics for the management of those with symptomatic COVID-19. To date, eleven agents have received emergency use authorization by the United States Food and Drug Administration (US FDA) for the management of patients with COVID, including one antiviral agent, the adenosine nucleoside analog remdesivir[3]. Other investigational agents are also being evaluated in clinical trials, including the ribonucleoside analog Molnupiravir (MLV), also known as EIDD-2801 or MK-4482[4,5].
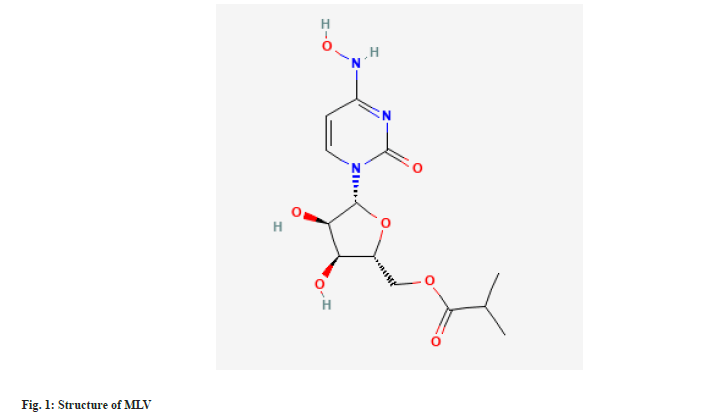
MLV is chemically known as [(2R,3S,4R,5R)-3,4-Dihydroxy-5-[4-(hydroxyamino)-2-oxypyrimidin-1-yl]oxolan-2-yl]methyl 2-methylpropanoate (fig. 1)[6]. MLV has been authorized by Drug Controller General of India for the phase III clinical trials in moderate COVID patients[7]. MLV is the isopropyl ester pro-drug of N4-hydroxycytidine[8]. With improved oral bioavailability in non-human primates, it is hydrolysed in vivo and distributes into tissues where it becomes the active 5'-triphosphate form[9]. The active drug incorporates into the genome of RNA viruses, leading to an accumulation of mutations known as viral error catastrophe[10]. Recent studies have shown MLA inhibits replication of human and bat corona viruses including SARS-CoV-2, in mice and human airway epithelial cells[8].
Comprehensive literature survey reveals bio analytical method for the estimation of MLV in Human Plasma and Saliva[11]. MLV is not official in any pharmacopoeia, hence no official method is available for the estimation of MLV in their dosage forms. According to detailed literature survey, there is no stability indicating Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) method reported for determination of MLV in their combined dosage forms. Hence the objective of this work was to develop suitable stability indicating RP-HPLC method for drug product containing MLV.
Materials and Methods
Chemicals and reagents:
A working standard of MLV was procured from JSK Chemicals, Ahmedabad, Gujarat, India (Potency: 99.83 % w/w on as is basis). MLV capsules were purchase form local pharmacy. The HPLC grade solvents and Potassium dihydrogen orthophosphate, triethylamine, hydrochloric acid, ortho phosphoric acid, sodium hydroxide, methanol, acetonitrile, hydrogen peroxide, (Merck, Mumbai, India) were used in the analysis. HPLC grade water was prepared using Millipore purification system.
Instrumentation:
A HPLC instrument (Agilent Technologies separations module) consisting of Ultraviolet (UV) Photodiode Array Detector (DAD) equipped with Open Lab CDS Software at wavelength 236 nm was used for this analysis. The chromatographic separations were performed on Eclipse Plus C18 (150×4.6 mm) column by keeping it on 25° using a flow rate of 1ml/min with the run time of 12 min. Injection volume was set as 10 µl.
Mobile phase and diluent:
The mobile phase was a mixture of buffer (pH=4.5) and methanol (70:30 v/v), filtered through 0.22 µm finer porosity nylon membrane filter and degassed prior to use. The buffer (pH=4.5) was prepared by dissolving about 0.68 g of potassium dihydrogen phosphate in 1000 ml of water and adjusting the pH to 4.5±0.05 with ortho phosphoric acid. Water and acetonitrile in the ratio of 30:70 was used as diluent.
Standard Preparations:
Standard stock solution was prepared by dissolving 50 mg of MLV standard in 50 ml of diluent. Further 5 ml of this solution is diluted up to 50 ml with diluent for final standard concentration of 100 ug/ml.
Validation:
The developed method has been validated for the assay of MLV in capsules, 200 mg capsule strength using following parameters as per International Conference on Harmonization Q2 (R1) guideline[12].
System suitability:
Five replicate injections of MLV standard preparation were injected into HPLC. The tailing factor for the MLV peak from the first injection of the standard preparation should be less than 2.0 and the column efficiency determined from MLV peak from the first injections of the standard preparation should not be less than 1500 theoretical plates. The relative standard deviation for the mean area calculated for MLV peak from the five replicate injections of standard preparation should be less than 2.0 %.
Specificity:
Specificity is the ability of the method to measure the analyte response in the presence of its potential impurities. Specificity consisted of interference and forced degradation studies.
Interference study: This was demonstrated by preparing a placebo containing all excipients and injecting a sample prepared from the same.
Force degradation study: This was demonstrated by carrying out forced degradation of the sample with 1 N HCl, 1 N NaOH, 30 % H2O2, heating in water bath at 60° for 30 mins and keeping under UV light for 24 h. The samples were prepared as per sample preparation and injected into HPLC system with a photodiode array detector.
Acid degradation: Transferred accurately weighed capsule filled powder equivalent to 200 mg of MLV into 100 ml volumetric flask. Added 50 ml of diluent and sonicate for 5 min. Add 0.5 ml of 1 N HCl and heated in a water bath at 60° for 30 min. Cooled and neutralized with 0.5 ml of 1 N NaOH. Made up the volume with diluent and mixed well. Centrifuged to get clear supernatant. Diluted 5 ml this solution upto 100 ml with diluent. Injected this solution in to the HPLC system.
Alkali degradation: Transferred accurately weighed capsule filled powder equivalent to 200 mg of MLV into 100 ml volumetric flask. Added 50 ml of diluent and sonicated for 5 min. Add 0.5 ml of 1 N NaOH at room temperature for 10 min and neutralized with 0.5 ml of 1 N HCl. Made up the volume with diluent and mixed well and centrifuge it to get a clear supernatant. Dilute 5 ml of this solution to 100 ml with diluent. Inject this solution in to the HPLC system.
Peroxide degradation: Transferred accurately weighed capsule filled powder equivalent to 200 mg of MLV into 100 ml volumetric flask. Added 50 ml of diluent and sonicated for 5 min. Add 0.5 ml of 30 % H2O2 and heated in a water bath at 60° for 15 min. Cooled and made up the volume with diluent and mixed well. Dilute 5 ml of this solution to 100 ml with diluent. Inject this solution in to the HPLC system.
Thermal degradation: Transferred accurately weighed capsule filled powder equivalent to 200 mg of MLV into 100 ml volumetric flask. Added 50 ml of diluent and sonicate for 5 min, heated in a water bath at 60° for 30 min. Cooled and made up the volume with diluent and mixed well. Dilute 5 ml of this solution to 100 ml with diluent. Inject this solution in to the HPLC system.
UV degradation: Transferred accurately weighed capsule filled powder equivalent to 200 mg of MLV (Previously kept in UV light for 24 h) into 100 ml volumetric flask. Added 50 ml of diluent and sonicate for 5 min and made up the volume with diluent and mixed well. Dilute 5 ml of this solution to 100 ml with diluent. Inject this solution in to the HPLC system. The peak purity of MLV peak should pass.
Linearity:
From the standard stock solution, a series of solutions were prepared at concentration levels ranging from 50 % to 150 % of standard concentration. The peak area responses of solutions at all levels in duplicate were measured. The peak response verses concentration data was treated by linear regression analysis and the linearity of response for MLV was determined by calculating correlation coefficient (acceptance criterion: correlation coefficient should not be less than 0.999).
Accuracy:
For accuracy study, known amount of placebo was taken separately into different volumetric flasks and spiked with known quantities of MLV active pharmaceutical ingredient at three different levels, in triplicate. Accuracy has been performed at about 50 % (Level 1), 100 % (Level 2) and 150 % (Level 3) of sample concentration. The samples were analysed by the proposed method and the amount of MLV recovered was calculated (acceptance criterion: percentage recovery shall be in the range of 98-102 %. Individual and overall % RSD of % recovery should not be more than 2.0).
Precision:
In precision study, system precision, method precision and intermediate precision have been carried out. The system precision was examined by analysing standard solution in five replicates. % RSD of area counts of MLV peak was calculated. In method precision, six preparations of a single batch of MLV capsules, 200 mg capsule against MLV standard solution were examined.
Intermediate precision was repeated using different analysts, on different days, on different instruments and using column of different lot. Overall RSD for assay between the two sets of data was calculated.
Limit of Detection (LOD) and Limit of Quantification (LOQ):
By preparing a series of standard preparation of different concentrations of MLV standards were prepared by using linearity 50 % solution and injected in to HPLC to determine LOD and LOQ.
Robustness:
Robustness of the method was investigated by varying the instrumental conditions such as wavelength of detection (±5 nm), column oven temperature (±5°), minor component in mobile phase (±2 % absolute), flow rate (±10 %) and pH of Buffer (±0.2 unit). System suitability of the standard solution was checked at each variable condition. The theoretical plates, tailing factor of MLV peak from standard solution and % RSD of area counts of standard solution for each set of data were calculated. Sample solution of MLV capsules, was prepared in triplicate and analysed under each condition and % label claim of MLV was calculated. Robustness of the method was indicated by the overall % RSD between the data of method precision and data at each variable condition (acceptance criterion: System suitability should pass and overall % RSD of % Assay shall not be more than 2.0).
Stability of analytical solution:
Freshly prepared Standard and sample solutions of MLV capsules were kept at 25°. Both the solutions were analysed initially and at different time intervals. Calculate percentage deviation form mean initial area count (acceptance criterion: % Deviation shall not be more than ±2.0 %).
Results and Discussion
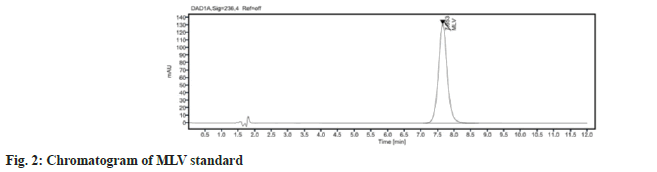
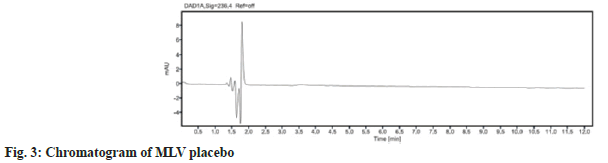
The chromatographic method was optimized by changing various parameters, such as the mobile phase composition and pH of the buffer used in the mobile phase[6]. Retention time and separation of peak of MLV were dependent on the pH of the buffer and the percentage of methanol. Different mobile phases were tried, but satisfactory separation and good symmetrical peak were obtained with the mobile phase consisting of buffer (pH=4.5) and methanol in the ratio of 70:30 % v/v with Eclipse plus C18 (150×4.6mm), 5 µ column with run time of 12 min. The column selection and shorter run time depicts the cost effectiveness of the method. A typical chromatogram obtained by using the aforementioned mobile phase and 10 µl of the injected assay preparation is illustrated in fig. 2. Retention time for MLV was found to be 7.6 min. No blank peak and placebo interference at the retention time of known peak was obtained as shown in fig. 3.
Standard solution was injected on different days during the validation study. Using the system suitability software, theoretical plates and tailing factor for MLV peak were calculated. Also, % RSD for five replicate injections was calculated. The tailing factor of MLV standard peak from the first injection of the standard preparation was 1.07, theoretical plates was 4161 and the % RSD calculated for MLV peak from the five replicate injections of standard preparations was 0.11 %. The above three system suitability parameters were met during the course of entire validation. System precision results are summarised in Table 1.
| System precision | Method precision | Intermediate precision | |||
|---|---|---|---|---|---|
| Injection No. | Area Count (mAU) | Sample No. | Assay (% Label claim) | Sample No. | Assay (% Label claim) |
| 1 | 2410.71 | 1 | 102.68 | 1 | 101.49 |
| 2 | 2416.59 | 2 | 100.2 | 2 | 100.67 |
| 3 | 2414.08 | 3 | 103.58 | 3 | 101.96 |
| 4 | 2417.27 | 4 | 100.88 | 4 | 100.96 |
| 5 | 2415.82 | 5 | 102.31 | 5 | 100.97 |
| 6 | 101.25 | 6 | 100.63 | ||
| Mean | 2414.89 | Mean | 101.82 | Mean | 101.46 |
| ±SD | 2.62 | ±SD | 1.26 | ±SD | 1.03 |
| % RSD | 0.11 | % RSD | 1.23 | % RSD | 1.01 |
Table 1: Precision Data.
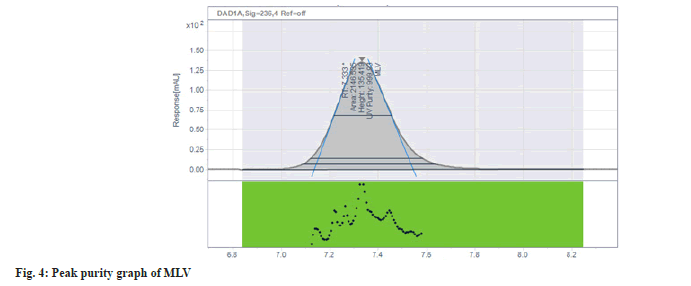
The result of interference study indicates that there are no coeluting peaks from placebo as well as no interference of MLV impurities with MLV peak as the peak purity indicates that the MLV peak is homogeneous. The purity angle is less than purity threshold for the MLV peak in the samples fig. 4.
During forced degradation study, degradation was observed when MLV exposed to acid and alkali hydrolysis, Peroxide degradation, Thermal and UV degradation. Minor degradation was observed when sample exposed to acid, thermal and photolytic conditions. Results from peak purity testing confirmed the main compound peak obtained by analysis of all the stress samples was homogenous and pure and unaffected by the presence of its degradation products, confirming the stability indicating nature of the method. The results from forced degradation studies are summarized in Table 2.
| Mode of degradation | Condition | Assay | % Degradation | Purity angle | Purity threshold |
|---|---|---|---|---|---|
| Sample as such | No treatment | 101.78 | - | 0.049 | 0.294 |
| Acid degradation | 1 N HCl-0.5 ml/60°/30 min | 97.43 | 4.35 | 0.048 | 0.295 |
| Alkali degradation | 1 N NaOH-0.5 ml/RT/10 min | 89.1 | 12.68 | 0.046 | 0.292 |
| Peroxide degradation | 30% H2O2-0.5 ml/60°/30 min | 80.78 | 21 | 0.045 | 0.296 |
| Thermal degradation | 60° for 30 min | 99.38 | 2.4 | 0.048 | 0.295 |
| UV degradation | 24 h at UV Chamber | 101.82 | 0 | 0.048 | 0.29 |
Table 2: Results of Force Degradation Study.
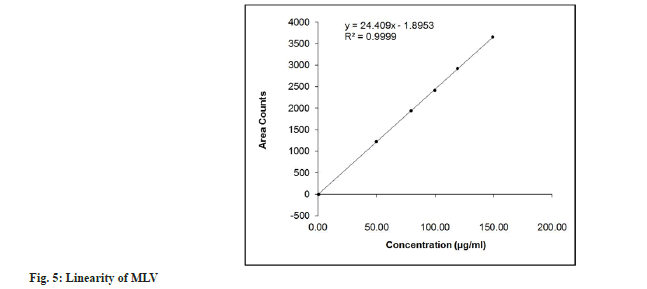
The method validated in the range of 49.80 to 149.40 μg/ml of standard concentration is significant. The regression equation from peak response vs. concentration data obtained Y=24.409X–1.8953 and correlation coefficient was 0.9999 indicating that the response is linear over the specified range. The result data is shown in Table 3 and the plot of Linearity shown in fig. 5.
| Concentration (µg/ml) | Mean Area Counts |
|---|---|
| 49.8 | 1223.05 |
| 79.68 | 1935.14 |
| 99.6 | 2412.99 |
| 119.52 | 2920.32 |
| 149.4 | 3652.91 |
| Slope | 24.41 |
| Intercept | -1.90 |
| Correlation Coefficient (r) | 1.000 |
Table 3: Linearity of MLV.
The recovery of three sample preparation at each level was examined and ranged from 99.89 % to 101.34 %. As percentage recovery is found within the acceptance criteria, also individual and overall % RSD of % recovery is found within the acceptance criteria, the method is accurate. Results are summarized in Table 4.
| Recovery levels | Amount added Conc. (µg/ml) | Amount recovered Conc. (µg/ml) | % Recovery |
|---|---|---|---|
| Level 1 (50 %) | 50.8 | 50.4 | 99.21 |
| 50.4 | 50.50 | 100.20 | |
| 51.2 | 50.70 | 99.02 | |
| Level 2 (100 %) | 100.4 | 100.13 | 99.73 |
| 100.8 | 99.98 | 99.19 | |
| 100.2 | 100.16 | 99.96 | |
| Level 3 (15 0%) | 150.2 | 151.29 | 100.73 |
| 150.8 | 151.47 | 100.44 | |
| 151.0 | 151.16 | 100.11 | |
| Mean | 99.84 | ||
| ±SD | 0.60 | ||
| % RSD | 0.60 | ||
Table 4: Accuracy Results.
The HPLC system has an acceptable level of precision as the acceptance criterion for individual and overall % RSD should not be more than 2.0 for precision, which was achieved successfully. The system is found to be precise as the %RSD of the area counts for five injections of the standard solution was 0.11 (Table 5). The method is also precise as the % RSD of amount present for MLV in the sample was 1.23. In intermediate precision, individual % RSD of amount present for MLV was found to be 1.23 for Set I (Method precision data), 1.01 for Set II and overall % RSD value is 1.12. Hence the method is found to be rugged. Results are summarized in Table 1.
Experimentally LOQ was found 0.5 µg/ml and LOD was found 0.25 µg/ml so we can say that method is sensitive and can detect and quantify very small amount of Concentration. Results are summarized in Table 5.
| Injection | Area count | ||||||
|---|---|---|---|---|---|---|---|
| 4.98 (µg/ml) | 2.49 (µg/ml) | 1.25 (µg/ml) | 0.75 (µg/ml) | 0.5 (µg/ml) | 0.25 (µg/ml) | 0.05 (µg/ml) | |
| 1 | 123.41 | 62.32 | 31.44 | 14.24 | 11.17 | 5.12 | ND |
| 2 | 121.73 | 60.26 | 30.29 | 15.23 | 13.61 | 8.27 | ND |
| 3 | 121.99 | 61.45 | 30.00 | 15.86 | 12.59 | 6.41 | ND |
| Mean | 122.38 | 61.34 | 30.57 | 15.11 | 12.45 | 6.60 | - |
| ±SD | 0.90 | 1.03 | 0.76 | 0.82 | 1.22 | 1.58 | - |
| % RSD | 0.73 | 1.68 | 2.49 | 5.4 | 9.83 | 23.99 | - |
Note: ND: Not detected
Table 5: LOD and LOQ Study.
In all the deliberate varied chromatographic conditions (wavelength of detection, column oven temperature, composition of mobile phase, flow rate and pH of buffer) the results obtained were well within the limit. Results were summarized in Table 6.
| Method parameters | Standard Solution | Assay of MLV (% Claim) | ||
|---|---|---|---|---|
| Theoretical plate | Tailing factor | % RSD | ||
| As such (Method precision) | 4161 | 1.07 | 0.11 | 101.82 |
| Variation in wavelength (234 nm) | 4216 | 1.08 | 0.12 | 102.68 |
| Variation in wavelength (238 nm) | 4158 | 1.07 | 0.15 | 100.20 |
| Variation in column oven temperature (20°) | 4072 | 1.05 | 0.15 | 100.88 |
| Variation in column oven temperature (30°) | 4198 | 1.02 | 0.13 | 102.31 |
| Variation in minor component in mobile phase (- 2 % of methanol) | 3892 | 1.14 | 0.21 | 101.25 |
| Variation in minor component in mobile phase (+ 2 % of methanol) | 4254 | 1.05 | 0.16 | 100.96 |
| Variation in flow rate (0.8 ml/min) | 4098 | 1.16 | 0.14 | 100.97 |
| Variation in flow rate (1.2 ml/min) | 3941 | 1.04 | 0.13 | 101.49 |
| Variation in pH of Buffer solution (pH 4.3) | 4028 | 1.05 | 0.14 | 101.96 |
| Variation in pH of Buffer solution (pH 4.7) | 4121 | 1.02 | 0.15 | 100.63 |
Table 6: Robustness Data.
In standard solution, % deviation form mean initial area counts up to 24 h at room temperature condition is 0.8 %. In sample solution, % deviation form mean initial area counts up to be 24 h at room temperature condition is 1.1 %. As the % deviation form mean initial area counts of standard solution and sample solution of MLV, within the acceptable limits ± 2.0 %, it reveals that standard and sample are stable in analytical solution for at least 24 h at room temperature. Results are summarized in Table 7.
| Time (h) | Standard solution | Sample solution | ||
|---|---|---|---|---|
| Area count | % Deviation | Area count | % Deviation | |
| Initial | 2410.71 | 0.0 | 2489.05 | 0.0 |
| 4 | 2425.78 | 0.6 | 2492.90 | 0.1 |
| 10 | 2423.56 | 0.5 | 2498.92 | 0.4 |
| 15 | 2431.25 | 0.8 | 2505.86 | 0.6 |
| 24 | 2426.51 | 0.6 | 2517.03 | 1.1 |
Table 7: Solution Stability.
In conclusion, the present HPLC method for the determination of assay of MLV in capsule dosage form is simple, rapid, economical, precise, accurate and rouged. The method has been validated and satisfactory results were observed for all the tested validation parameters. The developed method can be conveniently used for determination of assay of MLV in marketed formulation and stability samples. Moreover, the lower solvent consumption along with the short analytical run time of 12 min leads to cost effective chromatographic method.
Acknowledgments:
The authors are thankful to Dr. Manju Mishra for extending her support in procurement of materials to carry out the research work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20(5):533-4.
[Crossref] [Google Scholar] [PubMed]
- Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol 2020;15:359-86.
[Crossref] [Google Scholar] [PubMed]
- Emergency Use Authorizations (EUAs). U.S. Food and Drug Administration. 2020:1-20.
- Painter WP, Holman W, Bush JA, Almazedi F, Malik H, Eraut NC, et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother 2021;65(5):10-128.
[Crossref] [Google Scholar] [PubMed]
- Painter WP, Sheahan T, Baric R, Holman W, Donovan J, Fang L, et al. Reduction in infectious SARS-CoV-2 in treatment study of COVID-19 with molnupiravir. Topics Antiviral Med 2021:304-5.
- Molnupiravir. PubChem 2020.
- Recommendations of the SEC meeting to examine COVID-19 related proposal under accelerated approval. Central Drug Standard Control Organisation. 2021.
- Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med 2020;12(541):eabb5883.
[Crossref] [Google Scholar] [PubMed]
- Toots M, Yoon JJ, Cox RM, Hart M, Sticher ZM, Makhsous N, et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med 2019;11(515):eaax5866.
[Crossref] [Google Scholar] [PubMed]
- Hampton T. New flu antiviral candidate may thwart drug resistance. JAMA 2020;323(1):1-17.
[Crossref] [Google Scholar] [PubMed]
- Parsons TL, Kryszak LA, Marzinke MA. Development and validation of assays for the quantification of β-D-N4-hydroxycytidine in human plasma and β-D-N4-hydroxycytidine-triphosphate in peripheral blood mononuclear cell lysates. J Chromatogr B 2021;1182:122921.
[Crossref] [Google Scholar] [PubMed]
- International Conference on Harmonization (ICH). Validation of analytical procedures: text and methodology Q2 (R1). ICH Harmonized Tripartite Guideline. 2005.