- Corresponding Author:
- Manisha puranik
Department of Quality Assurance, Institute of Pharmaceutical Education and Research, Borgaon (Meghe), Wardha442 001, India.
E-mail: manisha68_12@yahoo.com
| Date of Submission | 07 July 2005 |
| Date of Revision | 13 March 2006 |
| Date of Acceptance | 25 November 2006 |
| Indian J Pharm Sci, 2006, 68 (6): 737-739 |
Abstract
Two simple, accurate, and precise methods for simultaneous estimation of tramadol hydrochloride and chlorzoxazone in combined dosage form have been described. The first method employs formation and solving of simultaneous equations using 272.20 and 248.30 nm as two analytical wavelengths. The second method is absorption ratio method, which uses 272.20 and 257.50 nm as two analytical wavelengths. Both the methods allow the simultaneous determination of tramadol hydrochloride and chlorzoxazone in concentration ranges employed for this purpose with the standard deviation of <1.0% in the assay of tablet.
A new fixed dose combination containing tramadol hydrochloride (TRM) and chlorzoxazone (CLZ) is available in the market in tablet dosage form for relieving low back pain and cervical spondylosis. USP monograph describes high performance liquid chromatography (HPLC) [1] method for assay of CLZ tablet and European pharmacopoeia describes potentiometric [2] method for assay of TRM hydrochloride. Literature survey reveals that reports are available for estimation of TRM hydrochloride by GC3 in plasma and brain tissue of mice and rats, using HPLC [4-6] in plasma and urine, and spectrohotometry [7,8] in pharmaceutical formulations. Several spectrophotometric [9,10] and HPLC [11-13] methods are reported for estimation of CLZ in combination with other drugs from pharmaceutical formulations and biological fluid. However, no method is yet reported for simultaneous estimation of tramadol hydrochloride and chlorzoxazone in combined dosage form. Hence, two spectrophotometric methods have been developed to estimate these two drugs from tablet dosage form.
Materials and Methods
UV/Vis. double beam spectrophotometer, model Shimadzu UV 2401 PC with 1 cm quartz cells was used.
Preparation of solutions
TRM hydrochloride standard stock solution (0.6 mg/ml) was prepared by transferring accurately weighed 60 mg portion of TRM in 100 ml volumetric flask and volume was made up with 0.1 N NaOH in methanol to give concentration of 600 μg/ml. CLZ standard stock solution (0.1 mg/ml) was prepared by transferring accurately 10 mg portion of CLZ in 100 ml volumetric flask and volume was made up with 0.1 N NaOH solution in methanol to give concentration of 100 μg/ml.
Simultaneous equations method (Method 1)
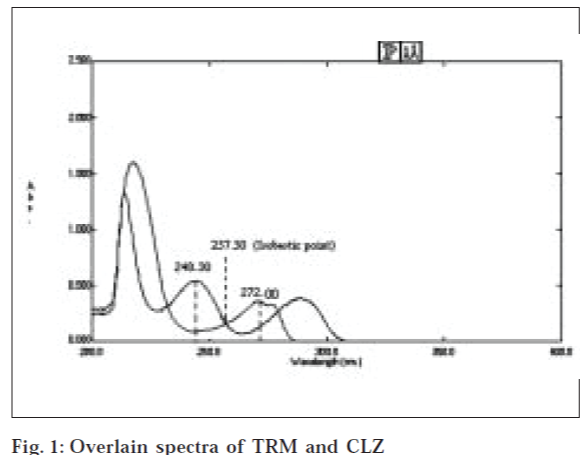
Selection of analytical wavelengths was done by taking pure samples of TRM and CLZ which were separately dissolved in 0.1 N NaOH in methanol to give two solutions of 60 and 10 μg/ml, respectively. They were scanned in the wavelength range of 200-400 nm. From the overlain spectra (fig. 1), wavelengths 272.20 and 248.30 nm were selected for the formation of simultaneous equations. For constructing a calibration curves, two series of different concentrations in range of 30-300 μg/ml for TRM and 5-50 μg/ml for CLZ were prepared from stock solutions. The calibration curves were plotted at 272.20 and 248.30 nm. The absorptivities (A1%, 1 cm) of both the drugs at both the wavelengths were determined. These calculated values were the mean of five independent determinations.
The absorbance and absorptivities values at the particular wavelengths were calculated and substituted in the following equations to obtain the concentrations: A1 = 0.56 × 102 Cx + 2.77 × 102 Cy- (1) and A2 = 0.04 × 102 Cx + 4.01 × 102 Cy- (2), where A1 and A2 are absorbance of sample solution at 248.30 and 272.2 nm, respectively. Cx and Cy are concentration of TRM hydrochloride and CLZ, respectively (in mole/l) in sample solution. By substituting the value of Cx from Equation (1) into Equation (2), the value of Cy can be obtained. Now substituting this value of Cy in one of the equations, the value of Cx can be obtained. The validity of formed equations was checked by preparing five mixed standards, measuring their absorbances at respective wavelengths and comparing these with the absorbances calculated using above formed equations.
Absorption ratio method (Method 2)
From the overlain spectra of TRM and CLZ 272.20 nm taken as λmax for TRM and 257.50 nm as isobestic point for estimation of TRM and CLZ, respectively. Series of different concentrations in range of 30-300 μg/ ml for TRM and 5-50 μg/ml for CLZ were prepared from stock solutions. The calibration curves were plotted at 272.20 and 257.50 nm. The absorptivities (A1%, 1 cm) of both the drugs at both the wavelengths were determined. These calculated values were the mean of five independent determinations.
Estimation from tablets
Twenty tablets of brand Muzox (Stedman Pharmaceuticals Ltd., Label claim CLZ 250 mg and 50 mg TRM) were weighed and finely powdered. Accurately weighed tablet powder equivalent to 100 mg was taken in 100 ml volumetric flask. To it 580 mg of pure TRM was added and sonicated for 5 min with 50 ml 0.1 N methanolic NaOH. The volume was made to mark. Aliquot portion of this solution was further diluted to achieve final concentration of 60 μg/ml for TRM and 10 μg/ml for CLZ. The absorbances were noted at respective wavelengths. The concentration of each drug in tablet formulation was determined using above methods.
Results and Discussion
The overlain spectra of TRM and CLZ in the concentration ratio of 6:1 showed that the peaks are well Fig. 1: Overlain spectra of TRM and CLZ resolved thus satisfactory criteria for obtaining maximum precision based on absorbance ratios The criteria being the ratios (A2A1/aX2aX1) for drug Y and (aY2aY1/A1A2) for drug X should lie outside the range of 0.1-2.0 where A1 and A2 represent absorbance of tablet solution at λ1 and λ2 , aX1 and aX2 represents absorptivities of X at λ1 and λ2 and aY1 and aY2 denote absorptivities of Y at λ1 λ2 respectively. In the present contest, the above criteria found to be satisfied for TRM (X) and CLZ (Y) where λ1 is 272.20 nm and λ2 is 248.30 nm. In overlain spectra, CLZ shows two distinct peaks, one at around 248.30 and other at 292.00 nm. The peak at 248.30 nm was found to be prominent hence for simultaneous equations method; the peak was used for determination of CLZ. Since only one prominent peak exists for TRM at 272.20 nm, the same was used for its determination. Absorbance was determined at both the wavelengths. Calibration curves were plotted and regression analysis was carried out (Table 1). The absorbitivity was then calculated and substituted in Equations 1 and 2 along with absorbance values to obtain concentration of drugs.
| Method | Drug | Wavelength | Concentration range | Intercept (RSD) | Slope | r2 |
|---|---|---|---|---|---|---|
| (nm) | (µg/ml) | |||||
| 1 | TRM | 272.20 | 30–300 | 0.0082 | 0.0059 | 0.999 |
| 248.30 | 30–300 | 0.006 | 0.006 | 0.9959 | ||
| CLZ | 272.20 | 5–50 | 0.0326 | 0.0234 | 0.9952 | |
| 248.30 | 5–50 | 0.0295 | 0.049 | 0.998 | ||
| 2 | TRM | 272.20 | 30–300 | 0.0087 | 0.0059 | 0.999 |
| 257.50 | 30–300 | 0.016 | 0.0019 | 0.997 | ||
| CLZ | 272.20 | 5–50 | 0.00331 | 0.0234 | 0.9955 | |
| 257.50 | 5–50 | 0.0265 | 0.0422 | 0.9972 |
Method 1 is the simultaneous equation method while Method 2 is absorption ratio method, RSD is relative standard deviation and r2 is correlation coefficient
Table 1: Regession Analysis of the Calibration Curves
In absorption ratio method, two wavelengths are selected from overlain spectra out of which one is isobestic point and another isλmax of one of the drugs. The spectra of TRM and CLZ when overlaid indicated that the isobestic point was at 257.50 nm at which estimation of CLZ was done and estimation of TRM was done at its λmax, 272.20 nm.
Both the methods were successfully used to estimate the amounts tramadol hydrochloride and chlorzoxazone in marketed tablet formulation containing tramadol hydrochloride 50 mg and chlorzoxazone 250 mg. The results obtained were comparable with the corresponding labeled amounts (Table 2).
| Tablet | Method 1 | Method 2 | ||
|---|---|---|---|---|
| % TRM | % CLZ | % TRM | % CLZ | |
| MUZOX | 100.11% ±0.75 |
101.29% ±0.80 |
100.86% ±0.85 |
99.93% ±0.87 |
Method 1 is the simultaneous equations method while Method 2 is absorption ratio method
Table 2: Assay Results of Tramadol Hydrochloride and Chlorzoxazone in Marketed Formulation
By observing the validation parameters (Table 3), both the methods were found to be specific, accurate, precise, repeatable, and reproducible. However, Absorption ratio method has an advantage of simpler calculations over the simultaneous equations method. Hence, both methods can be employed for routine analysis of tablet for assay as well as dissolution testing.
| Parameters | Method 1 | Method 2 | |||
|---|---|---|---|---|---|
| TRM | CLZ | TRM | CLZ | ||
| Linearity range (µg/ml) Correlation coefficient (r2) Precision (R.S.D.) Ruggedness Intraday (n=3) Interday (n=3) Accuracy (%) Reproducibility Specificity |
30–300 at 248.30 0.9959 at 272.20 0.9990 0.0310 0.7609 0.5461 100.27 Reproducible R Specific |
5-50 0.9980 0.9952 0.0090 0.4336 0.5918 99.78 eproducible Specific |
30–300 at 257.50 0.9970 at 272.20 0.9990 0.00104 0.5244 0.8543 100.19 Reproducible Specific |
5-50 0.9972 0.9955 0.0166 0.5819 0.9314 99.79 Reproducible Specific |
|
Method 1 is the simultaneous equation method while Method 2 is absorption ratio method and r2 is correlation coefficient
Table 3: Summary of Validation Parameters
Acknowledgments
The authors thank Neon Laboratories Ltd., Palghar (Thane) for the sample of pure tramadol hydrochloride (Thane) for the sample of pure tramadol hydrochloride pure chlorzoxazone.
References
- The United States Pharmacopoeia 24, US PharmacopoeialConvention , Rockville, 2000, 302.
- European Pharmacopoeia, 5th Edi., European Department forthe Quality of Medicines within the Council of Europe, Strasbourg, 2005, 2607.
- Tao,Q.,Stone,D.J.,Borenstein,M.R.,Jean,B.,Valerie,C.,Ellen,E., Timothy,P.,Desai,K.D.,Liao,S.andRaffa,R.B.,J.Chromatogr., B,2001, 763,165.
- Pederson, R.S., Brosen, K. and Nielsen, F., Chromtographia,2003, 57, 279.
- Nobilis, M., Kopecky, J., Kvetina, J., Chladek, J., Svoboda, Z., Varisek, V., Perlik, F., Pour, M. and Kunes, J., J. Chromatogr. A, 2002, 949, 11.
- Gan, S. H. and Ismail, R., J. Chromatogr. B, 2001, 759, 325
- Abdellatef, H.E., J. Pharm. Biomed. Anal., 2002, 29, 835
- Rajput, S .J. and Trivedi, P.D., Indian Drugs, 2001, 38, 100
- Kale, U.N., Naidu, K.R. and Shingare, M.S., Indian J. Pharm. Sci., 2002, 64, 168
- Mashru, R.C. and Banerjee, S.K., Eastern Pharmacist, 1998, 41, 141
- Frey,R.F.andStiff,D.D.,J.Chromatogr.B,1996,686,291.
- Gangawal, S. and Trivedi, P., Eastern Pharmacist, 2000, 45,139
- International Conference on Harmonization, Guidance for Industry In; Q2B Validation of Analytical procedures: Methodology, 1996, 2