- Corresponding Author:
- N. S. Rana
Parul Institute of Pharmacy and Parul Institute of Pharmacy and Research, P. O. Limda, Waghodia, Vadodara-391 760, India
E−mail: rananikesh@ymail.com
| Date of Submission | 02 March 2013 |
| Date of Revision | 02 July 2013 |
| Date of Acceptance | 05 July 2013 |
| Indian J Pharm Sci 2013;75(5):599-602 |
Abstract
A rapid and sensitive RP-HPLC method with UV detection (244 nm) for routine analysis of montelukast sodium and ebastine in a pharmaceutical formulation (Ebast-M) was developed. Chromatography was performed with mobile phase containing a mixture of methanol:acetonitrile:ammonium acetate (80:10:10, % v/v/v), pH of mobile phase was adjusted 5.5 using glacial acetic acid and flow rate was 1.2 ml/min. The method was validated for linearity, accuracy, robustness and intermediate precision. The linearity was established over the concentration range of 0.01−0.06 mg/ml for both drugs. The correlation coefficients (r 2) for ebastine and montelukast were 0.9989 and 0.9955, respectively. Statistical analysis of the data showed that the method was precise, accurate, reproducible and selective for the analysis of ebastine and montelukast drugs. The method was successfully employed for the determination of ebastine and montelukast in commercially available tablet dosage form.
Keywords
Ebastine, method validation, montelukast, reversed−phase high−performance liquid chromatography, tablets
Montelukast Sodium (MNK) chemically, (S, E)−2−(1−((1−(3−(2−(7−chloroquinolin−2−yl) vinyl) phenyl) 3−(2−(2−hydroxypropan−2yl) phenyl) propylthio) methyl) cyclopropyl) acetic acid[1,2] is a cysteinyl leukotriene receptor antagonist used for the maintenance treatment of asthma and to relieve symptoms of seasonal allergies[3−5]. Literature survey reveals that assay of MNK in bulk and tablet dosage form is official in Indian Pharmacopoeia 2010[6]. Ebastine (EBA), chemically, 4−(4−benzhydryloxy−1−piperidyl)−1−(4−tert−butylphenyl) butan−1−one is a nonsedating H1 antihistamine. Assay of ebastine in bulk form is official in British Pharmacopoeia[7]. Literature survey reveals that analytical methods, including UV spectrophotometer, and HPLC methods, are available for the determination of montelukast in pharmaceutical dosage forms. The analytical methods reported for estimation of MNK and EBA alone and with other drug combination are UV spectrophotometry[8−11], HPLC[12−14], HPLC/PDA[15], LC−MS[16] and HPTLC[17−19] methods. The combination of MNK and EBA has recently been introduced into the market. However, so far, no method was reported for the simultaneous estimation of MNK and EBA, in combination. The proposed method is rapid, simple, accurate and reproducible, and can be successfully employed in the routine analysis of both these drugs simultaneously, in tablet dosage form. The proposed method is optimised and validated as per the ICH guidelines[20]. In the present work, a successful attempt has been made to estimate both these drugs simultaneously using RP−HPLC method. This study attempts to describe a rapid and sensitive HPLC method with UV detection, useful for routine quality control of MNK and EBA in pharmaceutical formulation. The structure of MNK and EBA was depicted in fig. 1.
A pure drug MNK was obtained as gift sample from Alembic Pharmaceuticals, Vadodara and EBA was procured as gift sample from Kivi Labs Pvt, Vadodara. Methanol AR was used as solvent. Calibrated glassware was used throughout the work. The marketed formulation studied was Ebast−M tablets manufactured by Micro Labs Pvt Ltd. Each tablet contains 10 mg MNK and 10 mg EBA.
The HPLC instrument was a Shimadzu chromatographic system equipped with an injection valve (Rheodyne 033381); Shimadzu LC 10AT UV dual absorbance detector (SPD 10A) was used. A reversed−phase C18 column (Phenomenex−Gemini 150×4.6 mm, 5 μm) was used. Peak area integration was performed using Sprinchrome software. A mixture of methanol:acetonitrile:ammonium acetate (80:10:10, % v/v/v) pH adjusted 5.5 using glacial acetic acid was used as a mobile phase with 1.2 ml/min flow rate.
Accurately weighed quantity of MNK (100 mg) and EBA (100 mg) were transferred to two separate 100 ml volumetric flasks, dissolved in little amount of methanol and diluted to the mark with methanol and was denoted as stock solutions (1000 μg/ml of MNK and 1000 μg/ml of EBA). From each of this solution, 5 ml was diluted to 50 ml with methanol to give 100 μg/ml of MNK solution and 100 μg/ml of EBA solution.
Ten tablets were weighed and crushed to obtain a fine powder. An accurately weighed tablet powder equivalent to about 10 mg of MNK and 10 mg of EBA was transferred to 100 ml volumetric flask and dissolved in 50 ml of mobile phase. The volume was made up to the mark using mobile phase as solvent. The resulting solution was filtered through vacuum filter and 2 ml of this filtrate was appropriately diluted to get concentration of 20 μg/ml of MNK and 20 μg/ml of EBA.
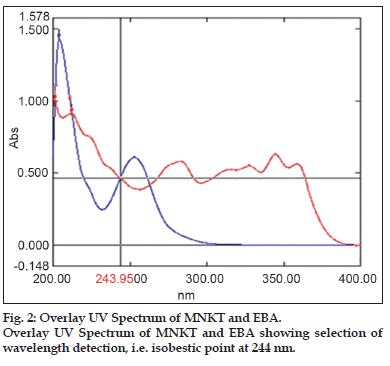
UV spectrum of both the standard solutions was taken separately. After overlay, it was found that at 244 nm both drugs showed same absorbance (isobestic point); hence 244 nm was selected as analytical wavelength for both drugs. The standard solution of MNK and EBA were scanned under the chromatographic condition described above.
The calibration curve was plotted with six concentrations of 10−60 μg/ml for MNK and EBA. The curve was repeated thrice for each dilution. The linearity was evaluated by linear regression analysis, which was calculated by the least square regression method. Before injecting solutions, the column was equilibrated for at least 30 min with the mobile phase flowing through the system. Peak area was recorded for all the solutions. The correlation graph was constructed by plotting the peak area obtained at the optimum wavelength of detection versus the injected amounts.
The mobile phase was a mixture of methanol:acetonitrile:ammonium acetate (80:10:10% v/v/v), pH of mobile phase was adjusted 5.5 using glacial acetic acid and flow rate was 1.2 ml/min. The UV detector wavelength was set at 244 nm (fig. 2) and the temperature was set at 26±2°C.
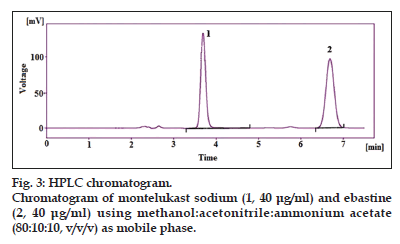
The applied chromatographic conditions permitted a good separation of MNK and EBA, (fig. 3), no drug decomposition was observed during the analysis. Results obtained in the study of the solution (both reference and sample solution) where it can be noticed that solutions were stable for 48 h, as during this time the results does not decrease below the minimum percentage (96%). The chromatographic separation, as explained above was carried out with HPLC to evaluate the chromatographic parameters, column efficiency (N), tailing factor and resolution between two consecutive peaks (Table 1). Resolution observed for MNK and EBA was 5.940. Number of plates observed for MNK and EBA were 4659.66 and 4312, respectively. Tailing factors observed for MNK and EBA were 0.995 and 1.119, respectively. The data for accuracy for MNK and EBA are presented in Table 2. The recovery range for MNK and EBA were found to be 98.97−99.83% and 98.75−99.63%, respectively. The calibration curve for MNK and EBA was found to be linear over the range of 10−60 μg/ml for both drugs. The %RSD for repeatability was found to be 1.758 and 1.403 for MNK and EBA, respectively. The range for intraday precision %RSD was found to be 1.772−1.86% for MNKT and 1.096−1.944% for EBA. The range for intraday precision %RSD was found to be 0.563−1.248% for MNK and 0.347−1.396% for EBA. The %RSD for pH Change (±0.2) were found to be 1.028−1.284 and 1.046−1.169 for MNK and EBA, respectively, and for mobile phase ratio change were found to be 1.207−1.462 and 1.138−1.308 for MNK and EBA, respectively. The limit of detection for MNK and EBA was found to be 0.819 μg/ml and 0.667 μg/ml, whereas the limit of quantification for MNK and EBA was found to be 2.484 μg/ml and 2.02 μg/ml, respectively. All validation parameters were summarised in Table 3. The proposed highperformance liquid chromatographic method has been evaluated over the linearity, precision, accuracy and specificity and proved to be convenient and effective for the quality control of MNK and EBA in pharmaceutical dosage forms. The measured signal was shown to be precise, accurate and linear over the concentration range tested with a correlation coefficient better. Method validation has been demonstrated by various tests for linearity, accuracy, precision and ruggedness. In order to demonstrate the stability of both standard and sample solutions during analysis, both solutions were analysed over a period of 5 h at room temperature. The results show that for both solutions, the retention time and peak area of MNK and EBA remained almost unchanged (% RSD less than 2.0) and no significant degradation within the indicated period, thus indicated that both solutions were stable for at least 5 h, which was sufficient to complete the whole analytical process.
| System suitability parameters |
Drugs | Observed values |
IP’ 2007 specification |
|---|---|---|---|
| Resolution (Rs) | Between MNK and EBA |
5.94 | >1.5 |
| Number of theoretical plates (N) |
MNK | 4966 | Not less than 2000 |
| EBA | 4464 | ||
| Tailing factor (Tf) | MNK | 1.034 | Not greater than 2.0 |
| EBA | 1.152 |
Table 1: System Suitability Parameters Of Mnk And Eba.
| Amt of sample (μg/ml) | Amt. of drug added (μg/ml) | Amt. recovered mean±SD (n=3) (μg/ml) | % recovery n=3 | ||||
|---|---|---|---|---|---|---|---|
| MNK | EBA | MNK | EBA | MNK | EBA | MNK | EBA |
| 10 | 10 | 9.915±0.065 | 9.963±0.62 | 99.15 | 99.63 | ||
| 10 | 10 | 20 | 20 | 19.91±0.28 | 19.75±1.55 | 99.55 | 98.75 |
| 10 | 10 | 30 | 30 | 29.95±0.26 | 29.79±0.32 | 99.83 | 99.3 |
| 10 | 10 | 40 | 40 | 39.59±0.25 | 39.69±0.42 | 98.97 | 99.22 |
Table 2: Accuracy Data Of Mnk And Eba.
| Parameters | MNK | EBA |
|---|---|---|
| Linearity range | 10-60 μg/ml | 10−60 μg/ml |
| Correlation coefficient | 0.9989 | 0.9955 |
| Precision (% RSD) | ||
| Repeatability | 1.758 | 1.403 |
| Intraday (n=3) | 1.772-1.86 | 1.096-1.944 |
| Interday (n=3) | 0.563-1.248 | 0.347-1.396 |
| Mean % recovery | 98.87−99.83 | 98.75−99.3 |
| Robustness | ||
| pH Change (±0.2) | 1.028-1.284 | 1.046-1.169 |
| Mobile phase ratio change | 1.207-1.462 | 1.138-1.308 |
| Limit of detection | 0.819 μg/ml | 0.667 μg/ml |
| Limit of quantification | 2.484 μg/ml | 2.02 μg/ml |
Table 3: Summary Of Validation Parameters.
Acknowledgements
The authors thank to Alembic Pharmaceuticals, Vadodara and Kivi Labs, Vadodara for providing gift samples of standards of montelukast sodium and ebastine, respectively.
References
- Anonymous, In-process Revision. Pharmacopeial Forum; 2010.p. 36.
- The Merck Index. 14th ed. New Jersey: Merck Research laboratories; 2006. p. 591, 1081.
- Rang HP, Dale MM, Ritter JN, Moore PK. Pharmacology. 6th ed. Edinburgh, New York: Churchill Livingstone; 2008. p. 361-3.
- Satoskar RS, Bhandarkar SD. Pharmacology and Pharmacotherapeutics. 20th ed. Mumbai: Popular Prakashan; 2008. p. 357-8.
- Tripathi KD. Essential of Medical Pharmacology. 6th ed. New Delhi: Jaypee Brothers Ltd.; 2008. p. 222-3.
- Indian Pharmacopoiea, Vol. 2. New Delhi: Controller of Publication, Govt. of India, Ministry of Health and Family Welfare; 2010. p. 1704-6.
- British Pharmacopoiea, Vol 1. London: HMSO Publication; 2009. p. 735.
- Patel DJ, Patel SA, Patel SK. Simultaneous determination of montelukast sodium and bambuterol hydrochloride in tablet dosage form by ultraviolet spectrophotometry (Dual wavelength method). Int J Pharm Biol Res 2010;1:71-5.
- Pawar V, Pai1 S, Rao GK. Development and validation of UV spectrophotometric method for simultaneous estimation of montelukast sodium and bambuterol hydrochloride in bulk and tablet dosage formulation. Jordan J Pharm Sci 2008;1:152-8.
- Kamyar P, Zahra MK, Alireza G, Mahmoud RS, Hossein A. Spectrophotometric Determination of CetrizineAndMontelukast In Prepared Formulations. Int J Pharm PharmSci 2011;3:128-30.
- Chavda RS, Vaghela JP, Patel PB, Shah JS. UV spectrophotometic methods for simultaneous estimation of montelukast sodium and desloratadinein combined tablet dosage form. Inventi Rapid: Pharm Analysis and Quality Assurance; 2012. p. 412-6.
- Patil S, Pore YV, Kuchekar BS, Mane A, Khire VG. Determination of montelukast sodium and bambuterol hydrochloride in tablets using RP-HPLC. Indian J Pharm Sci 2009;71:58-61.
- Ravisankar M, Uthirapathy S, Thangadurai A, Dhanapal K. simultaneous estimation of fexofenadine hydrocloride and montelukast sodium in bulk drug and marketed formulation by RP-HPLC method. Int Res J Pharm 2012;3:356-9.
- Eswarudua MM, Junapudia S, Charya TN. RP-HPLC method development and validation for simultaneous estimation of montelukast sodium and levocetirizinedihydrochloride in tablet dosage form. Int J Pharm World Res 2011;2:1-18.
- Radhakrishna T, Narasaraju A, Ramakrishna M, Satyanarayana A. Simultaneous determination of montelukast and loratadine by HPLC and derivative spectrophotometric methods. J Pharm Biomed Anal 2003;31:359-68.
- Kang W, Liu KH, Ryu JY, Shin JG. Simultaneous determination of ebastine and its three metabolites in plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B AnalytTechnol Biomed Life Sci 2004;813:75-80.
- Suparna ST, Snehal JM, Atul SR, Ajinkya RN, Lohidasan S, Kakasaheb RM. Method development and validation for the simultaneous determination of fexofenadine hydrochloride and montelukast sodium in drug formulation using normal phase high-performance thin-layer chromatography. Anal Chem 2012, Article ID 924185, 7 pages.
- Rathore AS, Sathiyanarayanan L, Mahadik KR. Development of validated HPLC and HPTLC methods for simultaneous determination of levocetirizinedihydrochloride and montelukast sodium in bulk drug and pharmaceutical dosage form. Pharm Anal Acta 2010;1:106-11.
- Rote A, Niphade V. Determination of montelukast sodium and levocetirizinedihydrochloride in combined tablet dosage form by
- HPTLC and first-derivative spectrophotometry. J LiqChromatogrRelatTechnol 2011;34:155-67.
- ICH, Q2 (R1): Validation of Analytical Procedures: Text and Methodology, Geneva; 2005.