- *Corresponding Author:
- Aruna Jadhav
Department of Quality Assurance, Bharati Vidyapeeth’s College of Pharmacy, Belapur, Navi Mumbai 400614, India
E-mail: aruna.jadhav@bvcop.in
| Date of Received | 22 July 2021 |
| Date of Revision | 11 January 2022 |
| Date of Acceptance | 26 July 2022 |
| Indian J Pharm Sci 2022;84(4):1083-1088 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Oxitard capsules, a polyherbal formulation, is an effective natural antioxidant that prevents oxidative stress-related tissue damage and is helpful for coronary artery disease. The aim of the present work was systematic development and validation of the high-performance thin-layer chromatographic method for simultaneous quantitative estimation of mangiferin and gallic acid from oxitard capsules. Both markers, mangiferin and gallic acid were separated on a pre-coated silica gel 60 F254 thin-layer chromatographic plate, using toluene:ethyl acetate:formic acid:methanol (4:6:0.8:2 v/v/v/v) as mobile phase. The retention factor of mangiferin and gallic acid was found to be 0.28±0.03 and 0.78±0.03, respectively. The linear regression analysis data for the calibration plots of drugs scanned at isosbestic point 299 nm showed a good linear relationship with r2=0.9931 and r2=0.9901, over the concentration range of 300 to 700 ng/spot and 500 to 900 ng/spot for mangiferin and gallic acid, respectively. The validation parameter followed for simultaneous estimation of these polyherbal drugs were linearity, precision, accuracy, robustness, ruggedness and sensitivity (limit of detection and limit of quantitation). The method was found to be precise, accurate, sensitive and robust meeting all the parameters as per International Council for Harmonisation guideline Q2 (R1). Thus, the developed high-performance thin-layer chromatography method can conveniently be employed for simultaneous detection and quantification of mangiferin and gallic acid in commercial polyherbal formulation.
Keywords
High performance thin layered chromatography, simultaneous estimation, mangiferin, gallic acid, oxitard capsule
Herbal medicines have existed since the prehistoric period worldwide with long recorded history. They were used in ancient Chinese, Greek, Egyptian and Indian medicine for different therapies purposes; whereas the Native Americans and African use herbs in their healing rituals as a part of their culture. Due to the scientific evolution today, more and more pharmacologically active ingredients of the Ayurvedic medicines as well as their usefulness in drug therapy have been identified. The desired healing effect of herbals are obtained from the phytochemical constituent present in it, such as saponins, tannins, alkaloids, alkenyl phenols, flavonoids, terpenoids, phorbol esters and sesquiterpenes lactones. Polyherbalism gives some benefits because of its synergic effect which is not available in a single herbal formulation[1].
Himalaya oxitard capsule is a polyherbal formulation that helps to control coronary artery disease, dermatitis and antioxidants. Due to their gastroprotective properties, these capsules also ensure ideal gastrointestinal functions. Himalaya oxitard capsules are also known to possess immunomodulatory properties which help to boost the body's ability to fight off infections[2]. The formulation consists of nine different herbs. Each capsule contains extracts of amra kernel (Mangifera indica, Anacardiaceae family) 94 mg, ashwagandha root (Withania somnifera, Solanaceae family) 71 mg, gajara root (Daucus carota, Apiaceae family) 47 mg, ashtimadhu root (Glycyrrhiza glabra, Fabaceae family) 29 mg, dhraksh fruit (Vitis vinifera, Vitaceae family) 12 mg, powder of amalaki fruit (Phyllanthus emblica, Phyllanthaceae family) 141 mg, lavanga flower bud (Syzygium aromaticum, Myrtaceae family) 29 mg, Yashada bhasma 2.5 mg and also oil of godhuma wheat germ (Triticum aestivum, Poaceae family) 6.5 mg.
Oxitard capsule contains extracts of amra kernel and amalaki fruit powder in higher proportion. Mangiferin is present in the amra kernel. Gallic acid is found to be present in amalaki fruit, amra kernel, lavanga flower bud and draksha fruit. Therefore, for standardization of oxitard capsules, mangiferin and gallic acid were selected as markers as shown in fig. 1.
Mangiferin is a C-glucosyl xanthone present in considerable levels in higher plants and different parts of the mango fruit, such as the peel, stalks, leaves, barks, kernel and stone[3]. Numerous studies confirm that mangiferin, through different mechanisms, has various biological activities such as anti-cancer[4], antioxidant[5], anti-inflammatory, anti-diabetic[6], cardiovascular protection[7], neuroprotective[8], antiviral, enhanced immunity, gastroprotective effect, analgesic activity and radioprotection, as seen in the experiments on mice. The neuroprotective effect was patented, also anti-allergic properties and hepatoprotective activity have been published[9]. Gallic acid, a phenolic compound, also known as 3,4,5-trihydroxybenzoic acid, is a naturally occurring secondary metabolite found in various plants, vegetables, nuts and fruits[10]. Gallic acid can be useful as an additive in the food industry as it can inhibit the oxidation and rancidity of oils and fats ascribed to their free radical scavenging and antioxidant nature. Besides the edible uses of gallic acid and its ester derivatives such as lauryl gallate, propyl gallate, octyl gallate, tetradecyl gallate and hexadecyl gallate, as flavoring agents and preservatives in the food industry, there are various pharmacological activities of these phytochemicals, with emphasis on antioxidant[11], antimicrobial[12], anti-inflammatory[13], anticancer[14], cardioprotective, gastroprotective and neuroprotective effects[15].
The present work deals with analytical method development for standardization of oxitard capsules and method validation, which was carried by using International Council for Harmonisation (ICH) guidelines Q2 (R1)[16]. Reference standards mangiferin and gallic acid were procured from Yucca enterprises, Mumbai. Analytical grade reagents and solvents used were ethyl acetate, toluene, formic acid, methanol (SD Fine Chem, Limited, Mumbai). Himalaya oxitard capsules were purchased from a pharmacy.
The High Performance Thin Layer Chromatography (HPTLC) system used consisted of a Camag Linomat V sample applicator containing a 100 µlHamilton syringe. The samples and standard solutions of markers were spotted in the form of bands (6 mm width) at the bottom of the chromatographic plates at a distance of 10 mm. Aluminum Thin Layer Chromatography (TLC) plates (10×10 cm, 250 mm thickness Merck) precoated with silica gel 60 F254 were used for spotting. The slit dimension was kept at 5×0.45 mm and a scanning speed of 10 mm/s. Analysis was carried out using Camag TLC scanner-III for scanning at 299 nm, which was found to be the absorptive wavelength of both mangiferin and gallic acid. Camag WinCATS software was used for application and scanning. Camag glass twin-trough a glass chamber (10×10 cm) saturated with mobile phase was used for the development of the plates. The mobile phase consisted of toluene:ethyl acetate:formic acid:methanol (4:6:0.8:2 v/v/v/v) was used for chromatogram development in the ascending mode to the migration distance of 90 mm. Before the study, the chamber was saturated with the mobile phase for 20 min at room temperature.
A separate stock solution of mangiferin and gallic acid was prepared by dissolving 10 mg of precisely weighed marker in a 10 ml volumetric flask with methanol to get a 1000 ppm stock solution. From this stock solution, working solutions were prepared by accurately diluting aliquots of the stock solution. 10 capsules were taken and weighed precisely and individually and the amount of capsule content equivalent to 10 mg of extract of amra kernel and 10 mg of powder of amalaki fruit was dissolved separately in 10 methanol followed by sonication for 30 min. This solution was filtered through whatman filter paper no. 42 and then the volume was made up to 10 ml using methanol.
Validation of the developed method was carried out according to the ICH guidelines Q2 (R1). The parameters checked were linearity, specificity, Limit Of Detection (LOD), Limit Of Quantification (LOQ), precision, accuracy (recovery) and robustness. The linearity of an analytical procedure is its ability (within a given range) to obtain test results that are directly proportional to the concentration (amount) of analyte in the sample. Linearity was observed by the plotting graph of drug concentration against peak area for each standard.
The precision of an analytical procedure expresses the closeness of agreement (degree of scattering) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. The present method was validated for intraday and interday precision. Intraday precision was determined in triplicate with the same method on the same day for three different concentrations of mangiferin (300, 500 and 700 ng/spot) and gallic acid (500, 700 and 900 ng/spot). The interday precision of the method was done by performing a similar method on different days under the same set of experimental situations. The results were reported as percent Relative Standard Deviation (% RSD).
The accuracy of an analytical procedure expresses the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and the value found. Recovery studies were carried out using the addition of standard methods. Known concentrations of the sample were applied and then spiked with 80, 100 and 120 % w/w amount of analyte in triplicate and the accuracy was calculated as percent of analyte recovered.
The robustness of an analytical procedure is a measure of its capacity to stay unchanged by small, but deliberate variations in method parameters and indicates its reliability during normal usage. The robustness was studied in triplicate at 300 and 700 ng/spot for mangiferin and 500 and 900 ng/spot for gallic acid by deliberately making small changes in the mobile phase composition (3.8:5.8:0.8:2 v/v/v/v) and (4.2:6.2:0.8:2 v/v/v/v) and change in saturation time (20±5 min).
The ruggedness of an analytical method is the degree of reproducibility of test results obtained by the analysis of the same samples under a variety of conditions, such as different laboratories, different analysts, different instruments, different reagents, etc. Specificity is the ability to assess unequivocally the analyte in the presence of components that may be expected to be present. The specificity of the method was checked by overlaying the spectra of standards with the spectra of the marketed formulation. The method was found to be specific since the spectra of both standards matched with the spectra of the spots in the formulation at the same Retention Factor (Rf) values as that of standards.
The sensitivity of this method was studied in terms of LOD and LOQ. The LOD of an analytical procedure is the lowest amount of analyte present in a sample which can be detected but not necessarily quantitated as an exact value. The Detection Limit (DL) may be expressed as DL=3.3 standard deviation/slope of the calibration curve (σ/S). The LOQ of an analytical procedure is defined as the lowest amount of analyte within a sample which can be quantitatively determined with suitable precision and accuracy. The Quantitation Limit (QL) may be expressed as QL=10 σ/S.
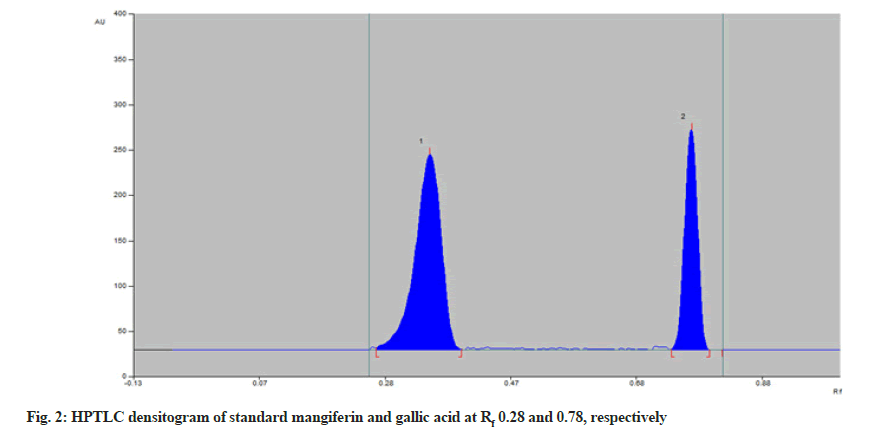
Standard solutions of mangiferin and gallic acid were used for chromatographic separation. The spots of mangiferin and gallic acid were applied on TLC plates with Linomat V Sample applicator. Various trials were made using many solvents in different proportions for the development of the mobile phase. The optimized mobile phase consisting of toluene:ethyl acetate:formic acid:methanol (4:6:0.8:2 v/v/v/v) showed satisfactory resolution at Rf 0.28 for mangiferin and 0.78 for gallic acid (fig. 2) respectively. Densitometric measurements were obtained with Camag TLC scanner III operated by winCATS software at 299 nm. The linearity of an analytical method was determined by the response of mangiferin and gallic acid by applying different concentrations 300 to 700 ng/spot and 500 to 900 ng/spot respectively. Mangiferin and gallic acid showed linear relationship with r2 values 0.9931 (y=10.759x+234.5) and 0.9901 (y=6.7733x+293.5), respectively (Table 1). Precision (intraday and interday) of the method was calculated for each drug. The result of intraday and interday precision was studied as % RSD (Table 2). The low % RSD indicated the method is precise.
| Parameters | Mangiferin | Gallic acid |
|---|---|---|
| Rf value | 0.28 | 0.78 |
| Linearity range (ng/spot) | 300-700 | 500-900 |
| Correlation coefficient (r2) | 0.9931 | 0.9901 |
| Regression equation | y=10.759x+234.5 | y=6.7733x+293.5 |
| LOD (ng/spot) | 67.45 | 51.13 |
| LOQ (ng/spot) | 204.41 | 154.96 |
| Percent recovery (n=3) | 100.52 % | 101.19 % |
| Precision (% RSD) | Precise | Precise |
| Robustness | Robust | Robust |
| Ruggedness | Unaffected | Unaffected |
| Specificity | Specific | Specific |
Note: n=3 represents each is an average of three observations
Table 1: Results of Method Validation Studies.
| Compound | Concentration (ng/spot) | Intraday | Interday | ||||
|---|---|---|---|---|---|---|---|
| Mean area (AU) | SD | % RSD | Mean area (AU) | SD | % RSD | ||
| Mangiferin | 300 | 3477.36 | 48.49 | 1.39 | 3329.26 | 36.19 | 1.08 |
| 500 | 5866.73 | 76.5 | 1.3 | 5637.3 | 67.2 | 1.19 | |
| 700 | 8723.06 | 145.44 | 1.55 | 9008.26 | 50.57 | 0.56 | |
| Gallic acid | 500 | 5564.06 | 92.73 | 1.66 | 5485.06 | 62.22 | 1.13 |
| 700 | 7132.66 | 61.01 | 0.85 | 7191.33 | 54.79 | 0.76 | |
| 900 | 8889.4 | 181.97 | 2.04 | 9091.53 | 34.48 | 0.37 | |
Note: Each result is an average of three observations. Concentration levels used for a precision parameter was 300, 500, 700 ng/spot for mangiferin and 500, 700, 900 ng/spot for gallic acid
Table 2: Intraday and Interday Precision Results for Mangiferin and Gallic Acid.
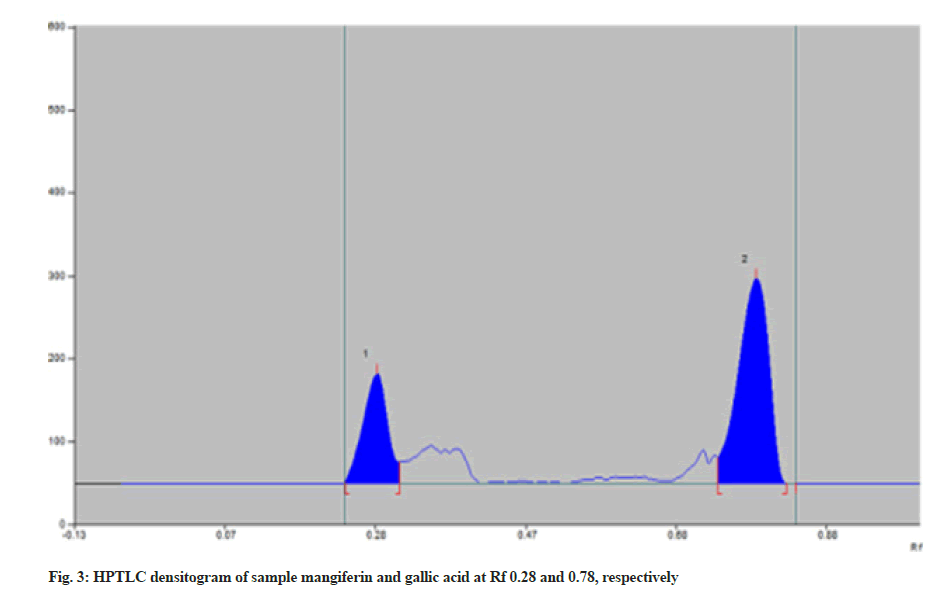
The recovery of mangiferin and gallic acid was found to be 100.52 % and 101.19 %, respectively (Table 3). Results from accuracy studies were in an acceptable range (98 %-102 %), indicating that the recovery of the proposed method was good. The effect of deliberate changes in the composition of mobile phase and saturation time was studied as % RSD. Low % RSD indicated the method is robust (Table 1). Ruggedness was determined by changing the analytes and different batches of chemicals. Two concentrations of each marker were spotted in triplicate i.e., 300 ng/spot and 700 ng/spot for mangiferin and 500 ng/spot and 900 ng/spot for Gallic acid. Low % RSD indicated the method is unaffected. The LOD values for mangiferin and gallic acid were 67.45 and 51.13 ng, respectively and the LOQ values were 204.41 and 154.96 ng, respectively which shows the sensitivity of the method (Table 1). The specificity of the method was determined by analyzing standard drugs and samples. The spots for mangiferin and gallic acid in the sample were confirmed by comparing Rf and spectra of spot with that of standard. The purity of the proposed method was determined by superimposing the spectrum of both standard and sample peaks and confirmed for its purity. The samples were spotted in triplicate on a TLC plate and developed. In the sample densitogram, gallic acid and mangiferin were observed at Rf 0.28 and 0.78 respectively (fig. 3). The quantified amount of mangiferin and gallic acid from the Himalaya’s oxitard capsules was found to be 2.03 % w/w and 0.44 % w/w, respectively.
| Drug | % Level | Initial amount (ng) | Total amount (ng) | Amount of drug recovered (ng) | % Recovery | % Average |
|---|---|---|---|---|---|---|
| Mangiferin | 80 | 115 | 207 | 206 | 99.74 | 100.52 |
| 100 | 115 | 234 | 234 | 101.7 | ||
| 120 | 115 | 254 | 254 | 100.39 | ||
| Gallic acid | 80 | 126 | 226 | 228 | 100.88 | 101.19 |
| 100 | 126 | 252 | 257 | 101.98 | ||
| 120 | 126 | 277 | 279 | 100.72 |
Note: Each result is an average of three observations performed at 80 %, 100 % and 120 % levels
Table 3: Recovery Study of Mangiferin and Gallic Acid.
A rapid, precise, accurate and sensitive HPTLC method was successfully developed for simultaneous estimation of mangiferin and gallic acid in the oxitard capsules. The developed method was validated as per ICH Q2 (R1) guidelines. This method can also be used for the standardization of other formulations containing these phytoconstituents.
Conflict of interests:
The authors declared no conflict of interests.
References
- Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: Concept of ayurveda. Pharmacogn Rev 2014;8(16):73-80.
[Crossref] [Google Scholar] [PubMed]
- Oxitard: Natural Antioxidant. Himalaya wellness (India); 2021.
- Imran M, Arshad MS, Butt MS, Kwon JH, Arshad MU, Sultan MT. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis 2017;16(1):84.
[Crossref] [Google Scholar] [PubMed]
- Mei S, Ma H, Chen X. Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem Toxicol 2021;149:111997.
[Crossref] [Google Scholar] [PubMed]
- Mendoza-Sarmiento G, Rojas-Hernández A, Galano A, Gutiérrez A. A combined experimental–theoretical study of the acid–base behavior of mangiferin: Implications for its antioxidant activity. RSC Adv 2016;6(56):51171-82.
- Iliya IA, Mohammed B, Akuyam SA, Yaro JD, Bauchi ZM, Tanko M, et al. Immunohistochemical evaluation of the antidiabetic potentials of S-allyl-cysteine (garlic) and mangiferin (mango) in type 2 diabetic rat models. S Afr J Med 2016;3(1):25-31.
- Suchal K, Malik S, Khan SI, Malhotra RK, Goyal SN, Bhatia J, et al. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK pathways. Sci Rep 2017;7(1):42027.
[Crossref] [Google Scholar] [PubMed]
- Lum PT, Sekar M, Gan SH, Pandy V, Bonam SR. Protective effect of mangiferin on memory impairment: A systematic review. Saudi J Biol Sci 2021;28(1):917-27.
[Crossref] [Google Scholar] [PubMed]
- Morozkina SN, Nhung Vu TH, Generalova YE, Snetkov PP, Uspenskaya MV. Mangiferin as new potential anti-cancer agent and mangiferin-integrated polymer systems: A novel research direction. Biomolecules 2021;11(1):79.
[Crossref] [Google Scholar] [PubMed]
- Bai J, Zhang Y, Tang C, Hou Y, Ai X, Chen X, et al. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed Pharmacother 2021;133:110985.
[Crossref] [Google Scholar] [PubMed]
- Badhani B, Sharma N, Kakkar R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv 2015;5(35):27540-57.
- Sarjit A, Wang Y, Dykes GA. Antimicrobial activity of gallic acid against thermophilic Campylobacter is strain specific and associated with a loss of calcium ions. Food Microbiol 2015;46:227-33.
[Crossref] [Google Scholar] [PubMed]
- Karimi-Khouzani O, Heidarian E, Amini SA. Anti-inflammatory and ameliorative effects of gallic acid on fluoxetine-induced oxidative stress and liver damage in rats. Pharmacol Rep 2017;69(4):830-5.
[Crossref] [Google Scholar] [PubMed]
- Subramanian AP, John AA, Vellayappan MV, Balaji A, Jaganathan SK, Supriyanto E, et al. Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Adv 2015;5(45):35608-21.
- Kahkeshani N, Farzaei F, Fotouhi M, Alavi SS, Bahramsoltani R, Naseri R, et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J Basic Med Sci 2019;22(3):225.
[Crossref] [Google Scholar] [PubMed]
- ICH guidelines. Validation of Analytical Procedures: Text and Methodology Q2 (R1). ICH Harmonised Tripartite Guideline, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 1994. p. 1-13.