- *Corresponding Author:
- M. Mansour
Department of Pharmaceutical Sciences, College of Pharmacy, Riyadh 11481, Saudi Arabia
E-mail: mansoura@ksau-hs.edu.sa
| Date of Received | 11 December 2024 |
| Date of Revision | 18 December 2024 |
| Date of Acceptance | 26 December 2024 |
| Indian J Pharm Sci 2024;86(6):1994-1999 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study, a validated method was used to analyze two Metoclopramide products available in the market like premosan and primperan. The chromatographic separation involves a mobile phase with a 50:50 mixture of acetonitrile and buffer 4.6 (v/v). A C18 rapid resolution column (4.6×100 mm, 3.5 μm, Agilent high performance liquid chromatography column) was used for the separation. The λ max of metoclopramide was found to be 248 nm. The analysis was performed with a 20 μl injection volume and a 3.0 min run time. The method showed linearity for metoclopramide within the concentration range of 2-10 μg/ml. Method validation followed International Council for Harmonisation guidelines, assessing specificity, selectivity, linearity, accuracy, precision and the lower limits of quantification and lower limits of detection. The lower limits of quantification and lower limits of detection for metoclopramide were determined to be 0.80 μg/ml and 0.26 μg/ml, respectively. These methods were found to be effective for the precise quantitative analysis of metoclopramide in pharmaceutical formulations.
Keywords
Metoclopramide, validation, percentage assay, high performance liquid chromatography
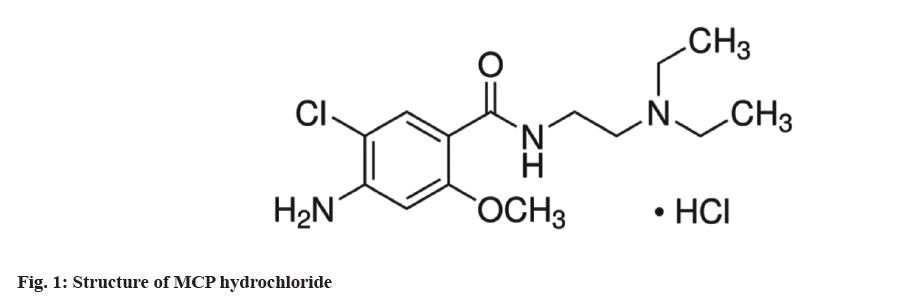
Metoclopramide (MCP), commonly referred to as 4-amino-5-chloro-[N]-[2-(diethylamino)ethyl]-2- methoxybenzamide (fig. 1), is primarily used in adult and pediatric medicine as an antiemetic and gastrointestinal prokinetic drug. It is also used to treat gastroparesis in diabetic patients[1-4]. Additionally, it helps alleviate nausea, vomiting, satiety, and loss of appetite. Numerous studies have focused on its determination in different dose forms due to its broad application and excellent therapeutic effect in empirical and clinical medicine. A number of analytical techniques, including High Performance Liquid Chromatography (HPLC), spectrofluorimetric, electrochemical, chemiluminescence and others, have been proposed for the quantification of MCP in pharmaceutical products and biological fluids as tandem mass spectrophotometry’s[5-12]. According to the United States Pharmacopeia (USP) and the British Pharmacopoeia (BP) liquid chromatography is the recognized technique for Methyl Cellulose Precipitable (MCP) assay[13,14]. Many of the aforementioned methods need heating, are difficult for routine analysis and require expensive or complex equipment, or have relatively low selectivity.
Perhaps the most often reported methods for determining MCP in medicines are titration and visible spectrophotometry[15-22]. Furthermore, reviews of the literature showed that the spectrophotometric approach was used to estimate MCP in the injection dosage form using 0.1 M Hydrogen Chloride (HCl) as the solvent and direct Ultraviolet (UV) spectroscopy at a wavelength of 270 nm with maximum absorbance[23]. Compared to other dose forms like tablets or liquids, injections have minimal to no interference because they hardly include any excipients. In order to quantify MCP in bulk using UV spectrophotometry when its degradation products are present, a stability indicating approach has been developed. The results showed that the Limit of Quantification (LOQ) was 9.89 μg/ml and the Limit of Detection (LOD) was 3.26 μg/ml[24]. One potential substitute for the many analytical techniques currently in use is the redox titrimetric approach, which is not only sensitive and economical, but also somewhat accurate. Through the use of redox techniques, this study creates straightforward, accurate and economical methods for determining MCP in pure preparation, injectable, and tablets.
The N-oxidation reaction, which used potassium hydrogen peroxy-mono-sulfate as the titrant, served as the basis for the titrimetric approach. After a predetermined amount of time, a known excess of reagent is added, and the remaining reagent is measured iodometrically. In order to produce a yellow-brown chromogen (triiodide) with a wavelength of maximum absorption at 350 nm, the spectrophotometric approach relies on the oxidation of MCP with Oxone® in an alkali medium (pH: 9.9) and subsequent coupling with iodide in an acidic medium (pH: 4.0)[25].
The main aim of the present study was to develop a simple, cost-effective, rapid and sensitive spectrophotometric method for determining MCP in pharmaceutical formulations (tablets) and to compare the sensitivity of this method with all previously reported methods. In addition, we will modify a new HPLC method for MCP using different column and mobile phase.
Materials and Methods
Materials:
MCP standard powder (MCP hydrochloride, Glentham, United Kingdom (UK)) was used to prepare the serial dilutions of stock standard solution.
Reagents and chemicals:
Acetonitrile Chromasolv™, for HPLC, ≥ 99.9 %, was procured and collected from Honeywell (United States of America (USA)), ultra-pure water system (Milli-Q® Integral 5 (ZRXQ005T0) Millipore®) (USA), was used to have HPLC grade water and sodium acetate, Loba Chemie, India.
Chromatographic conditions:
HPLC (1260 Infinity, Agilent Technologies) with a Variable Wavelength Detector (VWD) was used for data acquisition, recording and chromatographic integration. The instrument’s software (OpenLab ChemStation) was employed for method validation and development of MCP.
Mobile phase preparation:
The aqueous mobile phase consists of a mixture of acetonitrile and buffer (50:50, v/v) under isocratic conditions. The mobile phase was filtered through a fine filter (pore size 0.45 μm), then sonicated and degassed in an ultrasonic bath (CPX5800H-E, Bransonic®).
Preparation of stock standard solution:
A standard solution of MCP was prepared by dissolving 1000 mg of MCP powder in double-distilled water. The flask was gently shaken to ensure complete dissolution of the powder. The final concentration of the MCP stock solution was 1.00 mg/ml.
Preparation of standard serial dilution:
Preparation of standard calibration curve samples: The stock standard solution of MCP (1.00 mg/ml) was diluted with double-distilled water to concentrations ranging from 2 to 10 μg/ml, and the final volume was adjusted to 100 ml to prepare the calibration standard solutions. A portion of the standard solution was then pipetted using a micropipette (1000 μl) into a glass vial (2 ml screw vial, 8 mm, Thermo Fisher Scientific Inc.) for injection into the HPLC system.
Preparation of quality control samples: Regarding the preparation of low, medium and high-quality control samples, a stock standard solution of MCP (1.00 mg/ ml) were diluted with the double distilled water over the range of 2 to 10μg/ml to complete volume to 100 ml. Then, each serial solution was mixed very well and the final concentrations were 3, 5, and 9 μg/ml (two solutions prepared for each concentration). The glass vial (2 ml screw vial 8 mm, Thermo Fisher Scientific Inc.) was filled with standard solution and injected into the HPLC system.
Preparation of pharmaceutical samples assay test of tablet formulation: Using the analytical balance, 10 tablets of premosan and primperan were precisely weighed individually and the average weights were then noted. Separately, 10 tablets of each substance were ground into a fine powder. Each tablet was weighed to determine the average weight. After being moved to the volumetric flask, the powder was diluted with mobile phase until it reached the desired level. For every pharmaceutical formulation, the concentration of the MCP assay test solution was 1 mg/ml. Immediately after, the sample was filtered through a 0.45 μm syringe. Using a micropipette 1000 μl, Eppendorf Research® plus, 10000 μl of the sample was pipetted into a 100 ml amber volumetric flask and diluted to the water mark. The concentration at the end was 100.0 μg/ml. For analysis, 3 solutions were prepared. 5 vials of glass (2 ml screw vial 8 mm, Thermo Fisher Scientific Inc.) were labelled and 20 μl of the sample was injected into the HPLC system.
Method development, optimization and validation: This study describes the development and validated method HPLC for the determination of MCP in pharmaceutical formulation.
Reverse-Phase HPLC (RP-HPLC):
Method development: For the determination of MCP, a straightforward, sensitive, accurate, and precise RP-HPLC approach was created. Initially, a number of columns and mobile phases were examined to ensure they had the proper chromatogram. Selectivity, sensitivity, and acceptable chromatographic parameters of the generated peaks in terms of peak sharpness, peak symmetry and tailing factor have been used to establish the appropriate column and mobile phase employed in the improved procedure. Using the ZORBAX Eclipse Plus C18 rapid resolution (4.6×100 mm, 3.5 μm, Agilent HPLC column), MCP was separated chromatographically. Using a mobile phase consisting of an isocratic combination of acetonitrile:buffer pH 4.6 (50:50, v/v) at a flow rate of 1.00 ml/min, the detection wavelength was 248 nm. The separation was accomplished with an injection volume of 20 μl at 25° with a run time of 3.0 min.
Method validation: The International Conference on Harmonization's (ICH, 2005) guidelines, which address issues including specificity, selectivity, linearity, accuracy, precision, LOQ and LOD, were followed in the validation of the suggested approach.
Specificity/selectivity: The ability of an analytical technique to distinguish and quantify the analyte in the presence of possible interfering compounds in the sample is known as selectivity. The analysis of selectivity showed that at the analyte's retention time, no discernible reaction attributed to interfering substances was found. It was discovered that MCP has a retention period of 1.4 min.
Even when there were other ingredients in the formulation, this technique showed excellent specificity in identifying the MCP analyte.
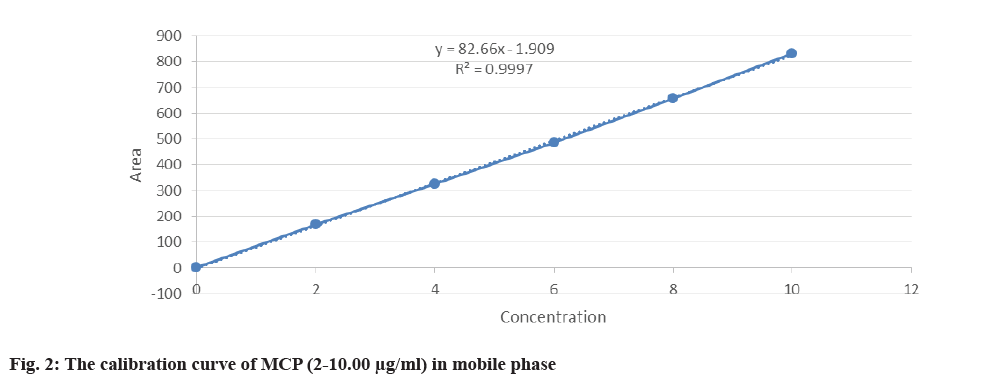
Linearity and range: According to ICH criteria, the analytical method was considered linear if it could produce test findings that were exactly proportionate to the analyte concentration in samples falling within a specified range. To establish the linearity of the suggested approach, several aliquots of the drug's standard solution were made from the stock solution and examined. The drug's correlation coefficient (r2≥0.997) indicated linearity in the 2-10 μg/ml range.
Accuracy and precision: A set of calibration standard curve samples was prepared for the intraday accuracy and precision investigation and ten Quality Controls (QCs) samples of each concentration; low (03.00 μg/ ml), medium (5.00 μg/ml) and high (9.00 μg/ml) were evaluated in a single day. Additionally, a set of calibration standard curve samples were prepared for the interday accuracy and precision research and 5 QC samples of each concentration low (3.00 μg/ml), medium (5.00 μg/ml) and high (9.00 μg/ml) were analyzed over the course of three days. For both intraday and interday validation, the mean, percentage accuracy, Standard Deviation (SD) and percentage Coefficient of Variation (CV) were computed.
Application of the proposed method for pharmaceutical formulation: Determining the exact amount of the active ingredient was the goal of the assay test for different strengths of MCP tablets. Five calibration standards, 5 QCs samples and 5 assay samples were prepared for each batch analysis with the goal of injecting them into the RP-HPLC procedure.
Results and Discussion
The main challenge of our study was developing and validating various techniques using different tools, while ensuring that the same set of calibration standards was consistently applied. The proposed HPLC methods were validated in accordance with ICH guidelines by assessing specificity, selectivity, linearity, accuracy, precision, LOQ, LOD and robustness.
In the concentration range of 2-10 μg/ml, the HPLC validated chromatographic findings showed excellent linearity, with a correlation coefficient (r2≥0.997). With a CV error of less than 10 %, the accuracy for both intraday and interday testing was within 100 %-180 % fig 2. Both Lower LOQ (LLOQ) and Lower LOD (LLOD) were established at 100 ng/ml.
Furthermore, the approach proved suitable for the highly sensitive study. Every validation parameter was carried out in compliance with ICH regulations. With an intraday and interday precision assessment (% CV) of less than 2 % (acceptable range ±10 %), the validation data demonstrated the sensitivity, accuracy, and precision of the HPLC technique in aqueous solutions. According to Table 1, the intraday and interday percentage accuracy of MCP varied from 101 % to 108 %, falling within the generally accepted range of 90 % to 110 %.
Proposed methods |
MCP concentration (µg/ml) | Mean | % accuracy | SD | % CV |
|---|---|---|---|---|---|
Intraday validation (accuracy and precision) |
3 | 3.2619 | 108.7307 | 0.0476 | 1.4603 |
| 5 | 5.173 | 103.4603 | 0.0780 | 1.5074 | |
| 9 | 8.9955 | 99.9500 | 0.1267 | 1.4081 | |
Interday validation (accuracy and precision) |
3 | 03.2078 | 106.928 | 0.0354 | 1.1044 |
| 5 | 04.9400 | 98.8004 | 0.0470 | 0.9508 | |
| 9 | 09.1127 | 101.2519 | 0.0929 | 1.0200 |
Table 1: Summary of Accuracy and Precision Results of the Proposed HPLC Method
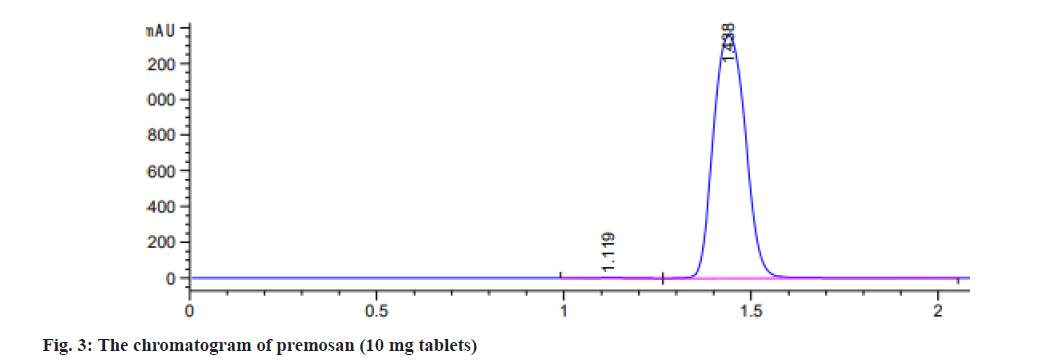
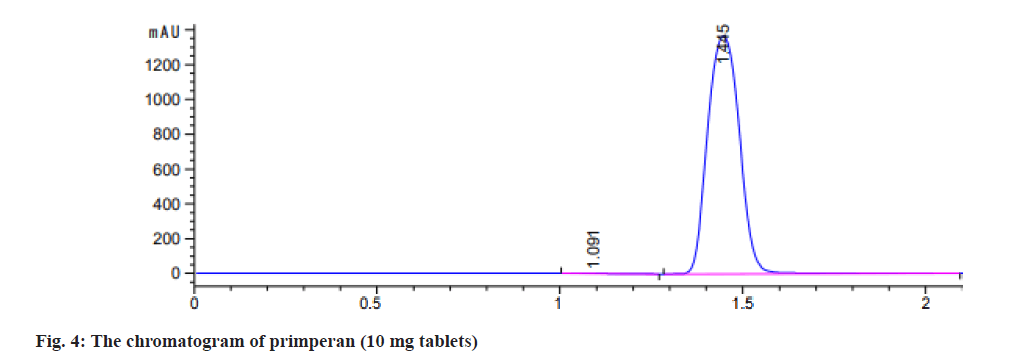
Additionally, using the equations (LLOD=3.3×SD/ slope) and (LLOQ=10×SD/slope), the chromatographic method demonstrates a LLOQ of 0.80 μg/ml and a LLOD of 0.26 μg/ml, respectively. The HPLC method was used to analyze 2 pharmaceutical formulations of MCP tablets: Primperan and premosan. A calibration curve was used to ascertain the amount of MCP present in the tablet formulations after the peak area of the sample solution was noted. Table 2 summarizes the assay results, which showed that the 2 MCP tablets active ingredient percentages varied from 84 % to 100 %, satisfying the ICH acceptance standards of 84 % to 110 %[26]. The chromatogram showed the separation of MCP (10 mg tablets) premosan and primperan both made to an injectable dilution of 20 μg/ml in (fig. 3 and fig. 4).
| S. No | Name of product | Strength (mg) | Test product concentration (µg/ml) | Average (of 6 samples) | % assay | STD | % RSD |
|---|---|---|---|---|---|---|---|
| 1 | Premosan | 10 | 100 | 84.705018 | 84.705018 | 0.058292 | 0.068818 |
| 2 | Primperan | 10 | 100 | 85.846362 | 85.846362 | 2.167737 | 2.525136 |
Table 2: MCP Assay Tests Were Analysed by the HPLC
In accordance with ICH recommendations, the current investigation employed validated analytical procedures to ensure sensitive and accurate MCP measurement. Notably, the HPLC approach demonstrated improved linearity, sensitivity, accuracy and precision. Numerous in-vitro applications, including stability and quality control tests, are ideally suited for these analytical techniques.
Acknowledgments:
The King Abdullah International Medical Research Center (KAIMRC) provided financing and assistance for this research proposal, for which the authors are grateful.
Author’s contribution:
Ibrahim Farh, Nouf Alnajim, Abdulrahman Alossimi, Jinan Alhuwayshil and Norah Aldeghaither supervised all practical work, rewrote the manuscript and reviewed it. Sultan Alqahtani and Mahmoud Mansour suggested the research topic and the experimental design.
Conflict of interest:
The authors declared no conflict of interests.
References
- Vandenplas Y, Hauser B. An updated review on gastro-esophageal reflux in pediatrics. Expert Rev Gastroenterol Hepatol 2015;9(12):1511-21.
[Crossref] [Google Scholar] [PubMed]
- Blazheyevskiy M, Alfred-Ugbenbo D, Mozgova OO, Moroz VP. Determination of metoclopramide hydrochloride in pharmaceutical formulations using N-oxidation caroate. Turk J Pharm Sci 2022;19(5):589.
[Crossref] [Google Scholar] [PubMed]
- Becker WJ. Acute migraine treatment in adults. Headache 2015;55(6):778-93.
[Crossref] [Google Scholar] [PubMed]
- The American society of health-system pharmacists. AHFS drug information-monograph of metoclopramide (1st ed). Maryland: American Society of Health-Systems Pharmacists, 2021.
- Kahali N, Khanam J. A novel HPLC method validation based on analytical techniques of metoclopramide benzamide derivative (metoclopramide base) and its determination from solid dispersion by solvent evaporation method. J Appl Pharm Sci 2018;8(2):018-26.
- Khan A, Khan J, Irfan M, Naqvi SB, Khan GM, Shoaib MH, et al. Validation and application of high performance liquid chromatographic method for the estimation of metoclopramide hydrochloride in plasma. Pak J Pham Sci 2017;30(1):143-7.
[Google Scholar] [PubMed]
- Elmansi H, Mohamed SA, Fathy ME. Simultaneous determination of metoclopramide and aspirin by spectrofluorimetric technique: Application to pharmaceutical formulations and human plasma. Anal Methods 2016;8(6):1281-92.
- Al-Haideri AM, Abdulla NI, Malih IK. Polymeric membrane sensors for the selective determination of metoclopramide hydrochloride and their applications to pharmaceutical analysis. Iraqi J Pharm Sci 2012;21(1):70-7.
- Patil SM, Pattar VP, Nandibewoor ST. Simultaneous electrochemical determination of acetaminophen and metoclopramide at electrochemically pre-treated disposable graphite pencil electrode. J Electrochem Sci Eng 2016;6(3):265-76.
- Ghonim OA. PVC membrane sensors for potentiometric determination of metoclopramide in pharmaceutical preparations and in presence of its degradate. Anal Bioanal Electrochem 2014;6:296-307.
- Hun X, Zhang Z. Electrogenerated chemiluminescence sensor for metoclopramide determination based on Ru (bpy)32+-doped silica nanoparticles dispersed in nafion on glassy carbon electrode. J Pharm Biomed Anal 2008;47(5):670-6.
[Crossref] [Google Scholar] [PubMed]
- Lee HW, Ji HY, Kim HY, Park ES, Lee KC, Lee HS. Determination of metoclopramide in human plasma using hydrophilic interaction chromatography with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877(18-19):1716-20.
[Crossref] [Google Scholar] [PubMed]
- British Pharmacopeia. Monograph of metoclopramide (volume II). London: The Stationary Office Ltd; 2013.
- Brown W, Marques MR. 14 The United States Pharmacopeia/National Formulary. InGeneric drug product development: Solid oral dosage forms. Boca Raton p: CRC Press. 2013:319.
- Naggar A, Elnasr T, Sayed Ali A, Kotb A, El Sayed A. Determination of metoclopramide hydrochloride in pharmaceutical formulations using three different spectrophotometric methods. Pharm Anal Acta 2017;8(538):2.
- Devi OZ, Basavaiah K, Vinay KB, Revanasiddappa HD. Sensitive spectrophotometric determination of metoclopramide hydrochloride in dosage forms and spiked human urine using vanillin. Arab J Chem 2016;9:S64-72.
- Thangamani A, Smith AA, Vedhapriya B. Development of analytical method for metoclopromide using UV-spectrophotometry. Int J Pharm Chem Biol Sci 2014;4(3).
- Jawad AA, Kadhim KH. Spectrophotometric determination of metoclopramide hydrochloride in bulk and pharmaceutical preparations by diazotization-coupling reaction. Int J Pharm Sci 2013;5:294-9.
- Dudhane NP, Vidhate SS, Borkar BH, Lohiya RT, Umekar MJ. Simultaneous UV spectrophotometric estimtion of metoclopramide hydrochloride and paracetamol in solid dosage form. J Pharm Sci 2010;2(1):48.
- M Al-Abbasi K, A Mohammed S, A Sarsam L. Spectrophotometric determination of metoclopramide hydrochloride in pharmaceutical preparations usingdiazotization reaction. Rafidain J 2011;22(5):76-88.
- Devi OZ, Basavaiah K, Vinay KB, Revanasiddappa HD. Determination of metoclopramide hydrochloride in pharmaceuticals and spiked human urine through diazotization reaction. J Food Drug Anal 2012;20(2):4.
- Khammas ZA, Abdulkareem HM. A new visible spectrophotometric approach for mutual determination of amoxicillin and metoclopramide hydrochloride in pharmaceuticals after cloud point extraction. Science 2016;4(5):66-76.
- Basheer MY, Aljaily A, Ibrahim MM, Osman HM. Development and validation of UV-spectroscopic method for assay of metoclopramide hydrochloride in bulk and injectable dosage form. Am J Res Commun 2017;5(3):22-33.
- Deokate UA, Gorde AM. A stability indicating UV. Spectrophotometric method for determination of metoclopramide hydrochloride. Int J Pharm Sci 2014;6(9):394-7.
- Blazheyevskiy M, Alfred-Ugbenbo D, Mozgova OO, Moroz VP. Determination of metoclopramide hydrochloride in pharmaceutical formulations using N-oxidation caroate. Turk J Pharm Sci 2022;19(5):589.
[Crossref] [Google Scholar] [PubMed]
- ICH HT. Validation of analytical procedures: text and methodology Q2 (R1). In International conference on harmonization, Geneva, Switzerland 2005 (pp. 11-2).