- *Corresponding Author:
- N. S. S. P. K. Chebolu
Department of Sciences and Humanities, Chemistry Division, Vignan’s Foundation for Science, Technology and Research, Vadlamudi, Andhra Pradesh 522213, India
E-mail: pavaniict@gmail.com
| Date of Received | 17 June 2021 |
| Date of Revision | 31 March 2022 |
| Date of Acceptance | 04 May 2023 |
| Indian J Pharm Sci 2023;85(4):895-902 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Favipiravir drug is fitted into antiviral medication criteria and mainly used in the treatment of influenza. The mechanism is associated with choosy inhibition of viral ribonucleic acid-dependent ribonucleic acid polymerase. Development and validation of dissolution method and filter compatibility studies are conducted with reverse phase ultra-performance liquid chromatography method for the quantitative analysis. The validation of this method was performed as per International Council for Harmonisation Q2 (R1) guidelines with the optimized experimental conditions. The proposed method was achieved on Acquity ultra-performance liquid chromatography HSS C18 (100 mm×1.8 μ) column and temperature maintained at 30° and run time was 8 min. The mobile phase consists of A-Methanol, B-0.1 % Trifluoroacetic acid (v/v) in water (pH=4.8). The injection volume of samples was 1 μl and ultraviolet detection was carried out at 210 nm. Linearity ranges were covered from 1 % to 300 % of the sample concentration level. The newly developed dissolution profile will show good repeatability and reproducibility in solid dosage forms and proved the filter compatibility studies. The projected method has capable to produce swift retention time and maintained well percentage recoveries throughout the dissolution profile. Hence this method can be used in customary quantitative analysis in quality control department for solid dosage forms and active pharmaceutical ingredients.
Keywords
Favipiravir, ultra-performance liquid chromatography method, dissolution, filter compatibility studies
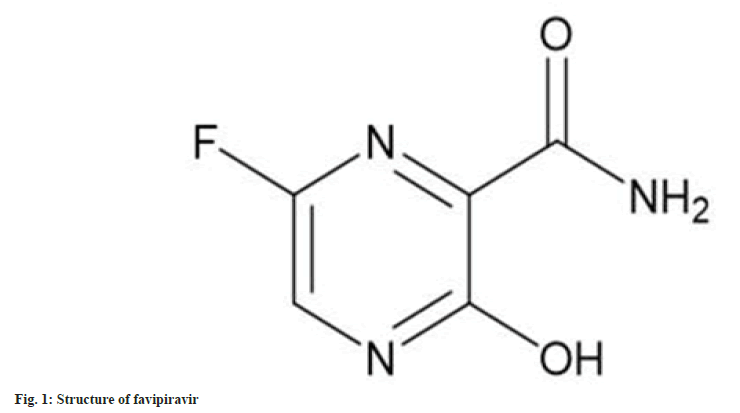
The chemical name of favipiravir is 6-Fluoro-3,4- dihydro-3-oxo-2-pyrazinecarboxamide. Favipiravir mechanism is mainly to control the entry and exit of antivirals. As per the studies, purine will reduce the drug activity and there is a competition between favipiravir ribofuranosyl-5'-triphosphate and purine ribonucleic acid (RNA)-dependent RNA polymerase. Favipiravir is broadly absorbed in the urine. Oral dosage of favipiravir is 600 mg is recommended daily twice a day. That is also 2-5 d only preferable. In recent days, favipiravir drug has been used in the treatment of Ebola virus, Lassa fever and Coronavirus Disease (COVID-19). Favipiravir, advertising with the trade names of FluGuard, Avigan, Favulous, and FabiFlu[1,2]. It has a molecular formula of C5H4FN3O2 with molecular weight 157.104 (fig. 1). Its nature is white to light yellow powder and sparingly soluble in acetonitrile, methanol and also in water.
Favipiravir will come under small molecule category and the drug is approved in 2014 in Japan for treatment of influenza cases[3]. More recently, it is exhibited its effectiveness for aiming different strains of influenza. It is also having 54 % protein bound in plasma protein. Favipiravir is one of the repurposed leading drugs used for the treatment of Ebola and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)[4]. In the list of COVID-19 drugs, favipiravir having the chance to cure 80 % as per the studies[5]. Primarily, Indian Council of Medical Research (ICMR) approved favipiravir drug for only emergency usage[6,7].
They can be fast-tracked conventional into the final phase of clinical development, Phase III and can be effortlessly estimated for their safety and effectiveness as COVID-19 treatment[8,9]. The convenience of this approach has apprehended the mind of pharma companies and researchers committed to quickly resolving the COVID-19 pandemic[10].
Materials and Methods
Drug substance:
Working standards favipiravir (99.9 %) was procured from Spectrum Pharma Labs, Hyderabad, India.
Instrumentation:
An Agilent-1100, Ultra Performance Liquid Chromatography (UPLC) consisting of ACQUITY UPLC Binary Solvent Manager (part numbers: 186015002), ACQUITY UPLC Sample Manager (part numbers: 186015005) and Waters Empower 2 workstation, Waters Acquity column manager (Part number: 186015007), Waters Photodiode Array Detector (PDA) detector (Part numbers: 186015032), supplied by M/s. Waters, USA. Dissolution apparatus (708 DS, M/s. Biocompare, USA), Mettler-Toledo analytical balance, model AG-245 capable of weighing 0.01 mg, supplied by M/s. Mettler AG, Switzerland. Sonicator supplied by M/s. Serwell instrument, India. Digital pH meter supplied by M/s. Serwell instruments, India.
Chemicals and reagents:
High-Performance Liquid Chromatography (HPLC) grade Methanol and HPLC water were purchased from Merck, India. AR grade Sodium acetate trihydrate, acetic acid, trifluoroacetic acid supplied by M/s. Rankem, Avantor performance materials, India used for present study, 0.45 μm pore size Nylon filter from Merck, India.
Preparation of mobile phase:
Mobile phase: A-0.1 % Trifluoroacetic acid (v/v), B-Methanol.
Preparation of standard solution:
Weigh accurately and transfer 20.0 mg of favipiravir standard into 100 ml volumetric flask, then add 25 ml of dissolution medium and allow dissolving completely by sonication and making up to volume with dissolution medium, shake well and transfer to vial.
Preparation of sample solution:
Set the parameters of dissolution apparatus as mentioned in dissolution parameters. Place previously weighed tablet in each of the six dissolution vessels and start the dissolution apparatus. At the end of specified time intervals, withdraw 10 ml of the sample solution from each of the dissolution vessel and filter the sample solution through 0.45 μm pore size Nylon filter (Make: mdi) and discard first 3 ml of the filtrate. Collect the filtrate and use as sample solution.
Chromatographic conditions:
The mobile phase was a combination of mobile phase A-0.1% trifluoroacetic acid (v/v) in Water, B-methanol. The contents of the mobile phase were filtered before use through 0.45 μm membrane filter, degassed for 10 min and pumped from the respective solvent reservoirs to the column at a flow rate of 0.2 ml/min, Acquity UPLC HSS C18 (100 mm×1.8 μ). The column temperature was preserved at 30° and run time 8 min. The injection volume was maintained as 1 μl. Ultra-Violet (UV) detection was carried out using a UV-PDA detector at 210 nm.
Method development:
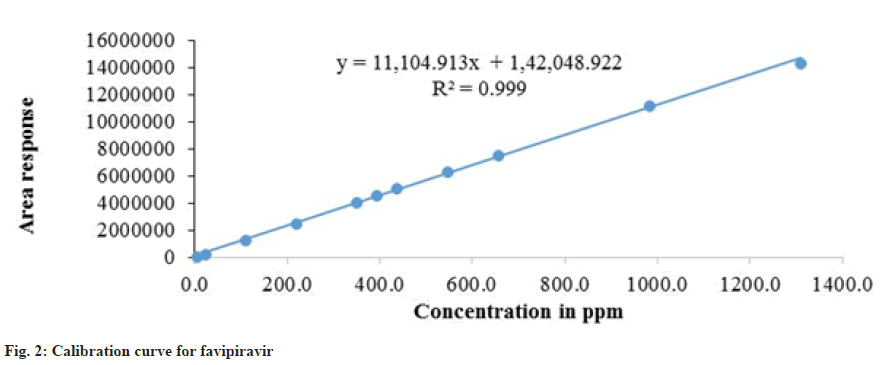
After several trials, optimal chromatographic conditions were fixed for better separations. The separate standard calibration lines were constructed to drug for dissolution profile. A series of aliquots are prepared from the above stock solutions using mobile phase to get the concentrations 1 % - 300 %). Each concentration was injected 3 times into chromatographic system. Each time peak area and retention time documented separately for the drug and impurities. Calibration curves were constructed by taking average peak area on Y-axis and concentration on X-axis separately for drug and impurities. From the calibration curves, regression equations were calculated as shown in the fig. 2.
Method validation:
In the validation, repeatability and reproducibility demonstrated for analytical method and was consistently produces the results for intended analytical applications[11,12]. The method was accomplished as per International Council for Harmonization (ICH) guidelines[13,14]. The developed method was supported by performing system suitability, linearity, Limit of Detection (LOD), Limit of Quantification (LOQ), precision, accuracy, selectivity and robustness.
System suitability parameters:
The system suitability, six replicates of favipiravir samples and favipiravir standard were injected and studied the parameters like tailing factor, and percentage of Relative Standard Deviation (% RSD). The results were revealed in Table 1.
| S No | Acceptance criteria | Result |
|---|---|---|
| 1 | Tailing factor for favipiravir peak from standard solution should be not more than 2.0 | 1.02 |
| 2 | The % RSD for favipiravir peak obtained from six replicate injections of standard solution should be not more than 2.0 % | 0.51 |
Table 1: System Suitability
Accuracy:
Accuracy was covered in range of 1, 50, 100 and 300 % with triplicate injections by adding a known amount of Active Pharmaceutical Ingredient (API) stock solution into Placebo and calculated the percentage of recoveries. The results were shown in Table 2.
| Level | Area response | Amount added in(mg) (actual) | Amount of recovered(mg) | % Recovery | % Mean | % RSD |
|---|---|---|---|---|---|---|
| Level-1 (1 %) | 28442.29 | 1.9789 | 2.23455 | 112.9 | 105.2 | 6.3 |
| 25660.28 | 1.9819 | 2.016 | 101.7 | |||
| 25458.24 | 1.9809 | 2.0001 | 101 | |||
| Level-2 (50 %) | 1267876 | 99.0299 | 99.6101 | 100.6 | 100.6 | 0.1 |
| 1268528 | 99.1735 | 99.66135 | 100.5 | |||
| 1268978 | 99.08435 | 99.6967 | 100.6 | |||
| Level-3 (100 %) | 2548125 | 198.2628 | 200.19225 | 101 | 101.1 | 0.1 |
| 2549591 | 198.139 | 200.30745 | 101.1 | |||
| 2551184 | 198.0994 | 200.4326 | 101.2 | |||
| Level-4 (200 %) | 7266246 | 594.2141 | 570.86935 | 96.1 | 96 | 0.1 |
| 7261917 | 594.1794 | 570.5292 | 96 | |||
| 7258355 | 594.12 | 570.24945 | 96 |
Table 2: Results of the Recovery Studies for Favipiravir
Precision:
Precision of the analytical method was demonstrated by analyzing six sets of sample solution. Favipiravir tablets (200 mg) of all six replicates sample solutions and calculated the mean percentage. Average relative peak area and % RSD were calculated and the obtained results were displayed in Table 3 and Table 4. Precision is the level of repeatability of results as reported for analyzed method. The precision test of the method, specifically, the method variation in the peak area of the drug formulation was calculated by using the below formula in terms of % RSD and the results were reported in the Table 3 and Table 4. Statistical results revealed that RSD of each drug for 6 times was less than 2.0.
| S. No | Retention time | Peak area |
|---|---|---|
| 1 | 3.961 | 2581812 |
| 2 | 3.921 | 2583062 |
| 3 | 3.933 | 2583338 |
| 4 | 3.941 | 2581117 |
| 5 | 3.923 | 2584478 |
| 6 | 3.922 | 2584096 |
| Mean | 3.934 | 2582984 |
| % RSD | 0.40 | 0.05 |
Table 3: System Precision
| S No. | Favipiravir |
|---|---|
| 1 | 94.8 |
| 2 | 95.1 |
| 3 | 95.8 |
| 4 | 96.1 |
| 5 | 95.4 |
| 6 | 95.8 |
| Mean | 95.5 |
| % RSD | 0.51 |
Table 4: Method Precision
% drug release calculation:
ATest/AStd×Wstd/DS×DTest/1×P/100×100/LC
Where, ATest=Average area response of favipiravir peak from sample solution, AStd=Average area response of favipiravir peak from standard solution, WStd=Weight of standard solution; DS=Dilution of standard solution, DTest=Dilution of sample solution; P=Potency of standard in % w/w on as is basis and LC=Label claim in mg/tablets
Dissolution (mg)=% Release×Label claim/100
Linearity:
The linearity of the method was determined in the concentration range of 1 % to 300 % for favipiravir solid dosage form. Linearity graph was plotted peak area against solution concentration and calibration curve was presented in fig. 2. The results were presented in Table 5.
| Level (%) | Concentration in ppm | Peak area | |
|---|---|---|---|
| 1 | 2.1816 | 23691.077 | |
| 5 | 10.90805 | 129671.26 | |
| 25 | 54.5402 | 642975.69 | |
| 50 | 109.08045 | 1242671 | |
| 80 | 174.5287 | 1976072.5 | |
| 90 | 196.3448 | 2313945.7 | |
| 100 | 218.16085 | 2554475.5 | |
| 120 | 272.7011 | 3200799.1 | |
| 150 | 327.2413 | 3761177.2 | |
| 200 | 490.86195 | 5117290.6 | |
| 300 | 654.4826 | 7179481.3 | |
| Correlation coefficient (r) | 0.999 | ||
| Regression coefficient (r2) | 0.999 | ||
| Slope | 11104.913 | ||
| y-intercept | 142048.922 | ||
| % intercept | 2.8 | ||
Table 5: Linearity of Favipiravir
Robustness:
The robustness of the dissolution method was established by introducing trivial changes in the chromatographic condition which included the temperature (27° and 33°), flow rate (0.1 ml/min and 0.3 ml/min) and mobile phase (60 Buffer:40 Methanol and 50 Buffer:50 Acetonitile). The results were given in Table 6.
| Method parameters | Conditions | Retention time (RT) favipiravir |
|---|---|---|
| Flow rate | 0.1 ml/min | 4.112 |
| Flow rate | 0.3 ml/min | 3.809 |
| Temperature | 27° | 4.211 |
| Temperature | 33° | 3.795 |
| Mobile phase | 60Buffer:40Methanol | 4.226 |
| Mobile phase | 50Buffer:50Methanol | 3.802 |
Table 6: Robustness
Specificity and selectivity:
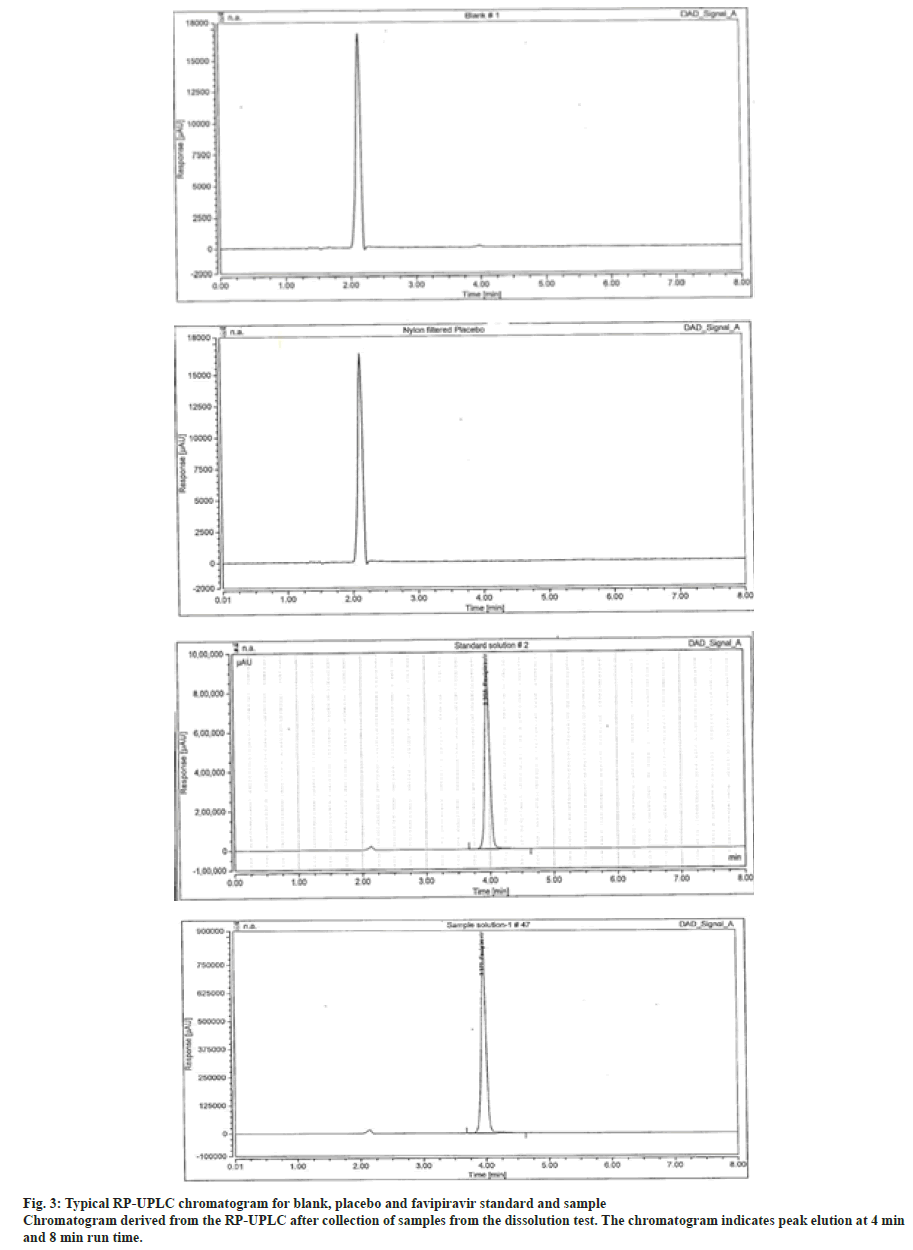
Specificity is the degree to which the procedure applies to a drug formulation and is checked in each analysis by probing samples and impurities for any interfering peaks. The specificity of the method was assessed with regards to interference. The reverse Phase-Ultra Performance Liquid Chromatography (RP-UPLC) chromatograms recorded for the drug substance showed no interfering peaks within retention time range. The respective chromatogram for blank, placebo and favipiravir standard and sample which shows the selected drug was effectively separated (fig. 3). Thus, the projected RP-UPLC method in this study was selective.
Filter compatibility study:
Filter Compatibility studies was ensured for dissolution testing in favipiravir tablets (200 mg) sample and placebo[15,16]. 0.45 μm Nylon filter is used for filtration and sub fractions are collected for filter (after discarding 3 ml and 6 ml). Collected the filtrate and used the same sample solution for analysis and results are tabulated in Table 7-Table 9.
| Sample details | Filter details | % Drug release | % Difference |
|---|---|---|---|
| Centrifuged sample | NA | 92.6 | - |
| 3 ml discarded sample | Nylon 0.45 µm | 92.8 | -0.2 |
| 6 ml discarded sample | Nylon 0.45 µm | 92.7 | -0.3 |
Table 7: Results of Filter Compatibility Studies Test Solution-1
| Sample details | Filter details | % Drug release | % Difference |
|---|---|---|---|
| Centrifuged sample | NA | 95.8 | - |
| 3 ml discarded sample | Nylon 0.45µm | 94.9 | -0.1 |
| 6 ml discarded sample | Nylon 0.45µm | 95.4 | -0.2 |
Table 8: Results of Filter Compatibility Studies Test Solution-2
| Sample details | Filter details | % Drug release | % Difference |
|---|---|---|---|
| Centrifuged sample | NA | 95.8 | - |
| 3 ml discarded sample | Nylon 0.45 µm | 94.9 | -0.1 |
| 6 ml discarded sample | Nylon 0.45 µm | 95.4 | -0.2 |
Table 9: Results of Filter Compatibility Studies Test Solution-3
Results and Discussion
The optimized dissolution profile and chromatographic conditions are stated above. Based on the dissolution profile, the best peak shape and extreme separation was achieved with mobile phase composition of A-0.1 % Trifluoroacetic Acid (v/v) in Water, B-Methanol. The best peak separation, peak symmetry and reproducibility were obtained on Acquity UPLC HSS C18 (100 mm×1.8 μ). The optimum wavelength for detecting the analyte was found to be 210 nm with a flow rate of 0.1 ml/ min. Most of the developed methods are reported High performance Liquid Chromatography (HPLC) till date use C8 column or C18 columns without dissolution profile. Also, most of them carried out with complex mobile phase compositions. Hence challenges were directed towards the development of a simple and better method on a commonly used C18 column with dissolution profile. Different logical amendments were tried to get good dissolution profile in solid dosage formulation. These changes included a change in mobile phase composition, column temperature.
The percentage recovery of favipiravir was attained in the range 1, 50, 100 and 300 % with triplicate injections by adding a known amount of API stock solution into placebo respectively. Related standard deviation value of replicated sets was less than 2.0 % which indicates that this method is highly accurate. The results are revealed in Table 2.
The precision of the method was determined by repeatability and method precision of favipiravir solid dosage formulation. The obtained results of repeatability and method precision were less than 2. Percentage of RSD value of replicated sets was less than 2.0 which indicates that this method is highly precise. The results are exposed in Table 3 and Table 4.
The calibration curve was plotted in the concentration range of 1 % to 300 % for favipiravir solid dosage form. The linearity graph is plotted for peak area against solution concentration and the correlation coefficient (r2) was attained (0.99). The standardization curve was constructed on regression equation. The statistical data exposed that the projected method was linear and the results were as shown in Table 5.
The robustness of the related substance method was established by introducing minor changes in the chromatographic condition which included the percentage of flow rate (0.1 and 0.3 ml/min), Mobile Phase (60Buffer:40 Methanol and 50 Buffer:30 Methanol) and temperature (27° and 33°). The developed method was unaffected by minor deliberated changes, which represents that the proposed method was robust. The results were shown in Table 6.
Filter compatibility study was established for dissolution testing in favipiravir tablets 200 mg sample and placebo and proved that 0.45 μm nylon filter is suitable for dissolution sample testing and the percentage drug release and percentage calculation was calculated for different test solutions and results are tabulated in Table 7-Table 9.
Dissolution profile with method development is an important part of drug formulation in development process and the pharmaceutical industries always show much interest in this area to effective release of drug in human body. A rapid, accurate and precise stability-indicating RP-UPLC analytical method with dissolution profile, filter compatibility study have been developed and validated for the quantitative analysis of favipiravir dosage formulation. The presented RPUPLC method with dissolution profile for separation of drug in placebo and found to be capable of giving swift retention times with accurate drug release in dissolution and with filter compatibility. Validation is followed as per International Council for Harmonization guidelines divulged that the method is more specific and stability-indicating. Henceforth, this method can be functional in the quality control samples and stability sample analysis. As per the current COVID pandemic situation, this method is valuable in pharmaceutical industries for fast release of batches in the market for the purpose of patient safety.
Acknowledgements:
Authors are thankful to VFSTR for constant support and encouragement. SK thankful to Spectrum laboratories, Hyderabad, India for providing favipiravir standard and sample as gift samples and thankful to Spectrum Laboratories, Hyderabad for providing instrumental facilities to carry out research work.
Conflict of interest:
All authors declare that they have no conflict of interest.
References
- Favipiravir. Drug bank Database. 2021
- Favipiravir. PubChem Database. 2021
- Monto AS. The role of antivirals in the control of influenza. Vaccine 2003;21(16):1796-800.
[Crossref] [Google Scholar] [PubMed]
- Nguyen TH, Guedj J, Anglaret X, Laouénan C, Madelain V, Taburet AM, et al. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis 2017;11(2):e0005389.
[Crossref] [Google Scholar] [PubMed]
- Pushpendra S, Kushwaha PP, Shashank K. Novel potent inhibitors of Plasmodium vivax dihydrofolate reductase: An in silico anti-malarial drug discovery. Indian J Pharm 2018;52(1):122-34.
- Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology 2002;36(S1):S237-44.
[Crossref] [Google Scholar] [PubMed]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med 2020;382(8):727-33.
[Crossref] [Google Scholar] [PubMed]
- de Clercq E. New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem Asian J 2019;14(22):3962-8.
[Crossref] [Google Scholar] [PubMed]
- Prajapat M, Sarma P, Shekhar N, Avti P, Sinha S, Kaur H, et al. Drug targets for corona virus: A systematic review. Indian J Pharmacol 2020;52(1):56-63.
[Crossref] [Google Scholar] [PubMed]
- Nehls C, Buonarati M, Cape S, Islam R, Satterwhite C, Briscoe C, et al. GCC consolidated feedback to ICH on the 2019 ICH M10 bioanalytical method validation draft guideline. Bioanalysis 2019;11(18s):1-228.
[Crossref] [Google Scholar] [PubMed]
- Little T. Design of experiments for analytical method development and validation. BioPharm Int 2014;27(3):40-5.
- Ye C, Liu J, Ren F, Okafo N. Design of experiment and data analysis by JMP®(SAS institute) in analytical method validation. J Pharm Biomed Anal 2000;23(2-3):581-9.
[Crossref] [Google Scholar] [PubMed]
- Guideline IH. Validation of analytical procedures: text and methodology. Q2 (R1). 2005;1(20):05.
- Guideline IH. Impurities in new drug substances Q3A (R2). InProceedings of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland 2006;25.
- Verma RK, Garg S. Compatibility studies between isosorbide mononitrate and selected excipients used in the development of extended release formulations. J Pharm Biomed Anal 2004;35(3):449-58.
[Crossref] [Google Scholar] [PubMed]
- Khalil SK, Shah MA, Naeem M. Laboratory studies on the compatibility of the entomopathogenic fungus Verticillium lecanii with certain pesticides. Agric Ecosyst Environ 1985;13(3-4):329-34.