- *Corresponding Author:
- R. B. Walker
Division of Pharmaceutics, Faculty of Pharmacy, Rhodes University, Grahamstown 6140, South Africa
E-mail: r.b.walker@ru.ac.za
| Date of Submission | 12 April 2017 |
| Date of Revision | 16 April 2018 |
| Date of Acceptance | 17 November 2018 |
| Indian J Pharm Sci 2019;81(1):45-56 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The applicability of a quality by design framework for the development of a sensitive, simple and selective, stability-indicating reversed-phase high-performance liquid chromatography analytical method for the analysis of captopril was investigated. Design of experiments using a central composite design approach was used for method development. Twenty experimental runs were performed with acetonitrile content ranging between 28 and 36 % v/v, pH from 2.8 to 3.6 and temperature between 22° and 32°. The experimental data obtained was used to derive a quadratic model for the retention time of captopril. The optimized method produced sharp peaks with good resolution (>2) for captopril and the internal standard with retention times of 3.1 and 6.2 min, respectively. The experimental data revealed that acetonitrile content in the mobile phase and pH are significant factors that affect the retention time and resolution of captopril. Normal probability plots revealed that the residual and predicted data fall approximately on a straight line, indicating that the experimental error for these studies was evenly distributed suggesting that the model could be used to navigate the design space. This approach is useful to expedite method development and optimization activities in analytical laboratories.
Keywords
Quality by Design, experimental design, forced degradation studies, stability indicating, captopril, RP-HPLC
Captopril (CP) is an orally active antihypertensive that acts by inhibiting the angiotensin converting enzyme and preventing the conversion of angiotensin I to angiotensin II [1]. Due to the lack of a strong chromophore, CP does not absorb UV radiation at high wavelengths. It is relatively unstable in solutions of pH >3.8 in which it oxidizes to form a disulphide dimer [2]. Several methods have been reported for the analysis of CP using spectrophotometry, fluorimetry gas chromatography [3], high pressure liquid chromatography with UVdetection [4-7], electro-chemical detection (ECD) [8] and capillary zone electrophoresis (CZE) [9]. Reversed-phase high performance liquid chromatography (RP-HPLC) is a method of choice for analysis in the pharmaceutical industry. A method using CZE and UV-detection for CP has been reported [9]. A stability indicating liquid chromatography analytical method for the analysis of CP has been reported, however, robustness and the method optimization approach used were not discussed [10] and a RP-HPLC method with ECD for CP using a design of experiments (DoE) approach was successfully developed but no information in respect of degradation studies was included [8]. The ICH Q1A (R2) [11] guideline suggested that stress testing of an active pharmaceutical ingredient can facilitate identification of likely degradation products, which in turn permits degradation pathways and the intrinsic stability of the molecule to be elucidated due to the stability-indicating power of the analytical procedure developed.
Routine practice when developing HPLC methods relies on the change “one factor at a time” approach which is time-consuming and does not completely demonstrate the flexibility of an analytical method [12,13]. During initial method development studies, parameters and interacting terms that affect the quality of an output are assessed by applying the principles of quality by design (QbD) [14]. The main reason for applying this approach is to define a method or product in terms of quality profiles and establish critical method parameters at the commencement of the process. The use of DoE facilitates the study of the design space (DS) for a particular process and can be used to establish the parameters of an analytical method with consistency and adequate quality. The DS consists of combinations of input variables that will ensure a quality output and a study of the impact of variables on the quality of the output or performance of a method ensures the development of a next level control strategy to guarantee a method meets or achieves predefined objectives. The application of QbD to the development of an analytical method can ensure the robustness and reproducibility of a method when used under different input conditions. From a pharmaceutical and regulatory perspective, functioning and continuing an operation within a DS does not constitute a significant change and obviates the need for submission of post-approval change protocols [15]. In a QbD framework, the impact of critical material and process variables on the ultimate quality of a process, in this case an analytical method, are usually evaluated. In order to evaluate the impact of different independent input variables in a process on the output of that process, multivariate regression models such as DoE can be used [16]. DoE is a mathematical and statistical approach that has been used effectively during the development and optimization of processes. Response surface methodology (RSM) is a widely used approach for the simultaneous study of multiple input factors on a response. QbD methodology using RSM, a full or fractional factorial, central composite (CCD) [17], Box-Behnken [18] or Doehlert [19] design can be used for the identification of experimental conditions for an optimized chromatographic separation. Identification of the factors that must be investigated and that affect key responses is necessary in order to define an experimental matrix that is fit for purpose so as to ensure that experiments are conducted in accordance and compliance with a well-defined experimental domain. The fitness of the model can be evaluated using mathematical approaches and polynomial equations and the final stage of analysis includes verification of the optimal input region of an operation for each of the responses monitored [20,21].
The objective of this study was to develop a simple, sensitive, selective, accurate and precise, stabilityindicating RP-HPLC method for the quantitation of CP in dosage forms, to optimize the method using RSM and subsequently validate the stability-indicating RP-HPLC method. To our knowledge this is the first report of a stability-indicating RP-HPLC method for the analysis of CP optimized using RSM in conjunction with QbD.
Materilas and Methods
CP was donated by Protea Chemicals (Midrand, South Africa), captopril disulphide (CD) USP reference standard was purchased from Sigma-Aldrich (Darmstadt, Germany) and phenobarbital (PB) was donated by Aspen Pharmacare (Port Elizabeth, South Africa). HPLC far UV grade acetonitrile (ACN) was purchased from Microsep (Port Elizabeth, South Africa) and 85 % w/v ortho phosphoric acid was procured from Merck Laboratories (Merck, Wadeville, South Africa). HPLC grade water was used for all analyses. Sample solutions were filtered through a 0.45 μm HVLP Durapore® membrane filters (Millipore, Bedford, MA, USA). CaptoHexal® 50 (Sandoz SA (Pty) Ltd., South Africa), Mylan Captopril 50 (Mylan (Pty) Ltd., South Africa), Adco-Captomax 50 (Adcock Ingram Ltd., South Africa) were purchased from a local pharmacy.
The HPLC system consisted of a Model 2695 Waters Alliance separation module equipped with a solvent delivery module, auto sampler, online degasser and a Model 2996 photodiode array detector (Waters, Milford, MA, USA). The stationary phase was a Phenomenex Luna C18 column (150×4.6 mm i.d. 5 μm) and data acquisition, processing and reporting were achieved using Waters Empower 2 software. A Model GLP21 pH-meter (Crison Instruments, Barcelona, Spain) was used in all studies and a Colora ultra-thermostat water bath (Colora, Lorch, Germany) was used to maintain temperature for stress testing studies. An Atlas Suntest CPS+ (Lisengericht, Germany) was used for photolytic degradation studies. Experimental design and data analysis calculations were performed using version 8.0.4.1 Design-Expert software (Minneapolis, MN, USA).
Preparation of stock solutions
Approximately 10 mg of CP and PB (the internal standard) were accurately weighed into two separate 100 ml A-grade volumetric flask and dissolved in mobile phase with the aid of a Branson B12 ultrasonic bath (Shelton, CN, USA). Appropriate aliquots of the stock solution were then serially diluted with mobile phase to produce seven working standards of 1, 5, 10, 20, 40, 60, and 80 μg/ml concentration and PB was prepared as a 10 μg/ml solution. The solutions were prepared on a daily basis.
Preparation of mobile phase
Milli-Q water (700 ml) and 300 ml of ACN were measured separately using a 1000 ml A-grade measuring cylinder and mixed in a 1000 ml Schott Duran bottle (Schott Duran GmbH, Wertheim, Germany), the pH of mobile phase was adjusted to 3 using 85 % w/v ortho-phosphoric acid. The mobile phase was filtered through a Millipore HVLP 0.45 μm filter membrane (Merck Millipore Ltd, Cork, Ireland) with the aid of an Eyela Aspirator A-2S vacuum pump (Rikakikai Co. Ltd, Tokyo, Japan) and degassed for 15 min after preparation using a Branson B12 ultrasonic bath (Shelton, CN, USA).
Chromatographic conditions
Separation of peaks was achieved using a Waters Alliance system fitted with a PDA detector set at 210 nm and a Phenomenex Luna C18 (150×4.6 mm i.d. 5 μm) stationary phase and mobile phase consisting of a mixture of ACN and water in a 30:70 % v/v ratio. A 0.8 ml aliquot of the CP (80 μg/ml) together with 0.5 ml PB solution (10 μg/ml) were transferred into an amber glass screw thread vial and this mixture was injected onto the system that was operated in isocratic mode at a flow rate of 1.0 ml/min and a column temperature of 24.0°.
QbD approach to analysis
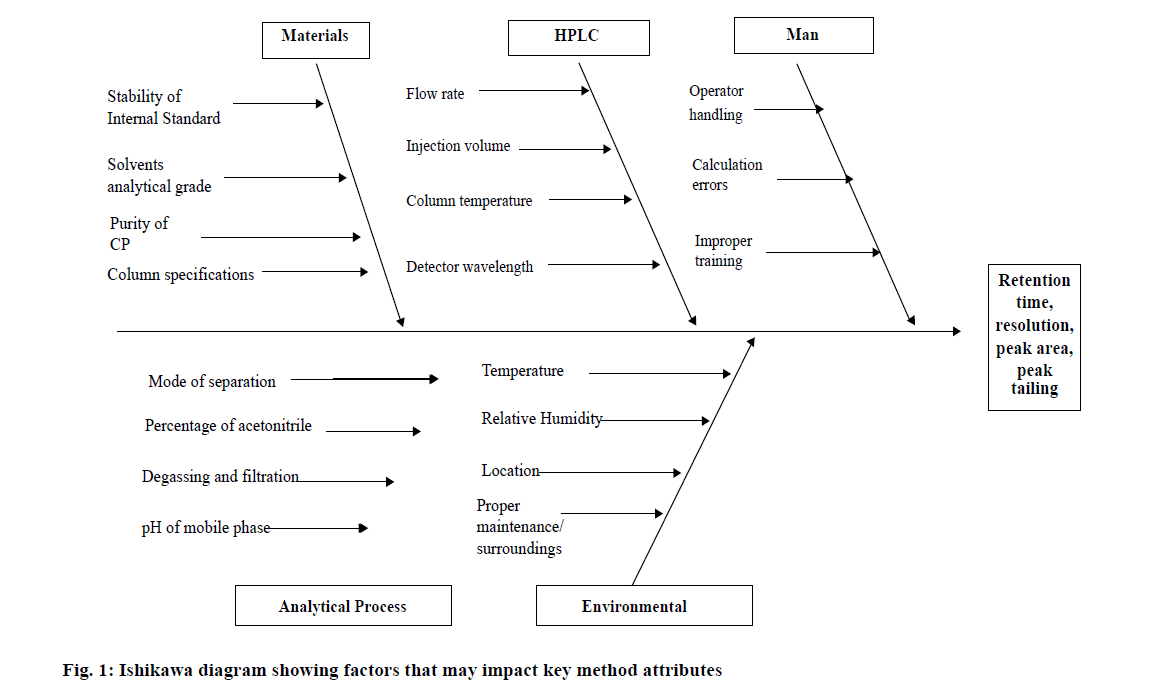
The application of QbD in HPLC method development commences with establishing analytical objectives based on sound science to ensure consistent method performance characteristics are achieved [22]. The use of QbD for an analytical method commences with defining the target analytical profile (TAP) in which the pre-defined objectives for method performance must be appropriately validated and documented [23,24]. Such analytical methods are, in fact, an indicator of a quality product and the robustness of that product for the duration on the lifecycle of that product. The main goal of any HPLC method is to separate and quantitate analyte(s) of interest from any impurity and/or excipients. Initially it is important to establish the critical quality attributes (CQA) of a system that may impact the quality of the analytical method. Quality attributes (QA) that may impact method performance were identified, based on knowledge gained during method development activities and scientific understanding of chromatographic separations. Information such as the physicochemical properties, ultraviolet absorption spectrum, pKa, solubility and chemical stability of CP were collected prior to commencement of experimental activities. Operating conditions such as temperature, humidity, nature of starting materials and purity of reagents, which may influence the quality of an analytical method were established and closely monitored during development of the HPLC separation. A common approach to screening QA is to undertake a structured risk assessment exercise by constructing a fishbone or Ishikawa diagram in which traditional knowledge used for analytical method development is combined with current state-of-the-art approaches applied to method development activities. The use of a fishbone diagram permits categorization and individualisation of input factors according to the instrument, materials, method, measurements and laboratory environment is discussed in the results [25,26].
Method validation, linearity
A calibration curve was constructed following replicate (n=6) analysis of seven standards of 1, 5, 10, 20, 40, 60 and 80 μg/ml concentration. The peak height ratio of CP to IS was calculated and plotted versus concentration after which least squares linear regression analysis of data was undertaken to establish the equation for the best fit line and the correlation coefficient (R2) was used to confirm linearity.
Precision
Intra-day (repeatability) precision was established following analysis of replicate samples (n=6) at three concentrations indicative of low, medium and high levels within the linear range viz., 5, 20, 60 μg/ml. Analysis was performed over a short period of time on the same day. Inter-day precision or reproducibility was assessed at low, medium and high concentration on three consecutive days and the percent relative standard deviation (% RSD) was used to assess intraand inter-day precision. An upper limit of 2 % was used to confirm precision in our laboratory.
Accuracy
A limit for % RSD was set at <2 % for accuracy. Accuracy was established by applying the optimized RP-HPLC method to the analysis of a CP standard of known purity at low, medium and high concentration and the percent recovery for each concentration calculated.
Limits of detection (LOD) and quantitation (LOQ)
Several approaches for the calculation of the LOD and LOQ of a method have been suggested in different guidelines and include visual evaluation, use of signalto- noise ratio, calculations based on standard deviation of a response and the slope of the calibration curve[27]. By convention, the LOD is estimated as one third of the LOQ. A series of samples of 0.25, 0.5, 1, 1.25, 1.75, 2.5, and 5 μg/ml was prepared and analysed using the optimized RP-HPLC method and the peak height ratio calculated. The LOQ was determined by establishing the lowest concentration of CP that resulted in a % RSD value for precision of <2 %.
Specificity
The specificity of an analytical method is defined as the ability of a method to ensure that the peak(s) of interest elute as distinct responses in the presence of excipients, impurities or degradation compounds [27].
Forced degradation studies
Approximately 10 mg of CP was accurately weighed and dissolved in 10 ml 1 M HCl, 0.1 M NaOH, water and 3 % v/v H2O2. A known quantity of CP was dispersed as a thin layer on a watch glass and placed in an oven (Gallenkamp®, Loughborough UK) set at 100° for 6 h to evaluate dry heat degradation and a similar sample was also exposed to UV/Vis radiation using an Atlas Suntest® CPS+ instrument for 24 h. Degradation was performed by refluxing 10 ml of the stock solution at temperatures 60°, 70°, 80°, 90° and 100° for 6 h using a Colora® Ultra-Thermostat water bath to maintain temperature. The peroxide solution was placed in the dark for 1 h at 22°. Aliquots (1 ml) were removed from test solutions, diluted with mobile phase and analysed using the RP-HPLC method. Solid samples were prepared for analysis following exposure, as previously described.
Application of analytical method
Commercially available CP tablets were used to assess the application of this method. Mylan Captopril 50, Adcock® Captomax 50 and Sandoz CaptHexal® 50 tablets were purchased from a local pharmacy. Twenty tablets were weighed and crushed in a mortar to form a fine powder. An aliquot of powder equivalent to the average weight of one tablet was weighed directly into a 100 ml A-grade volumetric flask. Approximately 70 ml mobile phase was added to the volumetric flask and the contents were sonicated for 15 min to dissolve the CP. Following sonication the solution was allowed to cool to room temperature (22°) and then made up volume with mobile phase. An aliquot of the solution was filtered through a 0.45 μm Millipore Millex-HV hydrophilic PVDF filter membrane and was further diluted to produce a solution of final concentration of 50 μg/ml. Replicates (n=3) of the test sample were analysed using the validated RP-HPLC method.
Results and Discussion
The use of a TAP is to ensure flexibility following method development, so as to facilitate continuous improvement of a product whilst avoiding the need for costly post-approval changes following market authorization [23]. The performance characteristics required to ensure that an analytical method is fit for the purpose should be stability indicating, have a short run time, produce sharp peaks that are well-resolved from impurities [28]. The method must conform to regulatory requirements and include an assessment of specificity, linearity, range, accuracy, precision, sensitivity and robustness as key parameters addressed during HPLC method validation as suggested in USP and in ICH guidelines [27,29]. Of these parameters, accuracy and precision are important performance characteristics for the quantitation of materials as is the range of the method. Linearity and specificity are not a requirement for a TAP as they are not directly implicated in the understanding of the extent of agreement of a measured and true value [24,30]. The set of objectives defined in the TAP includes critical factors that are necessary to establish the quality of a method.
In order to meet predefined TAP objectives, outputs or responses such as retention time, resolution between CP and IS and peak tailing were identified as CQA for this study. The first branch of the fish bone diagram (Figure 1) refers to the properties of the materials used such as for example the purity of CP, stability of the IS, age of the column and grade of solvent used. The purity of CP may result in variable peak height/area or an interaction between the IS and CP may alter the concentration and/or retention time of the analyte of interest. The analytical grade of the solvents used must be considered as any particulate matter in solvents may block the stationary phase and/or produce an unwanted peak in chromatograms since elution time of a compound is dependent on the affinity of that material to C18 chains of the stationary phase packing material. A new Phenomenex Luna 150×4.6 mm i.d 5 μm C18 column was used throughout the study. To minimize the effect of solvent impurities all analyses was performed using HPLC grade ACN. Process related variables of HPLC instruments and analytical techniques include mobile phase composition and pH, flow rate, injection volume, column temperature, detector wavelength and may have a serious impact on HPLC responses. Early method development studies facilitated identification of initial conditions for the separation. An injection volume of 20 μl and a flow rate of 1 ml/min and a wavelength of 210 nm were used for the analysis. Parameters such as flow rate, column, injection volume, instrumentation variables were considered controllable and were kept constant within the experimental domain whereas mobile phase composition, pH and column temperature were identified as critical process parameters that had to be investigated to establish the robustness of the analytical method [31]. Environmental factors such as temperature and relative humidity, proper maintenance and level of knowledge of the analyst when handling instruments may also interfere with the quality of the analysis.
An efficient and comprehensive experimental design based on systematic and simultaneous examination of three key components viz., ACN composition of the mobile phase, pH and column temperature was undertaken followed by optimization of chromatographic conditions using prediction software. Experimentally derived data was modelled and a number of chromatographic conditions predicted based on that data, were identified and evaluated. This approach to optimisation was selected after considering all method attributes and based on the assumption that the factors investigated would be reliable, thereby limiting the amount of work required to demonstrate the robustness of the analytical method.
DoE is an approach that enables scientists to evaluate the effect and interactions of a number of variables on an output simultaneously using a limited number of experiments. From the data generated in preliminary studies when developing a separation for CP, limits for experimental levels were identified. The five coded levels for the independent variables in the rotatable CCD were set at –α, –1, 0, +1 and +α and the corresponding experimental levels used, are listed in Table 1.
| Variables | Real values for the coded levels | ||||
|---|---|---|---|---|---|
| -α (1.68) | -1 | 0 | +1 | +α (1.68) | |
| ACN content (x1) % v/v | 26 | 28 | 32 | 36 | 38 |
| pH (x2) | 2.5 | 2.8 | 3.2 | 3.6 | 3.8 |
| Temperature (x3)° | 20 | 22 | 27 | 32 | 35 |
Table 1: Actual and coded levels for HPLC variables investigated
The upper and lower limits for ACN content (x1) were 28 and 36 % v/v, respectively. The axial points were set at 26 and 38 % v/v and for mobile phase pH (x2) were 3.6 and 2.8 with the lower and upper limits for the axial points set at 2.5 and 3.8. The minimum and maximum column temperatures (x3) were 22 and 32° and the lower and upper axial levels were set at 20 and 35°. The critical responses monitored were retention time (y1), resolution (y2) and peak tailing (y3). System suitability, precision, accuracy and specificity were considered as important method performance characteristics. The experiments were performed randomly in order to eliminate any possible experimental bias and the resultant HPLC data from 20 experiments with 8 factorial points, 6 axial and 6 centre points were analysed and are summarized in Table 2.
| Run Number | Standard | Point | ACN | pH | Temp | Retention time minutes | Resolution | Peak tailing |
|---|---|---|---|---|---|---|---|---|
| % v/v | (x2) | °C | (y1) | (y2) | (y3) | |||
| (x1) | (x3) | |||||||
| 14 | 1 | Axial | 0 | 0 | 1.68 | 2.79 | 3.08 | 1.25 |
| 16 | 2 | Centre | 0 | 0 | 0 | 2.81 | 3.24 | 1 |
| 10 | 3 | Axial | 1.68 | 0 | 0 | 2.36 | 2.15 | 1.25 |
| 5 | 4 | Factorial | -1 | -1 | 1 | 3.27 | 4.94 | 0.87 |
| 1 | 5 | Factorial | -1 | -1 | -1 | 3.31 | 4.8 | 0.83 |
| 18 | 6 | Centre | 0 | 0 | 0 | 2.86 | 3.09 | 1 |
| 6 | 7 | Factorial | 1 | -1 | 1 | 2.46 | 3.08 | 1.5 |
| 2 | 8 | Factorial | 1 | -1 | -1 | 2.48 | 2.84 | 1.16 |
| 9 | 9 | Axial | -1.68 | 0 | 0 | 3.69 | 4.77 | 1.12 |
| 15 | 10 | Centre | 0 | 0 | 0 | 2.84 | 3.4 | 1.16 |
| 20 | 11 | Centre | 0 | 0 | 0 | 2.85 | 3.18 | 1.25 |
| 17 | 12 | Centre | 0 | 0 | 0 | 2.85 | 3.17 | 1 |
| 7 | 13 | Factorial | -1 | 1 | 1 | 3.66 | 3.98 | 1 |
| 19 | 14 | Centre | 0 | 0 | 0 | 2.86 | 3.37 | 1 |
| 4 | 15 | Factorial | 1 | 1 | -1 | 2.9 | 2.02 | 1.75 |
| 12 | 16 | Axial | 0 | 1.68 | 0 | 3.5 | 2 | 1.25 |
| 8 | 17 | Factorial | 1 | 1 | 1 | 2.7 | 2.27 | 2 |
| 13 | 18 | Axial | 0 | 0 | -1.68 | 2.83 | 3.64 | 1 |
| 11 | 19 | Axial | 0 | -1.68 | 0 | 2.8 | 3.08 | 1.16 |
| 3 | 20 | Factorial | -1 | 1 | -1 | 3.43 | 4.75 | 1 |
Table 2: Coded levels for the CCD experimental domain and resultant responses
Statistical analyses were used to generate second-order quadratic equations that best fitted the experimental data in addition to prediction of responses. The polynomial equation that was generated following fitting of experimental data represents the quantitative values for coefficients of independent variables, first order main effects, higher order effects and their interactions for retention time. The HPLC variables x1, x2 and x3 and quadratic terms with positive coefficients were directly proportional, whereas the negative terms have an inverse relationship to the response. The interaction terms x1x2 and x2x3 had an agonistic effect on the retention time of CP whereas x1x3, had an antagonistic effect. The polynomial equations for critical quality responses are represented mathematically in Eqn. 1.1, 1.2 and 1.3, respectively. y1 = 2.84–0.39x1+0.17x2– 0.0070x3+0.068x12+0.11x22-0.0071x32+0.019x1 x2- 0.051x1 x3+0.011x2 x3 (Eqn. 1.1); y2 = 3.23–0.93x1- 0.33x2–0.079x3+0.18x12-0.15x22+0.14x32-0.077x1 x2+0.14x1 x3-0.11x2 x3 (Eqn. 1.2); y3 = 1.07+0.21x1+0 .11x2+0.077x3+0.059x12+0.066x22+0.038x32+0.099x1 x2+0.069x1 x3-0.016x2 x3 (Eqn. 1.3). ANOVA results suggest that the response surface quadratic model for the three responses was significant and adequate. The data for retention time, resolution and peak tailing are summarized in Table 3. The accurate response factors with a low standard deviation can be predicted by the model as the correlation coefficient R2 is close to unity. The results of modelling the data reveal that the R2 value was >0.9 indicating that there is a good correlation between the experimental and predicted responses for this model [32].
| Statistical parameter | Retention timeminutes (y1) | Resolution(y2) | Peak tailing(y3) |
|---|---|---|---|
| F-value | 507.12 | 99.65 | 13.83 |
| P-value | <0.0001 | <0.0001 | 0.0040 |
| Regression coefficient(R2) | 0.9852 | 0.9259 | 0.7107 |
| Predicted R2 | 0.8776 | 0.4573 | -0.9615 |
| Adjusted R2 | 0.9720 | 0.8592 | 0.4503 |
| Mean | 2.96 | 3.34 | 1.18 |
| Standard deviation | 0.064 | 0.34 | 0.21 |
| Adequate precision | 29.259 | 13.482 | 6.054 |
| C.V. % | 2.18 | 10.27 | 18.10 |
Table 3: ANOVA data for response surface quadratic model for retention time
The R2 values for responses y1, y2 and y3 were 0.9852, 0.9259 and 0.7107, and the predicted and experimental values were found to be in good agreement for y1 as the predicted R2 value was 0.8776, thereby indicating reasonably good agreement with the adjusted R2 value of 0.9720. However, the predicted R2 value of 0.4573 for response y2 is not close in value to the adjusted R2 value of 0.8592 and may be indicative of a large block effect for the prediction of this response when using this model. A negative predicted R2 value of –0.9615 was observed for response y3 implying that the overall mean value of 1.18 is a better predictor of the model responses than the coefficient of regression. Further, adequate precision measures the signal-tonoise ratio and a desirable ratio to produce an adequate signal should be >4. All responses exhibited adequate precision values >4, indicating that the model is adequate to navigate the DS. The relatively low value for the coefficient of variation indicates the precision and reliability of the experiments. ANOVA was used to estimate the quality of the quadratic regression models and to validate the response surface models [32].
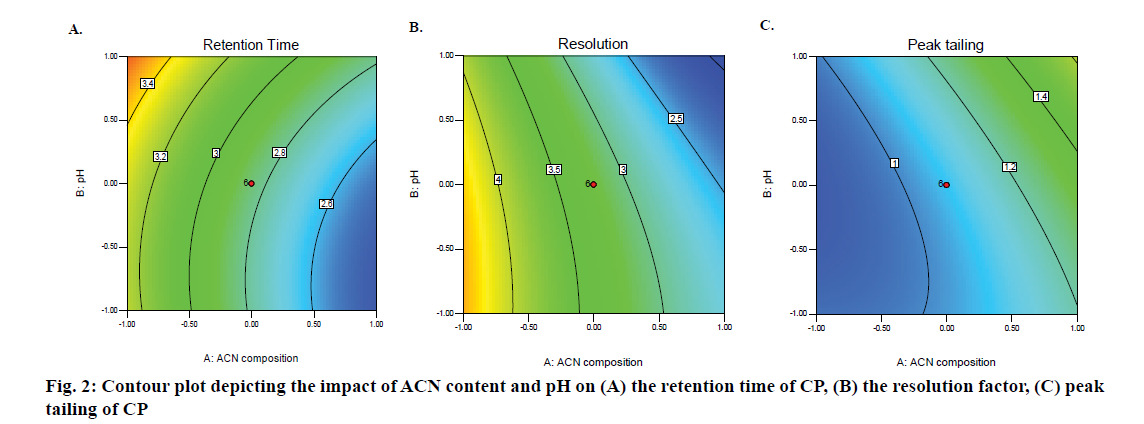
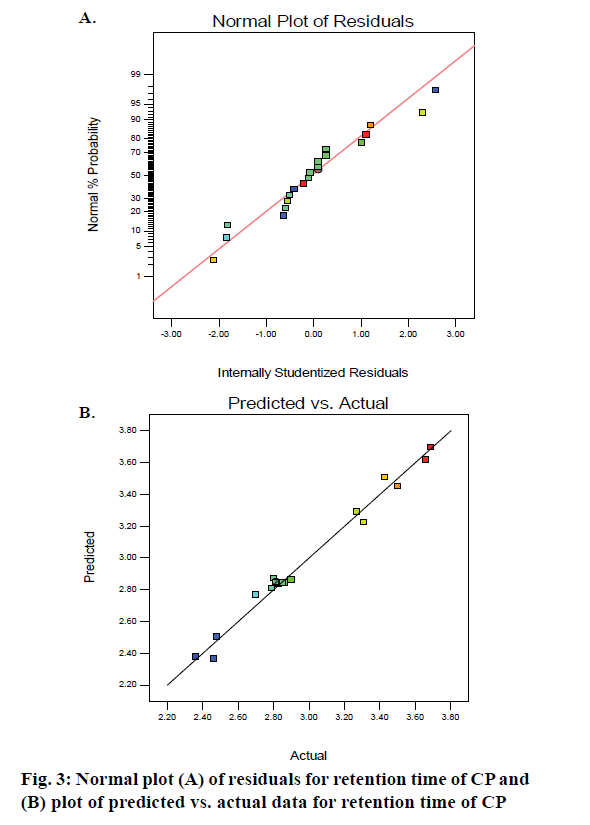
The F-values of 507.12 and 97.01 and p-values <0.001 for x1 and x2 indicate that these factors have a significant effect on the retention time of CP. The 2D contour depicted in Figure 2 reveals that an increase in ACN content in the mobile phase whilst maintaining a constant pH and temperature results in a decrease in the retention time of CP whereas an increase in the pH of mobile phase at constant temperature and ACN content results in longer retention times for the drug. A higher proportion of ACN in the mobile phase results in a high elution strength with a consequent reduction in the retention time of CP due to a change in the polarity of the mobile phase. CP is a weak acid with a pKa of 2.8 and therefore a change in the pH has a significant effect on the retention of CP and elution is therefore pH-dependent [33]. The normal probability plot of residuals for retention time reveals that the data points were distributed along a straight line (Figure 3A) indicating that the error was equally distributed across each individual point. The plot of actual versus predicted values (Figure 3B) reveals that all points fall nearly in the same region, confirming that the model could be used to predict response values for specific and independent input values.
The resolution (Rs) between CP and PB was considered an important factor for the development of this method. The 2D contour plot depicted in Figure 2 reveals that an increase in ACN content resulted in a significant decrease in resolution between the compounds. An increase in the pH of the mobile phase only, resulted in a slight decrease in resolution as pH influences the degree of ionization of CP and ionic species are favourably bound to silica sites on the stationary phase, reducing the resolution between the peaks yet resulting in acceptable resolution with Rs >2 for all studies. The addition of an organic solvent in RP-HPLC results in a low Rs due to interactions of the mobile phase components with the surfaces of the stationary phase in competition with analyte molecules for these sites. The resolution for the optimized chromatographic conditions was established as 2.7, which was considered as acceptable for the purposes of quantitation of CP.
Peak tailing was calculated using the USP approach and was significantly affected by an increase in ACN content in the mobile phase as depicted in the 2D contour plot (Figure 2). Based on these data, it is clear that column temperature and pH have only a minor effect on peak shape. It also reveals that an increase in ACN content results in an increase in peak tailing of CP due to preferential partitioning of the API into the stationary phase. The optimized method resulted in an acceptable peak tailing factor of 1.0 when the ACN content was 30 % v/v and pH was 2.8.
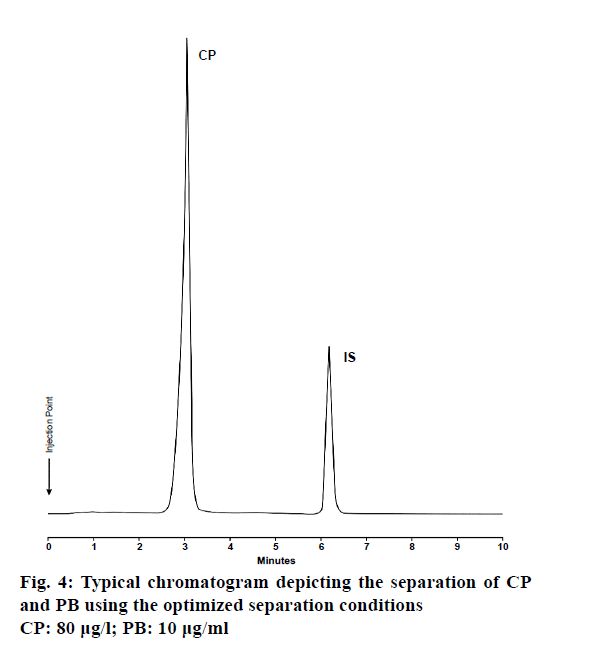
The optimized conditions for the quantitative analysis of CP were predicted using Design-Expert software and resulted in the improvement of conditions to ensure a superior response within a set of targeted experimental parameters. A typical chromatogram of a separation using these conditions is depicted in Figure 4. The predicted responses for the optimized method generated using Design-Expert software were found to be in close agreement with the experimental responses and the low percent predicted error suggests that the model is robust and furthermore indicates that RSM is an efficient tool for the optimization of analytical processes.
The results of linearity studies conducted on three consecutive days as summarized in Table 4 reveal that the method is linear over the ranges studied with high R2 values and low intercepts. The results for intra-day precision were all within the 2 % limit, indicating that the method is precise. Intra-day and inter-day precision results are summarized in Table 5. The data for both, intra and inter-day precision indicate the method is precise. The accuracy of the method established by evaluating low, medium and high level concentrations of CP in replicate (n=6) resulted in a mean percent recovery of 99.18, 100.15 and 99.93 %, respectively with % RSD values ranging from 0.13-0.20 % indicating that the method is accurate. The LOQ of the method established as described in the ICH Q2A guideline was 1 μg/ml with an associated % RSD of 1.79. By convention, the LOD was 0.3 μg/ml. The method was considered to be specific as no interfering peaks were evident during the analysis of commercially available tablets and during forced degradation studies.
| Parameter | Day | ||
|---|---|---|---|
| Day1 n=6 |
Day2 n=6 |
Day3 n=6 |
|
| Concentration range (µg/ml) | 1.01-80.6 | 1.1-80.5 | 1.1-80.6 |
| Correlation coefficient (R2) | 0.9997 | 0.9998 | 0.9998 |
| Regression equation | y = 0.0355x–0.0117 | y = 0.0355x–0.0117 | y = 0.0355x–0.0117 |
| %RSD | < 1.8 | < 1.5 | < 1.7 |
Table 4: Linearity data for CP on three consecutive days
| Intra-day | ||||
|---|---|---|---|---|
| CP level | Theoretical concentration µg/ml | Mean concentration ±SDµg/ml | % RSD | |
| Low | 5.05 | 5.12±0.001 | 0.47 | |
| Medium | 20.01 | 19.43±0.002 | 0.28 | |
| High level | 60.03 | 59.73±0.008 | 0.39 | |
| Inter-day | ||||
| CP Level | Day | Theoretical concentration µg/ml | Mean concentration ±SDµg/ml | % RSD |
| Low n=6 |
1 | 5.01 | 5.02±0.023 | 0.46 |
| 2 | 5.01 | 5.10±0.050 | 0.95 | |
| 3 | 5.01 | 4.98±0.030 | 0.64 | |
| Medium n=6 |
1 | 19.85 | 19.60±0.074 | 0.38 |
| 2 | 20.02 | 20.07±0.040 | 0.20 | |
| 3 | 20.01 | 19.88±0.090 | 0.44 | |
| High n=6 |
1 | 60.60 | 60.77±0.170 | 0.28 |
| 2 | 60.06 | 59.96±0.070 | 0.12 | |
| 3 | 60.30 | 60.34±0.130 | 0.22 | |
Table 5: Intra-day and inter-day precision studies for analysis of CP
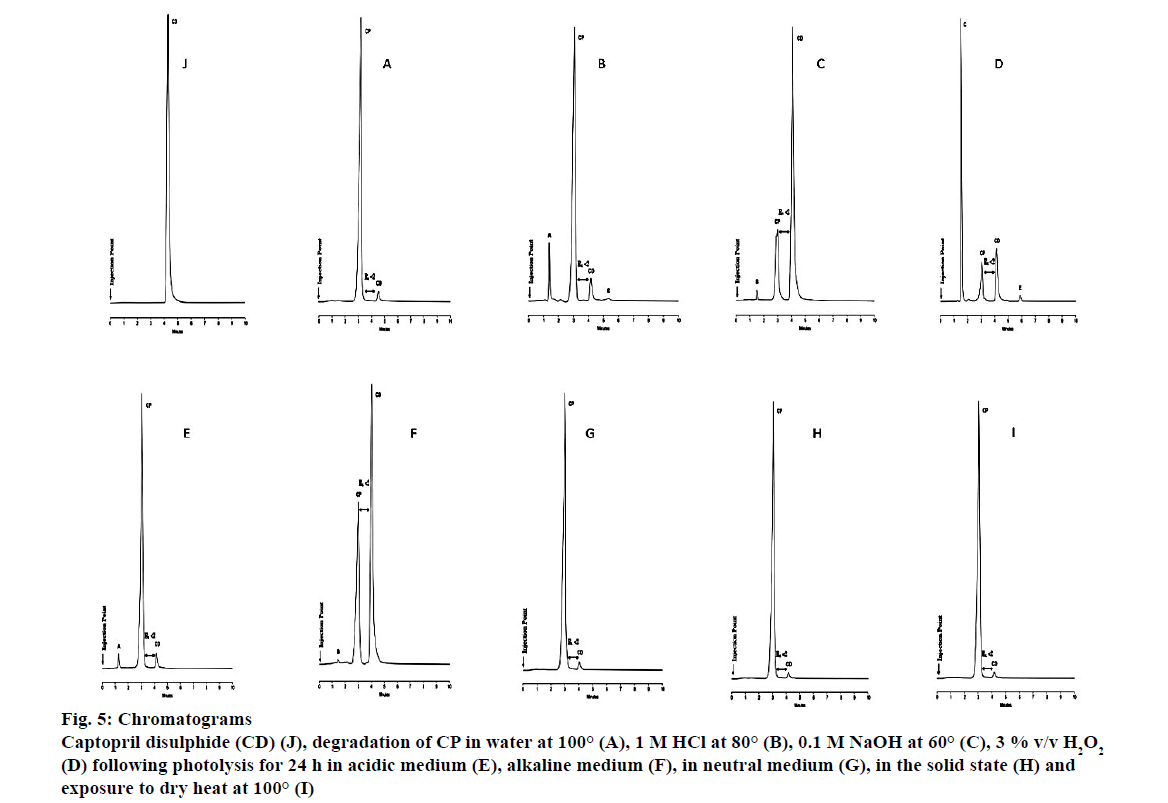
One of the major impurities of CP is CD, which can be detected for almost every stress condition evaluated [7,8,34]. The presence of CD was confirmed by including the USP reference standard of CD in testing and was resolved with a peak at retention time of 4.2 min, as depicted in Figure 5J. HPLC analysis of degradation (wet heat) samples revealed that no degradation of CP was observed at temperatures of 60°, 70°, 80° and 90°, however, approximately 3 % CP degraded at a temperature of 100° and a peak for CD was observed. The resultant chromatogram is depicted in Figure 5A. The amount of CP remaining in the mixture following acid degradation was 91.4 % and the resultant chromatogram (Figure 5B) revealed the presence of three degradation peaks represented as A, CD and D that were well resolved from the peak for CP. The amount of CP remaining following degradation under basic condition was 16.4 % and the resultant chromatogram is depicted in Figure 5C. As CP contains a thiol functional group, it readily oxidizes in the presence of 3 % v/v H2O2 and approximately 43.5 % CP was recovered following oxidation with three degradation products observed viz., C, E and CD in the chromatogram (Figure 5D). The results of photolytic degradation in an acidic medium resulted in the formation of degradation products labelled A and CD (Figure 5E) and in an alkaline medium, degradation peaks B and CD were observed and were well separated from the approximately 30.6 % CP recovered (Figure 5F). The degradation product CD was observed when CP was exposed to a neutral hydrolytic conditions (Figure 5G) and also during solid state studies (Figure 5H). Following exposure to dry heat at 100° only 3 % CP degraded and the chromatogram depicted in Figure 5I reveals the presence of CD.
Figure 5: Chromatograms
Captopril disulphide (CD) (J), degradation of CP in water at 100° (A), 1 M HCl at 80° (B), 0.1 M NaOH at 60° (C), 3 % v/v H2O2
(D) following photolysis for 24 h in acidic medium (E), alkaline medium (F), in neutral medium (G), in the solid state (H) and
exposure to dry heat at 100° (I)
The data from analysis of commercially available CP tablets are summarized in Table 6. The average amount of CP in each product was 48.51 to 50.50 mg equivalent to 97-101 % of the label claim. The percent recovery was calculated and falls within the specifications for assay in the USP.
| Product | Label claim | Mean CP±SD mg | Mean recovery ±SD % | % RSD |
|---|---|---|---|---|
| Mylan Captopril 50 | 50 mg | 50.10±0.21 | 100.17±0.42 | 0.42 |
| Adcock Captomax 50 | 50 mg | 48.53±0.11 | 97.03±0.22 | 0.23 |
| Sandoz CaptHexal®50 | 50 mg | 50.49±0.20 | 100.91±0.49 | 0.40 |
Table 6: Assay results for commercially available CP tablets
The use of a DoE and QbD approach facilitated the rapid development and optimization of an analytical method for the analysis of CP in raw material and dosage forms that was established as robust during the early stages of development. The use of the statistical tools ensured sufficient scientific knowledge about the impact of input variables on the critical quality parameters of the method was generated and interactions between input variables on a response are better understood. In addition the method, when operated in the defined DS, will ensure the generation of high quality analytical data from which informed formulation development decisions can be made. CP was found to be stable under acidic conditions but undergoes significant degradation under photolytic conditions in the solid state and in solution. This approach to method development was selected by considering all method attributes and assuming that several equipment and process factors would be reliable so as to limit the amount of work required to demonstrate the robustness of the analytical method. Whilst CP is an official USP compound and the analytical method reported in the USP is suitable for the analysis of the molecule, the retention time is 4.0 min [35] whereas this method exhibits an elution time for CP of 3.0 min. Furthermore the results of forced degradation studies revealed no shift in the elution time of CP, confirming a rapid, stability indicating method had been developed. The use of DoE and QbD ensured the identification of robust ranges for the operating conditions thereby ensuring the possibility of efficient application of the method in a regulatory context. In conclusion a new, rapid and comprehensive RP-HPLC method that is simple, precise, accurate, selective, specific and stability-indicating for the analysis of CP has been successfully developed using a QbD approach. The method could be operated effectively within the defined DS with the assurance that the data generated are reliable and valid.
Acknowledgments
The authors thank the Rhodes University Research Committee (RBW) and the National Research Foundation (NRF) of South Africa (KV) for funding.
Conflict of Interest
None.
References
- Kathleen P, Martindale W. Martindale: The Complete Drug Reference. 32nd ed. London, UK: Pharmaceutical Press; 1999.
- Mahmoud WMM, Kümmerer K. Captopril and its dimer captopril disulfide: Photodegradation, aerobic biodegradation and identification of transformation products by HPLC-UV and LC-ion trap-MSn. Chemosphere 2012;88:1170-7.
- Franklin ME, Addison RS, Baker PV, Hooper WD. Improved analytical procedure for the measurement of captopril in human plasma by gas chromatography--mass spectrometry and its application to pharmacokinetic studies. J Chromatogr B Biomed Sci Appl 1998;705:47-54.
- Mirza T, Tan HSI. Determination of captopril in pharmaceutical tablets by anion-exchange HPLC using indirect photometric detection; a study in systematic method development. J Pharm Biomed Anal 2001;25:39-52.
- Huang T, He Z, Yang B, Shao L, Zheng X, Duan G. Simultaneous determination of captopril and hydrochlorothiazide in human plasma by reverse-phase HPLC from linear gradient elution. J Pharm Biomed Anal 2006;41:644-8.
- Siddiqui FA, Sher N, Shafi N, Bahadur SS. Simultaneous Determination of metformin, captopril, lisinopril, and enalapril: its application to Pharmacokinetics. Arab J Chem 2013;11:35.
- Ivanovic D, Medenica M, Malenovic A, Jancic B. Validation of the RP-HPLC method for analysis of hydrochlorothiazide and captopril in tablets. Accredit Qual Assur 2004;9:76-81.
- Khamanga SM, Walker RB. The use of experimental design in the development of an HPLC-ECD method for the analysis of captopril. Talanta 2011;83:1037-49.
- Hillaert S, Van Den Bossche W. Determination of captopril and its degradation products by capillary electrophoresis. J Pharm Biomed Anal 1999;21:65-73.
- Stulzer HK, Tagliari MP, Kuminek G, Oliveira PR, Bertol CD, Silva MAS. Development and Validation of Stability Indicating LC Method to Quantify Captopril in Tablets of Controlled Release. Chromatographia 2009;69:123-8.
- Food and Drug Administration (FDA) Guidance for Industry. Stability testing of New Drug Substances and Products Q1A(R2). Available from: https://www.fda.gov/downloads/drugs/guidances/ucm073369.pdf.
- Rahmani A, Selamat J, Soleimany F. Ochratoxin A and zearalenone using an experimental design. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2011;28:902-12.
- Singh B, Raza K, Beg S. Developing “Optimized” Drug Products Employing “Designed” Experiments. Chem Ind Dig 2013;12:1-7.
- Sangshetti JN, Zahe Z. Application of QbD in development and validation of a rapid RP-HPLC method for determination of Atazanavir in bulk drug. J Med Chem Drug Discov 2015;32:385-401.
- Monks KE, Rieger HJ, Molnár I. Expanding the term “Design Space” in high performance liquid chromatography (I). J Pharm Biomed Anal 2011;56:874-9.
- Wu H, Khan MA. Quality‐by‐design (QbD): An integrated approach for evaluation of powder blending process kinetics and determination of powder blending end‐point. J Pharm Sci 2009;98:2784-98.
- Pebdan, AA, Shabani AMH, Dadfarnia S, Talebianpoor MS, Khodadoust S. Preconcentration of valsartan by dispersive liquid-liquid microextraction based on solidification of floating organic drop and its determination in urine sample: Central composite design. J Sep Sci 2016;39:1935-44.
- Sahu PK, Swain S, Prasad GVS, Panda J, Murthy YLN. RP-HPLC Method for Determination of Metaxalone Using Box-Behnken Experimental Design. J Appl Biopharm Pharmacokinet 2015;2: 40-9.
- Sathiyasundar R, Valliappan K. Experimental design approach to optimization of the new commercial RP-HPLC discrimination conditions for the estimation of paracetamol and zaltaprofen in pharmaceutical formulation. Int J Pharm Sci Res 2015;6:183.
- Ferreira SLC, Bruns RE, da Silva EGP, dos Santos WNL, Quintella CM, David JM, et al. Statistical designs and response surface techniques for the optimization of chromatographic systems. J Chromatogr A 2007;1158:2-14.
- Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008;76:965-77.
- Junker B, Zablackis E, Verch T, Schofield T, Douette P. Quality-by-Design: As Related to Analytical Concepts, Control and Qualification. In: Nunnally BK, Turula VE, Sitrin RD, editors. Vaccine Analysis: Strategies, Principles, and Control. Heidelberg, Berlin: Springer-Verlag; 2015. p. 479-520.
- Elder D, Borman P. Improving Analytical Method Reliability Across the Entire Product Lifecycle Using QbD Approaches. Pharm Outsourcing 2013;14:14-9.
- Peraman R, Bhadraya K, Reddy YP. Analytical Quality by Design : A Tool for Regulatory Flexibility and Robust Analytics. Int J Anal Chem 2015;2015:868727.
- Ishikawa K, Lu DJ. What is total quality control?: the Japanese way. New Jersey, United States: Prentice Hall; 1985.
- Vogt FG, Kord AS. Development of quality by design analytical methods. J Pharm Sci 2011;100:797-812.
- ICH Harmonised Tripartite Guideline. Validation of a analytical Procedures : text and methodology Q2(R1). Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf.
- Garg LK, Reddy VS, Sait SS, Krishnamurthy T, Vali SJ, Reddy AM. Quality by design: Design of experiments approach prior to the validation of a stability-indicating HPLC method for Montelukast. Chromatographia 2013;76:1697-706.
- Food and Drug Administration (FDA). Guidance for Industry: Analytical Procedures and Methods Validation for Drugs and Biologics. Center for Drug Evaluation and Research (CDER); 2014. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm386366.pdf.
- Schweitzer M, Pohl M, Hanna-Brown M, Nethercote P, Borman P, Hansen G, et al. Implications and opportunities of applying QbD principles to analytical measurements. Pharm Technol 2010;34:1-5.
- Garg LK, Sait SS, Krishnamurthy T, Kumar CHRP. Quality by Design (QbD): A Practical Experimental Design Approach by Blocking and Varying Certain Factors of a Stability-Indicating HPLC Method for Simultaneous Determination of Omeprazole and Ketoprofen. J Liq Chromatogr Relat Technol 2015;38:677-86.
- Ahuja M, Yadav M, Kumar S. Application of response surface methodology to formulation of ionotropically gelled gum cordia/gellan beads. Carbohydr Polym 2010;80:161-7.
- Watson DG. Pharmaceutical Analysis: A Textbook for Pharmacy Students and Pharmaceutical Chemists. Edinburgh, Scotland: Churchill Livingstone; 1999.
- Drummer OH, Jarrott B. Captopril disulfide conjugates may act as prodrugs: disposition of the disulfide dimer of captopril in the rat. Biochem Pharmacol 1984;33:3567-71.
- Leanpolchareanchai J, Suksiriworapong J. Validation of analytical method for captopril extemporaneous preparations by high performance liquid chromatography. Pharm Sci Asia 2015;42(2):85-92.