- Corresponding Author:
- D. A. Shah
Anand Pharmacy College, Opp. Town Hall, Anand - 388 001, India. E-mail: dimalgroup@yahoo.com
| Date of Submission | 7 September 2006 |
| Date of Revision | 14 June 2007 |
| Date of Acceptance | 20 July 2007 |
| Indian J Pharm Sci, 2007, 69 (4): 546-549 |
Abstract
A simple, specific and accurate reverse phase high performance liquid chromatographic method was developed for the simultaneous determination of atorvastatin calcium and aspirin in capsule dosage forms. A phenomenex Gemini C-18, 5 mm column having 250 x 4.6 mm i.d. in isocratic mode, with mobile phase containing 0.02 M potassiumdihydrogen phosphate: methanol (20:80) adjusted to pH 4 using ortho phosphoric acid was used. The flow rate was 1.0 ml/ min and effluents were monitored at 240 nm. The retention times of atorvastatin calcium and aspirin were 5.4 min and 3.4 min, respectively. The linearity for atorvastatin calcium and aspirin were in the range of 0.5-4 mg/ml and 5-25 mg/ml, respectively. The recoveries of atorvastatin calcium and aspirin were found to be in the range of 98.02-100.68% and 98.38-101.42%, respectively. The proposed method was validated and successfully applied to the estimation of atorvastatin calcium and aspirin in combined capsule dosage forms.
Keywords
Validation, RP-HPLC, atorvastatin calcium, aspirin
Atorvastatin calcium (ATV) is chemically [R– (R*,R*)]-2-(4-flurophenyl)-β,δ-dihydroxy-5- (1- methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]–1Hpyrrole- 1-heptanoic acid, calcium salt trihydrate. Atorvastatin calcium is an inhibitor of 3-hydroxy-3- methylglutaryl Coenzyme A (HMG-CoA) reductase. This enzyme catalyses the conversion of HMG-CoA to mevalonate, an early and rate limiting step in cholesterol biosynthesis [1,2]. Aspirin (ASP) is chemically 2-acetoxybenzoic acid and used as an analgesic, antipyretic, antiinflammatory and antithrombic agent. Combined dosage forms of ATV and ASP are available in the market. Clinical trials showed that combination therapy when used in dyslipidaemic patient with coronary heart diseases reduced cardiovascular events [3].
A literature survey regarding quantitative analysis of these drugs revealed that attempts were made to develop analytical methods for ATV using extractive spectrophotometry [4], HPLC [5-9], GC-MS [10], LC-MS [11], LC-electrospray tandem mass spectrometry [12-14] and HPTLC [15] methods. ASP is official in Indian pharmacopoeia and titrimetric [16] and HPLC [17] methods had been reported for its estimation. For estimation of ATV and ASP in combination, RP-HPLC [18] method had been reported. This paper describes a new RPHPLC method for the estimation of ATV and ASP combination in mixture using simple mobile phase.
Materials and Methods
The liquid chromatographic system consisted of the following components: Shimadzu HPLC model (VP series) containing LC-10AT (VP series) pump, Variable wavelength programmable UV/Vis detector SPD-10AVP and Rheodyne injector (7725i) with 20 µl fixed loop. Chromatographic analysis was performed using Spinchrom software on a Phenomenex Gemini C18 column with 250×4.6 mm i.d. and 5 µm particle size. The Shimadzu electronic balance (AX 200) was used for weighing purpose. Analytically pure ATV and ASP were obtained as gift samples from M/s Blue Cross Labs. Ltd., (Mumbai, India) and M/s Mercury Laboratories Ltd., (Vadodara, India). Acetonitrile, methanol, water (E. Merck, Mumbai, India) were of HPLC grade, while ortho-phosphoric acid and potassiumdihydrogen phosphate (S. D. Fine Chemicals, Mumbai, India) were of Analytical grade used for the preparation of mobile phase. Capsule formulation A (Ato Plus, Triton Pharma. Ltd., Mumbai, India) and B (Atchol ASP, Aristo Pharma. Ltd., India) containing labeled amount 10.34 mg ATV and 75 mg ASP were procured from local market.
Preparation of mobile phase and stock solutions
Potassium dihydrogen phosphate was weighed (0.544 g) and dissolved in 200 ml of water. This solution was mixed with 800 ml of methanol. Finally the pH was adjusted to 4.0 with ortho phosphoric acid (0.1 M). The solution was sonicated for 10 minutes and filtered using Whatman filter paper (No.1) and used. ATV and ASP were weighed (25 mg each) and transferred to two separate 25 ml volumetric flasks and dissolved in methanol, which gives 1000 µg/ ml of ATV and ASP, respectively. ATV and ASP solutions were further diluted with methanol to obtain final concentration of 50 µg/ ml and 100 µg/ ml, respectively.
Chromatographic conditions
A reverse phase C18 column equilibrated with mobile phase 0.02M potassiumdihydrogen phosphate-methanol (20:80) adjusted to pH 4 was used. Mobile phase flow rate was maintained at 1 ml/min and effluents were monitored at 240 nm. The sample was injected using a 20 µL fixed loop, and the total run time was 10 min. Appropriate aliquots of ATV and ASP stock solutions were taken in different 10 ml volumetric flasks and diluted up to the mark with mobile phase to obtain final concentrations of 0.5, 1, 2, 3, 4 µg/ ml of ATV and 5, 10, 15, 20, 25 µg/ml of ASP, respectively. The solutions were injected using a 20 µl fixed loop system and chromatograms were recorded. Calibration curves were constructed by plotting average peak area versus concentrations and regression equations were computed for ATV and ASP.
Determination of ATV and ASP in their combined dosage forms
The content of twenty capsules were taken and weighed. Powder equivalent to ATV 10.34 mg and 75 mg ASP was accurately weighed and transferred to a 50 ml volumetric flask and 20 ml of mobile phase was added to the same and flask was sonicated for 5 min. The flask was shaken, and the volume was diluted to the mark with the same mixture. The above solution was filtered using Whatman filter paper No.1. Appropriate volume of the aliquot was transferred to a 50 ml volumetric flask and the volume was made up to the mark with mobile phase to obtain 2.068 µg/ ml of ATV and 15 µg/ml of ASP. The solution was sonicated for 10 min. The solution was injected at above chromatographic conditions and peak areas were measured. The quantification was carried out by keeping these values to the straight line equation of calibration curve. The method was validated for accuracy, precision, specificity, detection limit, quantitation limit and robustness.
Accuracy
The accuracy of the method was determined by calculating recoveries of ATV and ASP by method of standard additions. Known amount of ATV (0, 0.5, 1, 1.5 µg/ml) and ASP (0, 2, 4, 6 µg/ml) were added to a pre quantified sample solution, and the amount of ATV and ASP were estimated by measuring the peak areas and by fitting these values to the straight-line equation of calibration curve.
Precision
The intra day and inter day precision study of ATV and ASP was carried out by estimating the corresponding responses 3 times on the same day and on 3 different days (first, second and fifth day) for 3 different concentrations of ATV (1, 2, 3 µg/ml) and ASP (10, 15, 20 µg/ml), and the results are reported in terms of relative standard deviation (RSD, Table 2). The Repeatability studies were carried out by estimating response of 3 different concentrations of ATV (0.5, 1, 2 µg/ml) and ASP (10, 15, 20 µg/ml) for triplicate and results are reported in terms of relative standard deviation (RSD).
Specificity
Commonly used excipients (starch, microcrystalline cellulose and magnesium stearate) were spiked into a pre weighed quantity of drugs. The chromatogram was taken by appropriate dilutions and the quantities of drugs were determined.
Detection limit and quantitation limit
A calibration curve was prepared using concentrations in the range of 0.1-2 µg/ml for ATV and 0.2-2 µg/ml for ASP (expected detection limit range). The standard deviation of y-intercepts of regression lines were determined and kept in following equation for the determination of detection limit and quantitation limit. Detection limit= 3.3σ /s; quantitation limit= 10σ/s; where σ is the standard deviation of yintercepts of regression lines and s is the slope of the calibration curve.
Robustness
Robustness of the method was studied by changing the composition of organic phase by ± % and the pH by ±0.2, and also by observing the stability of the drugs for 24 h at 35o temperature in the mobile phase.
Results and Discussion
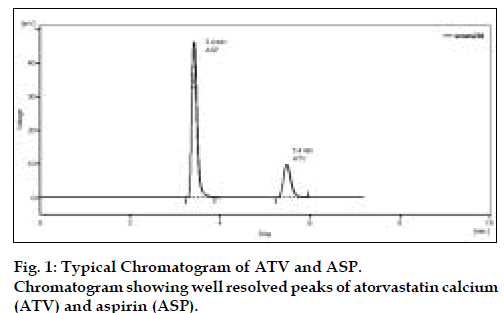
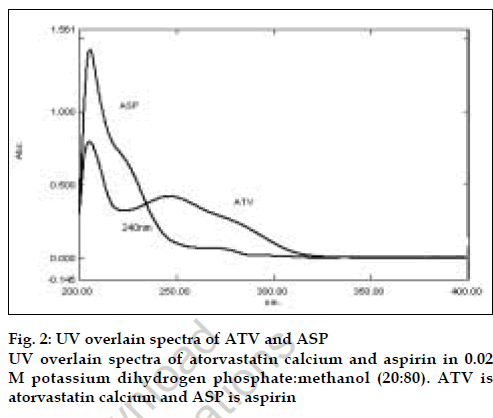
Optimization of mobile phase was performed based on resolution, asymmetric factor and peak area obtained for both ATV and ASP. The mobile phase 0.02 M potassiumdihydrogen phosphate:methanol (20:80) adjusted to pH 4 using ortho phosphoric acid was found to be satisfactory and gave two symmetric and well-resolved peaks for ATV and ASP. The resolution between ATV and ASP was found to be 8.1, which indicates good separation of both the compounds. The retention time for ATV and ASP were 5.4 min and 3.4 min, respectively (fig. 1). The asymmetric factors for ATV and ASP were 1.30 and 1.48, respectively. Overlain UV spectra of both ATV and ASP showed that both the drugs absorbs appreciably at 240 nm so, 240 nm was selected as the detection wavelength in liquid chromatography (fig. 2).
The calibration curve for ATV was obtained by plotting the peak area of ATV versus the concentration of ATV over the range of 0.5-4 µg/ml, and it was found to be linear with r= 0.9982. Similarly, the calibration curve for ASP was obtained over the range of 5-25 µg/ml and was found to be linear with r= 0.9993. The data of regression analysis of the calibration curves are shown in Table 1. The detection limit for ATV and ASP were 0.09 µg/ml and 0.21 µg/ ml, respectively. The quantitation limit for ATV and ASP were 0.28 µg/ml and 0.65 µg/ml, respectively, which suggest that a nanogram quantity of both the compounds can be estimated accurately. The validation parameters are summarized in Table 2.
| Parameters | ATV | ASP |
|---|---|---|
| Linearity range (µg/ ml) | 0.5-4 | 5-25 |
| Slope | 28.26 | 14.66 |
| Standard deviation of slope | 0.608 | 0.130 |
| Intercept | -1.55 | -0.518 |
| Standard deviation of intercept | 0.910 | 0.662 |
| Correlation coefficient (r) | 0.9982 | 0.9993 |
ATV is atorvastatin calcium and ASP is aspirin
Table 1: Regression analysis of the calibration curves for the proposed method
| Parameters | ATV | ASP |
|---|---|---|
| Detection limit (µg/ ml) | 0.09 | 0.21 |
| Quantitation limit (µg/ ml) | 0.28 | 0.65 |
| Accuracy (%) | 98.02-100.68 | 98.38-101.42 |
| Precision (RSDa, %) | ||
| Intraday (n=3) | 0.99-1.42 | 0.84-0.65 |
| Interday (n=3) | 1.14-3.82 | 0.71-1.21 |
| Repeatability (RSDa,n=3) | 0.59-0.95 | 0.26-0.34 |
aRSD indicates relative standard deviation; ATV is atorvastatin calcium and ASP is aspirin
Table 2: Summary of validation parameters
The recoveries of ATV and ASP were found to be in the range of 98.02–100.68% and 98.38–101.42%, respectively. The system suitability test parameters are shown in Table 3. The liquid chromatographic method was applied to the determination of ATV and ASP in their combined dosage forms (capsule formulation A and B). The results for ATV and ASP were comparable with the corresponding labeled amounts (Table 4).
| System suitability Parameters | ATV | ASP |
|---|---|---|
| Retention time (min) | 5.4 | 3.4 |
| Resolution | 8.1 | - |
| Theoretical plates | 5311 | 4474 |
| Tailing factor (asymmetric factor) | 1.30 | 1.48 |
Table 3: system suitability test parameters for ATV and ASP by the proposed method
| Formulations | Labelled amount (mg) | Amount obtained (mg)b | % Recoveryb | |||
|---|---|---|---|---|---|---|
| ATV | ASP | ATV | ASP | ATV | ASP | |
| A | 10.34 | 75 | 10.15 ± 0.08 | 74.18 ± 0.44 | 98.18 ± 0.76 | 98.91 ± 0.58 |
| B | 10.34 | 75 | 10.33 ± 0.17 | 73.64 ± 0.52 | 99.90 ± 1.63 | 98.19 ± 0.70 |
Table 4: Assay results of combined dosage form using proposed method
Proposed study describes a new RP-HPLC method for the estimation of ATV and ASP combination in mixture using simple mobile phase with low buffer concentration compared to the reported method. The method gives good resolution between both the compounds with a short analysis time (<10 min). The method was validated and found to be simple, sensitive, accurate and precise. Percentage of recovery shows that the method is free from interference of the excipients used in the formulation. Therefore, the proposed method can be used for routine analysis of ATV and ASP in their combined dosage form.
Acknowledgements
The authors are grateful to M/s Blue Cross Labs. Ltd., Mumbai for providing gift sample of atorvastatin calcium and M/s Mercury Laboratories Ltd., Vadodara for providing gift sample of aspirin.
References
- Budavari S, editor. The Merck Index. 12 th ed. Whitehouse station (NJ): Merck and Co. Inc: 1996. p. 897.
- Gennaro AE, editor. Remington's-The Science and Practice of Pharmacy. 20 th ed. Vol. II. Easton (PA). Mack Publishing Co: 2000. p. 1294.
- Athyros VG, Mikhailidis DP, Papageorgious AA, Bouloukos VI, Pehlivanidis AN, Symeonidis AN, et al . Effect of statins and aspirin alone and in combination on clinical outcome in dyslipidaemic patients with coronary heart disease: A subgroup analysis of the greace study. Platelets 2005;16:65-71.
- Erk N. Extractive spectrophotometric determination of atorvastatin in bulk and pharmaceutical formulations. Anal Lett 2003;36:2699-711.
- Shen HR, Liz D, Zhong MK. HPLC assay and pharmacokinetic study of atorvastatin in beagle dog after oral administration of atorvastatin self-micro emulsifying drug delivery system. Pharmazie 2006;61:18-20.
- Verd JC, Peris C, Alergret M, Diaz C, Hernandez ZG, Sanchez RM. Different effect of simvastatin and atorvastatin on key enzyme involved in VLDL synthesis and catabolism on high fat / cholesterol rabbit. Br J Pharmacol 1999;127:1479-85.
- Bleske BE, Willis RA, Anthony M, Casselberry N, Datwani M, Uhley VE, et al . The effect of pravastatin and atorvastatin on coenzyme Q10. Am Heart J 2001;142:262.
- Altuntas TG, Erk N. Liquid chromatographic determination of atorvastatin in bulk drug, tablets and human plasma. J LiqChromatogrRelatTechnol 2004;27:83-93.
- Erturk S, Aktas ES, Ersoy L, Ficicioglu S. An HPLC method for the determination of atorvastatin and its impurities in bulk drugs and tablets. J Pharm Biomed Anal 2003;33:1017-23.
- McKenney JM, McCormick LS, Weiss S, Koren M, Kafonek S, Blanck DM. A randomized trial of the effects of atorvastatin and niacin in patients with combined hyperlipidaemic or isolated hypertriglyceridemia, collaborative atorvastatin study group. Am J Med 1998;104:137-43.
- Nirogi RV, Kandikere VN, Shukla M, Mudigonda M, Maurya S, Boosi R, et al . Simultaneous quantification of atorvastatin and active metabolites in human plasma by LC-MS using rosuvastatin as internal standard. Biomed Chromatogr 2006;20:924-36.
- Miao XS, Metcalfe CD. Determination of cholesterol lowering stain dugs in aqueous samples using liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatography A 2003;998:133-41.
- Jemal M, Ouyang Z, Chen BC, Teitz D. Quantitation of the acid and lactone forms of atorvastatin and its biotransformation products in human serum by high performance liquid chromatography with electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom 1999;13:1003-15.
- Bullen WW, Miller RA, Hayes RN. Development and validation of a high performance liquid chromatography- tandem mass spectrometry assay for atorvastatin, ortho-hydroxy atorvastatin and para-hydroxy atorvastatin in human, dog and rat plasma. J Am Soc Mass Spectrom 1999;10:55-66.
- Yadav SS, Mhaske DV, Kakad AB, Patil BD, Kadam SS, Dhaneshwar SR. Simple and sensitive HPTLC method for determination of content uniformity of atorvastatin calcium tablet. Indian J Pharm Sci 2005;67:182-8.
- Indian Pharmacopoeia, Vol. 1. Published by Controller of Publications: Delhi; 1996. p. 69.
- Ramos MN, Aguirr-gomez F, Molina DA, Capitan LF. Application of liquid chromatography to the simultaneous determination of acetyl salicylic acid, caffeine, codeine, pyridoxine and thiamine in pharmaceutical preparation. J AOAC Int 2001;84:676-83.
- Manoj K, Shanmugapandiyan P, Anbazhagan S. RP- HPLC method for simultaneous estimation of atorvastatin and aspirin from capsule formulation. Indian Drugs 2004;41:284-9.