- *Corresponding Author:
- N. J. Shah
Shri B. M. Shah College of Pharmaceutical Education & Research, Modasa - 383 315, India
E-mail: nehal9175@yahoo.co.in
| Date of Submission | 28 September 2005 |
| Date of Revision | 21 August 2006 |
| Date of Acceptance | 16 March 2007 |
| Indian J Pharm Sci, 2007, 69 (2): 202-205 |
Abstract
A simple, precise, accurate and rapid high performance thin layer chromatographic method has been developed and validated for the estimation of telmisartan and hydrochlorothiazide simultaneously in combined dosage forms. The stationary phase used was precoated silica gel 60F254. The mobile phase used was a mixture of chloroform: methanol: toluene (2:5:5 v/v/v). The detection of spots was carried out at 272 nm. The method was validated in terms of linearity, accuracy, precision and specificity. The calibration curve was found to be linear between 250 to 500 ng/spot for telmisartan and 200 to 700 ng/spot for hydrochlorothiazide. The limit of detection and the limit of quantification for the telmisartan were found to be 75 and 190 ng/spot, respectively, and for hydrochlorothiazide 55 and 150 ng/spot, respectively. The proposed method can be successfully used to determine the drug content of marketed formulation.
The combination of telmisartan and hydrochlorothiazide has recently been introduced in the market. Chemically telmisartan is 3-N1-methyl-2-benzimidazole derivative of N1-4-(2-carboxyphenyl phenyl)-2-propyl, 4-methyl benzimidazole [1,2] and hydrochlorothiazide is 6-chloro-3, 4-dihydro-2H-1,2,4-benzothiadiazine-7-sulphonamide-1,1-dioxide. Telmisartan is a new angiotensin II receptor antagonist for the treatment of essential hypertension. It is useful in the treatment of mild to moderate hypertension, well tolerated with a lower incidence of cough than ACE inhibitors [3]. The addition of hydrochlorothiazide to telmisartan was more effective than each agent at lowering blood pressure in patients with hypertension [4]. Literature survey reveals that few HPLC methods are reported for the estimation of telmisartan and hydrochlorothiazide in the biological fluids [5,6] and formulations [7]. So far no HPTLC method has been reported for the estimation of telmisartan and hydrochlorothiazide in formulation. In the present investigation an attempt has been made to develop accurate and precise HPTLC method for the simultaneous estimation of telmisartan and hydrochlorothiazide in combined dosage forms.
Materials and Methods
Telmisartan and hydrochlorothiazide standard were procured as a gift sample from Sun Pharmaceuticals Ltd., -Baroda. Silica gel 60F254 TLC plates (10 × 10 cm, layer thickness 0.2 mm, E. Merck, Mumbai) were used as a stationary phase. All chemicals and reagents used were of analytical grade. Tablets containing telmisartan (40 mg) and hydrochlorothiazide (12.5 mg) were purchased from local market (Telsar-H, Unichem Labt. and Telpres-H, Nicholas Piramal India Ltd). A Camag HPTLC system comprising of Camag Linnomate V automatic sample applicator, Hamilton syringe (100 μl), Camag TLC Scanner 3, Camag WinCATS software, Camag Twin-trough chamber (10×10 cm) and ultrasonicator were used during study.
Preparation of standard and sample solutions
Telmisartan and hydrochlorothiazide (25 mg) each were weighed accurately, dissolved and diluted with chloroform to obtain the final concentration of 100 μg/ml of each drug. Twenty tablets were weighed accurately and ground to fine powder. Weight equivalent to 25 mg of telmisartan and hydrochlorothiazide were transferred to conical flask and mixed with chloroform. The solution was sonicated for 15 min. The extracts were filtered through Whatmann filter paper No. 41 and residue was washed with chloroform. The extracts and washing were pooled and transferred to a 250 ml volumetric flask and volume was made up to 250 ml with chloroform. Required dilutions were made to get 100 μg/ml of telmisartan and hydrochlorothiazide.
HPTLC method and chromatographic conditions
TLC plates were prewashed with methanol. Activation of plates was done in an oven at 500 for 5 min. The chromatographic conditions maintained were precoated silica gel 60F254 aluminum sheets (10 × 10 cm) as stationary phase, chloroform: methanol: toluene (2:5:5 v/v/v) as mobile phase, chamber and plate saturation time of 30 min, migration distance allowed was 72 mm, wavelength scanning was done at 272 nm keeping the slit dimention at 5×0.45 mm. A deuterium lamp provided the source of radiation. Four microlitres of standard solutions of telmisartan and hydrochlorothiazide were spotted and developed at constant temperature. Wavelength was selected by scanning standard solutions of both drugs over 200 nm to 400 nm wavelengths. Telmisartan show maximum absorbance at 299 nm and hydrochlorothiazide at 277 nm. Both components show reasonably good response at 272 nm, therefore photometric measurements were performed at 272 nm in reflectance mode with Camag TLC scanner 3 using Win CATS software.
Camag TLC scanner 3 using Win CATS software. Aliquots of 2.5, 3.0, 3.5, 4.0, 4.5, 5.0 μl of standard solution of telmisartan and 2.0, 3.0, 4.0, 5.0, 6.0, 7.0 μl of hydrochlorothiazide were applied on the TLC plate (100 μg/ml of drug). TLC plate was dried, developed and analyzed photometrically as described earlier. The standard calibration curve was generated using regression analysis with Microsoft excel.
Validation of the method [8-10]
The developed method was validated in terms of linearity, accuracy, limit of detection, limit of quantification, intra-day and inter-day precision and repeatability of measurement as well as repeatability of sample application.
Analysis of the marketed formulations
Four microlitres of sample solutions of the marketed formulation was spotted on to the same plate followed by development scanning. The analysis was repeated in triplicate. The content of the drug was calculated from the peak areas recorded.
Results and Discussion
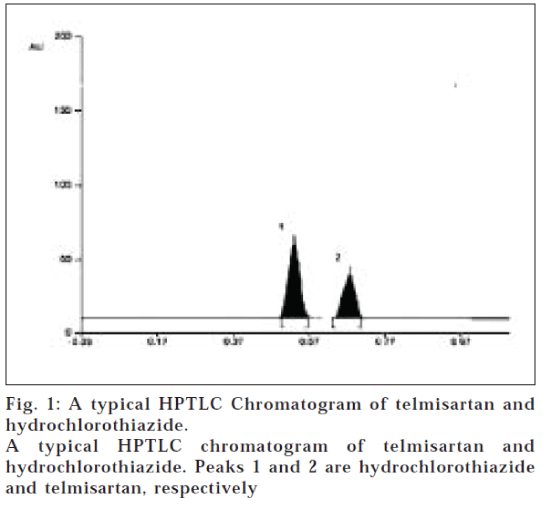
A solvent system that would give dense and compact spots with significant Rf values was desired for quantification of telmisartan and hydrochlorothiazide in pharmaceutical formulations. The mobile phase consisting of chloroform: methanol: toluene (2:5:5 v/v/v) gave Rf values of 0.53±0.04 and 0.68±0.03 for telmisartan and hydrochlorothiazide, respectively (fig.1). The linear regression data (n=5, Table 1) showed a good linear relationship over a concentration range of 250-500 ng/spot and 200-700 ng/spot for telmisartan and hydrochlorothiazide, respectively. The limit of detection and limit of quantification for telmisartan was found to be 75 and 190 ng/spot and for hydrochlorthiazide, 55 and 150 ng/spot, respectively.
| Parameters | Values | |
|---|---|---|
| Telmisartan | Hydrochlorthiazide | |
| Linearity range (ng/spot | 250-500 | 200-700 |
| Correlation coefficient (r) | 0.9963 | 0.9954 |
| Regression equation (y=mx+c) | 5.82 | 14.17 |
| Slope (m) | 497.48 | 692.87 |
| Intercept (c) | 75 ng/spot | 55 ng/spot |
| Limit of.detection (LOD | 190 ng/spot | 150 ng/spot |
| Limit of quantification (LOQ) Precision (%CV) Repeatability of application (n=5) | 1.17 | 1.53 |
| Repeatability of measurement (n=5) | 0.55 | 0.34 |
Table 1: Method Validation Parameters
The intra-day precision was determined by analyzing standard solutions in the concentration range of 250 ng/ spot to 500 ng/spot for telmisartan and 200 ng/spot to 700 ng/spot for hydrochlorothiazide for 3 times on the same day while inter-day precision was determined by analyzing corresponding standards daily for 3 day over a period of one week. The intra-day and inter-day coefficients of variation are given in Table 2. Repeatability of sample application was assessed by spotting 4 μl of drug solution 5 times on a TLC plate followed by development of plate and recording the peak area for 5 spots. The % RSD for peak area values of telmisartan and hydrochlorothiazide were found to be 1.17 and 1.53, respectively. Repeatability of measurement of peak area was determined by spotting 4 μl of telmisartan and hydrochlorothiazide solution on a TLC plate and developing the plate. The separated spot was scanned five times without changing the position of the plate and % RSD for measurement of peak area of telmisartan and hydrochlorothiazide were found to be
0.55 and 0.34, respectively. To confirm the specificity of the proposed method, the solution of the formulation was spotted on the TLC plate, developed and scanned. It was observed that the excipients present in the formulation did not interfere with the peaks of telmisartan and hydrochlorothiazide.
| Drug | Concentratio (µg/spot) | Intra-day precision % RSD | Inter-day precision % RSD |
|---|---|---|---|
| Telmisartan 40 (mg) | 3.5 | 0.68 | 1.30 |
| 4.0 | 0.7 | 1.42 | |
| 4.5 | 0.98 | 1.52 | |
| Hydrochlorthiazide 12.5 (mg) | 3.0 | 0.15 | 1.40 |
| 4.0 | 0.82 | 1.00 | |
| 5.0 | 0.45 | 1.45 |
Table 2: Precision Of Telmisartan And Hydrochlorothiazide
Recovery studies of the drugs were carried out for the accuracy parameter. These studies were carried out at three levels i.e. multiple level recovery studies. Sample stock solution from tablet formulation of 100 μg/ml of were prepared. Dilutions were made and recovery studies were performed. % Recovery was found to be within the limits as listed in Table 3. The assay value for the marketed formulation was found to be within the limits as listed in Table 4. The low RSD value indicated the suitability of the method for routine analysis of telmisartan and hydrochlorothiazide in pharmaceutical dosage forms.
| Brand name | Label claim mg/tablet | Amount added % | Total amount added (mg) | Amount recovered* (mg)±SD | % Recovery ± SD |
|---|---|---|---|---|---|
| Telsar-H | Telmisartan 40 | 33.33 | 13.33 | 13.54±0.21 | 101.58±1.57 |
| 50.00 | 20.00 | 20.21±0.79 | 101.05±0.80 | ||
| 66.67 | 26.66 | 26.96±0.99 | 101.16±1.01 | ||
| Telsar-H | Hydrochlorthiazide 12.5 | 33.33 | 4.16 | 04.15±0.04 | 99.95±0.04 |
| 66.67 | 8.33 | 08.27±1.18 | 99.30±1.18 | ||
| 100.00 | 12.5 | 12.5± 0.99 | 100.1±1.01 | ||
| Telpres-H | Telmisartan 40 | 33.33 | 13.33 | 13.13±1.31 | 98.55±1.30 |
| 50.00 | 20.00 | 19.89±0.33 | 99.45±0.33 | ||
| 66.67 | 26.66 | 26.96±1.63 | 101.16±1.65 | ||
| Telpres-H | Hydrochlorthiazide 12.5 | 33.33 | 4.16 | 04.15±0.33 | 99.81±0.33 |
| 66.67 | 8.33 | 08.32±0.05 | 99.97±0.05 | ||
| 100.00 | 12.5 | 12.55±0.01 | 100.45±0.02 |
Table 3: Recovery Studies Of Telmisartan And Hydrochlorothiazide
| Brandname | Label Claim mg/tablet | Amount found (mg) | % of drug found* | % RSD |
|---|---|---|---|---|
| Telsar-H | Telmisartan 40 | 40.15 | 100.38 + 1.45 | 1.45 |
| Telsar-H | Hydrochlorthiazide 12.5 | 12.45 | 99.60 + 1.83 | 1.83 |
| Telpres-H | Telmisartan 40 | 39.92 | 99.81 + 0.22 | 0.22 |
| Telpres-H | Hydrochlorthiazide 12.5 | 12.45 | 99.33 + 0.55 | 0.55 |
Table 4: Analysis Of Telmisartan And Hydrochlorothiazide
The developed HPTLC technique is simple, precise, specific and accurate and the statistical analysis proved that method is reproducible and selective for the analysis of telmisartan and hydrochlorothiazide in bulk drug and tablet formulations.
Acknowledgements
The authors thank the Sun Pharmaceuticals Ltd., Baroda for the gift sample of telmisartan and hydrochlorothiazide. Authors also thank the principal and the management for providing necessary facilities and encouragement.
References
- Ramsay, L.E., Brit. Med. J., 1999, 635, 1993.
- McClellan, K.J. and Markham, A., Drugs , 1998, 56, 1044.
- Anon., The Formulary , 1999, 18.
- McGill, J.B. and Reilly, P.A., Clin. Cardiol., 2001, 24, 72.
- White, W.B., Weber, M.A., Davidai, G., Neutel, J.M., Bakris, G.L. and Giles, T., Blood Press. Monit, 2005, 10, 163.
- Alocer, L., Fernandez, P., Campos, E., Ruiz, R.O., Bahena, J., Dela, J.J. and Segovia, A.C., Int. J. Clin. Pract. , 2004, 58, 39.
- Palled, M.S., Rajesh, P.M.N., Chatter, M. and Bhat, A.R., Indian J. Pharm. Sci., 2005, 5, 108.
- El-Gindy, A., El-Waily, A.F.M. and Bedari, M.F., J. Pharm. Biomed. Anal., 2000, 23, 341.
- Validation of Analytical Procedures, Methodology, ICH harmonized tripartite guidelines, 1996, 1.
- Thoppil, S.O., Cardoza, R.M. and Amin, P.D., J. Pharm. Biomed. Anal., 2001, 25, 15.