- *Corresponding Author:
- Sadhana J. Rajput
Department of Pharmaceutical Quality Assurance, Faculty of Pharmacy, The Maharaja Sayajirao University of Baroda, Vadodara-390 002, India
E-mail: sjrajput@rediffmail.com
| Date of Submission | 29 December 2016 |
| Date of Revision | 10 May 2017 |
| Date of Acceptance | 14 January 2018 |
| Indian J Pharm Sci 2018;80(2): 235-241 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
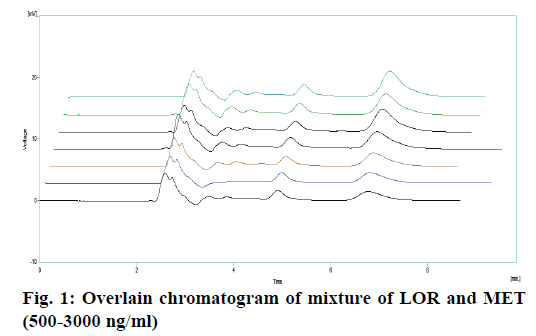
A rapid, precise, accurate, specific and simple high performance liquid chromatography method for estimation of lorcaserin hydrochloric in human plasma, using metoprolol as an internal standard, was developed and validated as per the regulatory requirements. Sample preparation included solid phase extraction and chromatographic separation was performed using a Phenomenex Luna C18 column (250×4.6 mm i.d, 5 µ particle size), with phosphate buffer (pH 3):acetonitrile:methanol (65:20:15) as the mobile phase at a flow rate of 1.0 ml/min. Wavelength of detection was 222 nm. Retention times of internal standard and lorcaserin HCl were found to be 5.15 and 7.19 min, respectively. The method was developed and tested in the linearity range of 500 to 3000 ng/ml. The method was validated for accuracy, precision, linearity, recovery and stability in compliance to international regulatory guidelines.
Keywords
Lorcaserin hydrochloride, metoprolol, bioanalytical method, HPLC
Lorcaserin hydrochloride (LOR) is an antiobesity (selective serotonin 2c receptor agonist), off-white to white powder, which is freely soluble in water, methanol, acetonitrile and dimethyl sulphoxide having log P value of 2.56 and pKa of 9.53. LOR is not official in IP, BP and USP and is available as 10 mg tablets.
A number of analytical methods were reported in literature to analyse LOR in tablet formulation. A liquid chromatography-electro spray ionization-tandem mass spectrometry (LC-ESI-MS/MS) [1] method was reported for in vivo and in vitro pharmacological characterization of LOR. Also a chiral LC-MS/MS method for the separation and quantitation of lorcaserin and its S-enantiomer has been reported [2]. Despite of the fact that various analytical methods are available for the estimation of lorcaserin, no solid phase extraction based bioanalytical high-performance liquid chromatography (SPE HPLC) method was available in the literature to the best of our knowledge. Thus, the main aim of the study was to develop a SPE HPLC method for the determination of LOR in plasma and its application to rat pharmacokinetic study. Metoprolol (MET) was used as internal standard (IS) for bioanalytical method development. MET is an antihypertensive agent and having solubility in water and official in IP, BP and USP having log P value of 1.88 and pKa of 9.67.
Materials and Methods
LOR was purchased from Swapnaroop Drugs Pvt. Ltd., Aurangabad and was certified to contain 99.70 % (w/w) on dried basis whereas MET of pharmaceutical grade was obtained as a gift sample from Torrent Pharmaceuticals Pvt. Ltd., Ahmedabad and was certified to contain 99.30 % (w/w) on dried basis. Methanol and acetonitrile used were of HPLC grade and were purchased from Rankem, Ankleshwar. Potassium hydrogen orthophosphate of HPLC grade was purchased from Qualigens, Ahmedabad and orthophosphoric acid was purchased from Spectrochem, Vadodara. Drug free EDTA human plasma was procured from Suraktam Blood Bank, Vadodara. The liquid chromatographic system was of Shimadzu, Mumbai and consisting of following components an isocratic pump, variable wavelength programmable UV/Vis detector, a manual injection facility with 20 μl fixed loop. The chromatographic analysis was performed using Spinchrom software on a Phenomenax-RP-18 column (250×4.6 mm, 5 μm particle size). In addition, an electronic balance (Shimadzu AX120ELB300), a pH meter (Lab India Pico+), sonicator (Spectra Lab, Selec XT 543), hot air oven (SK Industries), solid phase extractor (Orochem, Ezypress HT48), vortex shaker (SPINIX), membrane filter 0.22 micron (Pall Lifesciences, Ultipor Nylon), deep freezer (EIE Instruments), micropipette (Tarsons, accupipete), SPE cartridges (Phenomenex, Oasis HLB Cartridges), refrigerated centrifuge (Remi), refrigerator (Godrej, Pantacool) were used in this study. The pharmacokinetic study was carried out in Male Sprague Dawley rats. The experimental procedure was approved by Institutional Animal Ethics Committee, Pharmacy department under protocol number (MSU/ IAEC/2014-15/1419) on 22nd Sept 2014.
Preparation of mobile phase buffer
About 10 mM phosphate buffer was prepared by dissolving 0.136 g of potassium dihydrogen orthophosphate in sufficient water to produce 100 ml. The pH was adjusted to 3 using orthophosphoric acid. This buffer was filtered through 0.22 μ membrane filter and stored at ambient temperature.
Preparation of mobile phase
Appropriate volumes of phosphate buffer (pH 3, adjusted with orthophosphoric acid), acetonitrile and methanol were transferred into a reagent bottle, mixed thoroughly, sonicated for 5 min and filtered through 0.22 μm membrane filter and used as mobile phase. The HPLC analysis was performed on reversed-phase HPLC system with isocratic elution mode using a mobile phase of phosphate buffer:acetonitrile:methanol (65:20:15 v/v/v) on Phenomenex Luna C18 column (250×4.6 mm, 5 μm particle size) with 1 ml/min flow rate at 222 nm using UV detector.
Stock solutions of LOR and MET (1000 ppm)
About 10.8 mg of LOR hemihydrate (10.8 mg LOR hemihydrates is equivalent to 10 mg LOR) was weighed accurately, transferred into a 10 ml volumetric flask, dissolved in double distilled water and the volume made up to the mark to obtain the LOR stock solution. About 10 mg of MET (IS) was weighed accurately, transferred to a 10 ml volumetric flask, dissolved in double distilled water and the volume was made up to the mark to obtain a MET stock solution.
Calibration standards for LOR
Appropriate aliquots of LOR stock solution were taken in different 6 ml volumetric flasks and diluted up to the mark with mobile phase to obtain final concentrations of 10-60 μl/ml. To appropriate aliquots of calibration standards, 0.1 ml of 400 ppm IS was spiked and the final volume of 2 ml was made up with plasma to obtain final concentration of 500-3000 ng/ml. The linearity range was selected on the basis of reported peak plasma concentration (Cmax= 789 ng/ml) from literature.
To appropriate aliquots of calibration standards, 0.1 ml of 400 ppm IS was spiked and the final volume of 2 ml was made up with plasma to obtain final concentration of 500 ng/ml as lower limit of quantification control, 1000 ng/ml as low quality control, 2000 ng/ml as medium quality control (MQC), 3000 ng/ml as high quality control (HQC).
Mobile phase trials were taken on unextracted samples. Various mobile phases like water:methanol (50:50 and 20:80), water:acetonitrile (30:70), water:acetonitrile (pH 3; 60:40), phosphate buffer:acetonitrile (pH 3; 70:30), phosphate buffer:methanol:acetonitrile (60:15:25) and phosphate buffer:methanol:acetonitrile (65:15:20) were tried in which phosphate buffer:methanol:acetonitrile (65:15:20) at pH 3 gave the best peak.
Also trials for selection of appropriate IS were taken in which screening was done on the basis of structural resemblance, log P value, pKa value and availability. Chromatographic trials for bisoprolol fumarate and MET succinate were undertaken with conditions described earlier in which MET succinate gave good peak as shown in Table 1.
| Drug | Mobile phase | Column used | Flow rate | RT (min) | Peak shape and asymmetry |
|---|---|---|---|---|---|
| Bisoprolol fumarate |
Phosphate buffer:methanol:acetonitrile (65:15:20) pH 3.0 adjusted with orthophosphoric acid | Phenomenex Luna C18 (250×4.5 mm, 5 μm) | 1.0 ml/min | 4.674 | Bifurgated peak |
| Metoprolol succinate | Phosphate buffer:methanol:acetonitrile (65:15:20) pH 3.0 adjusted with orthophosphoric acid | Phenomenex Luna C18 (250×4.5 mm, 5 μm) | 1.0 ml/min | 5.130 | Good and sharp peak |
Table 1: Chromatography trials for Internal Standard Selection
The optimized method was validated as per the recommendations of USP [10,11] and ICH [12,13] for the parameters like accuracy, linearity, precision, detection limit, quantitation limit and robustness. Recovery of LOR in plasma was evaluated by comparing the mean peak responses of at least six injections of each low, medium and HQC sample, prepared in plasma, to mean peak responses of non-spiked samples prepared in elution solvent and external spiked matrix extracted sample. Recovery of IS in plasma was evaluated by comparing the mean peak responses of at least six MQC samples, prepared in plasma, to mean peak responses of non-spiked samples prepared in elution solvent and external spiked matrix extracted sample, replicates of aqueous samples of LOR. The mean standard deviation and % coefficient of variation (CV) for the peak area ratio and for the retention time of analyte and IS were calculated. Specificity and selectivity was carried out using six plasma samples. Blank (without IS) and zero sample (with IS) were analysed. The linearity of the method was determined over calibration range of 500 to 3000 ng/ml (Table 2). Calibration standards were prepared by spiking known concentration of LOR working standard solution. A linearity curve containing six non-zero concentrations was analysed (Figure 1). Back-calculated the concentrations of each level and plot the graph of back-calculated concentration against drug area ratio. The slope, y-intercept and correlation coefficient curve were calculated by suitable linear regression analysis as stated in Table 3. Accuracy and precision was measured on the samples spiked with known amounts of the analyte. Accuracy and precision were determined by replicate analysis of six determinations of low, medium and HQC sample, which covers the calibration range. Precision is expressed as the % CV. The accuracy and precision were evaluated as within batch and between-batch [14].
| Parameter (units) | LOR |
|---|---|
| Linearity range (ng/l) | 500-3000 |
| Correlation coefficient | 0.998±0.00038 |
| Recovery of LOR (%) | 86.856 |
| Recovery of IS (%) | 90.169 |
| Precision (% RSD) | |
| Interday (n= 3) | 1.23 |
| Intraday (n= 3) | 1.14 |
| Robustness | Robust |
| Retention time (min) for LOR | 7.19±0.2 |
| Retention time (min) for MET | 5.14±0.2 |
Table 2: Summary of Validation and System Suitability Test Parameters
| Batch ID | Back calculated concentrations for the standards | Slope | Intercept | R2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Standard 1 | Standard 2 | Standard 3 | Standard 4 | Standard 5 | Standard 6 | ||||
| Conc. (ng/ml) | 500 | 1000 | 1500 | 2000 | 2500 | 3000 | - | - | - |
| C1 | 445.168 | 844.276 | 1425.26 | 2063.28 | 2729.46 | 2948 | 0.023 | 6.418 | 0.996 |
| C2 | 448.688 | 948.956 | 1401.517 | 2023.453 | 2658.78 | 2996.62 | 0.022 | 7.966 | 0.998 |
| C3 | 475.74 | 958.35 | 1411 | 2031.47 | 2635.51 | 2961.56 | 0.021 | 9.336 | 0.999 |
| Mean | 456.532 | 917.194 | 1412.592 | 2039.401 | 2674.58 | 2968.727 | 0.022 | 7.906 | 0.997 |
| SD | 16.72746 | 63.32328 | 11.95132 | 21.06473 | 48.9281 | 25.08978 | 0.001 | 1.459 | 0.0015 |
| % CV | 3.664029 | 6.904023 | 0.846056 | 1.032888 | 1.82937 | 0.845136 | 4.5454 | 18.4657 | 0.1532 |
| % Mean | 91.306 | 91.72 | 94.172 | 101.968 | 106.983 | 98.718 | - | - | - |
Table 3: Back Calculated Concentrations for the standard Calibration Graphs
Bench top stability was performed at MQC level. Three replicates of MQC were withdrawn from deep freezer and were kept at room temperature for 12 h. These samples were preferred as stability samples after 12 h, prepared fresh samples of MQC concentrations of LOR in three replicates. These samples were referred as fresh or comparison samples. The freeze and thaw stability of analyte was determined after three freeze thaw (FT) cycles. The three sets of MQC samples were stored at –70±5 and subjected to three FT cycles at interval of 24 h. After the completion of three cycles of 12 to 24 h, the samples were analysed. Stability of samples was compared against freshly prepared samples.
The stability of LOR and IS in the stock solution were determined at room temperature for 7 h. Stock solution stability was performed by analysing three replicates of aqueous solutions prepared from freshly weighed stock solution against three replicates of aqueous solution prepared from aliquots of analyte and IS stored at room temperature for 7 h. The stability of LOR and IS in the stock solution were determined at 2-8° after 5 d. Refrigerated stock solution stability was performed by analysing three replicates of aqueous solution prepared from freshly weighed stock solution against three replicates of aqueous solution prepared from aliquots of analyte and IS stored at 2-8° after 5 d (stability samples; Table 4).
| Stability conditions | % Accuracy | |
|---|---|---|
| Bench top stability at RT for 12 h | 100.6736±1.60 | |
| Freeze thaw stability previously frozen at –70±5° and thawed at room temperature over three cycles | 99.12±5.04 | |
| Stock solution stability | 99.52±3.541 | |
| Short term (at RT for 7 h) | LOR | |
| MET | 101.5±2.835 | |
| Long term (at 2-8° for 5 d) | LOR | 97.89±3.915 |
| MET | 102.6±4.806 | |
Table 4: Summary of Stability Studies for LOR And MET
Pharmacokinetic studies
The pharmacokinetic study was carried out in male Sprague Dawley rats. The six healthy animals were selected for the study. The animals were fasted overnight (~14 h) and had free access to water throughout the experimental period. LOR was administered by oral gavage at a dose of 10 mg/kg, as solution of drug in water. Blood samples (0.5 ml) were collected from the retro orbital plexus sinus at designated time points (0.25, 0.5, 1, 2, 3 and 4 h) into micro centrifuge tubes containing 100 μl of heparin. Plasma was harvested by centrifuging the blood using cold centrifuge compufuge at 3000 rpm for 10 min. Plasma (300 μl) samples were spiked with IS and processed same as standards as described above.
Results and Discussion
Sample preparation technique used for the study plays a significant role with respect to bionalytical samples [15-20]. It is essential to reduce the effect of the biological and buffer matrix. Sample preparation is applied to remove interfering compounds. As a bonus, analytes can be concentrated during the extraction processes. Sample preparation procedure is tedious and time consuming. However, the cleanliness of the samples affects the overall performance of the analysis. Different extraction techniques tried were protein precipitation, liquid-liquid extraction and SPE. For selection and optimization of particular extraction techniques various trials were taken as described below. Initially protein precipitation method was tried using acetonitrile, methanol and acetone as precipitating agents but it showed greater plasma interference, greater sample transfer and greater sample evaporation steps. Samples obtained were unclean, which can be harmful to life of analytical instrument in long run. So this technique was not preferred. Following it liquid-liquid extraction technique was tried in which interference due to plasma matrix was reduced. However for sample transfer and sample evaporation, tedious multiple extraction steps were involved, which produced less consistent results. Various extracting agents used for the study included chloroform, ethyl acetate and methyl tertbutyl ether (MTBE). Best recovery of about 70-75 % was obtained with MTBE. Finally SPE technique was tried in which interference due to plasma matrix was very less as compared to other techniques. In it, sample transfer and sample evaporation steps are not involved, leading to consistent results. In addition this technique required less biological material and less time. The C18 cartridges were used for the extraction procedure. Two brands of C18 cartridges were tried for the study. The brands of C18 cartridges used were Oasis of Waters and Orochem. Best recovery of 89 % was obtained by using Orochem brand C18 SPE cartridge.
The optimized SPE method parameters used for the study included Orochem SPE cartridge, sample pretreatment by 0.5 ml plasma sample+0.5 ml water (sample dilution, 1:2) conditioning with 0.5 ml methanol, equilibrating with 0.5 ml water, loading of 0.5 ml pre-treated sample, washing with 0.5 ml water, drying with nitrogen purging for 1-2 min and finally eluting with 0.5 ml methanol.
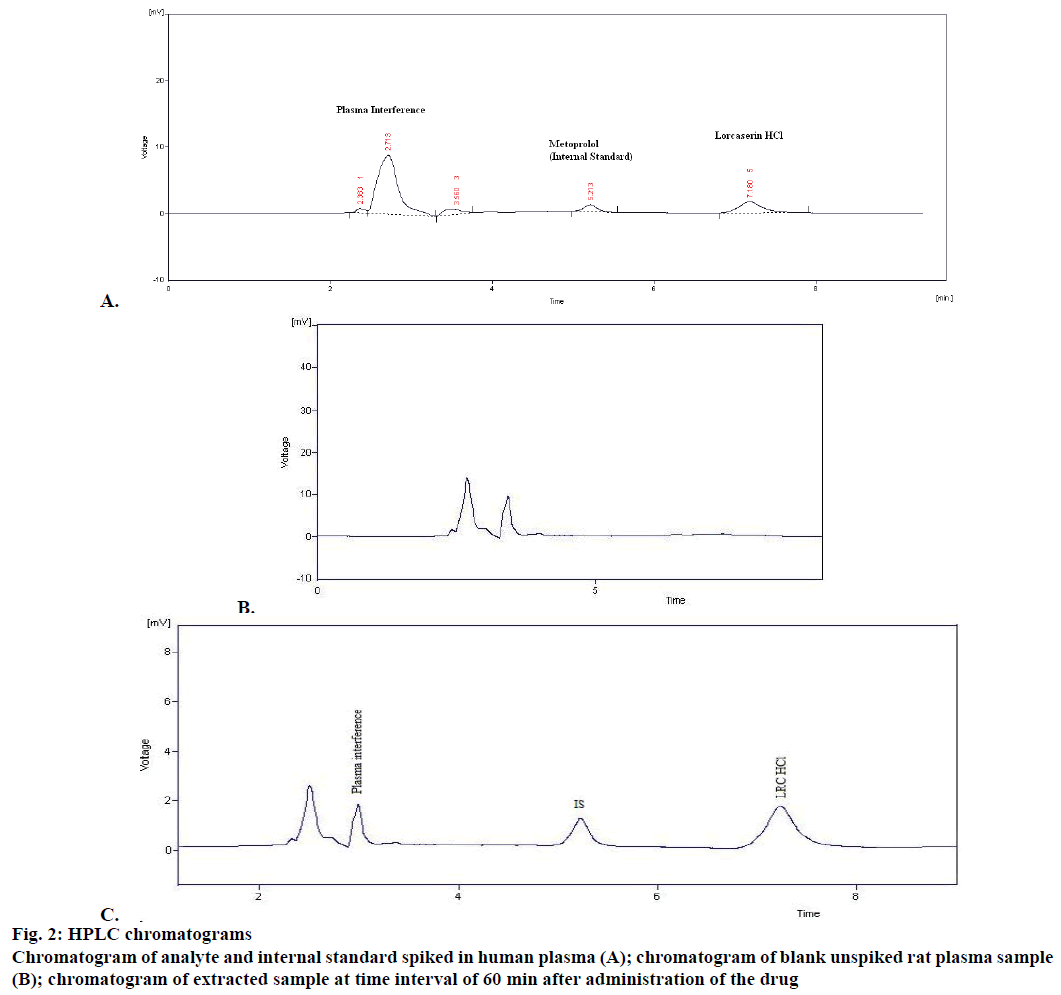
The extracted samples were retrieved from pre-labelled sample tubes stored in deep freezer at –20° and were pre-treated prior to extraction and then subjected to SPE. The extracted samples were subjected to HPLC. For development of analytical method for the estimation of LOR by RP HPLC, various chromatographic trials were taken. The various factors considered were flow rate, mobile phase composition, wavelength maxima and pH of mobile phase. The factors were varied on one factor at time basis [21-26]. In the HPLC method optimized on extracted samples, mobile phase consisted of phosphate buffer:methanol:acetonitrile (65:15:2, pH 3.0), at 1 ml/min flow rate, which gave two sharp, well-resolved peaks with minimum tailing factor for LOR and MET in human plasma as shown in Figure 2A. The retention times for LOR and MET were 7.19 and 5.14 min, respectively. UV overlain spectra of both LOR and MET showed that both drugs absorbed appreciably at 222 nm, so this wavelength was selected as the detection wavelength. The calibration curve for LOR was found to be linear over the range of 500- 3000 ng/ml (Figure 1). The data of regression analysis of the calibration curves is shown in Table 3. The proposed method was successfully applied to the determination of LOR in biological matrix. The developed method was also found to be specific, since it was able to separate drug in the biological matrix.
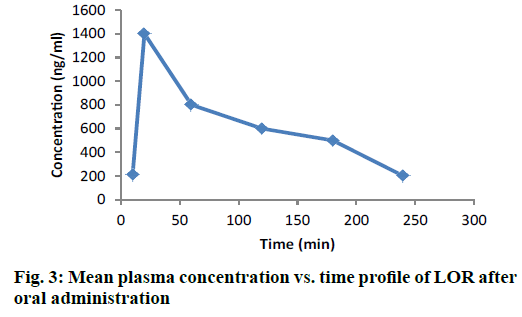
The chromatogram presented in Figure 2B is of blank (unspiked) rat plasma sample extracted using SPE extraction procedure as optimized below. The chromatogram depicted in Figure 2C showed some small peaks, well separated from the drug peak, but in absence of standard metabolite or any definite chromatographic pattern, it was difficult to identify the metabolite peak. All these peaks did not interfere with the drug analysis. Figure 3 depicted the changes in drug concentrations at various designated time points (0, 30, 60, 120, 180, 240, 300 min). The pharmacokinetic parameters were calculated with a non-compartmental model using Thermo Kinetica PK/PD analysis software (version 5.0 Thermo Fisher Scientific). The peak plasma concentration (Cmax) and the corresponding time (Tmax) were directly obtained from the raw data. The other pharmacokinetic parameters were obtained using non compartment model. AUCtotal was calculated using mixed log linear model. The pharmacokinetic data is presented in Table 5 [27-29].
| Parameters | Observed value | Reported value [31] |
|---|---|---|
| Cmax (ng/ml) | 837.2 | 789 |
| Tmax (h) | 0.5 | 0.25 |
| AUCtotal (µg/ml.h) | 2.964 | Dose dependent |
| T1/2 (h) | 1.539 | Dose dependent |
Table 5: Pharmacokinetic Parameters for Bioanalytical Method
From the results and discussions, it could be concluded that the method developed for the analysis of LOR in rat plasma is specific, accurate, precise and reproducible. The use of this method could enable the characterization of LOR and its pharmacokinetics after single oral dose without any interference from the metabolite. According to pharmacology and toxicology review by CDER, based on plasma profiles, overall pattern of metabolism in humans is most closely approximated the metabolite pattern seen in rats [30,31]. The assay can therefore be easily extended to quantitate LOR in plasma for routine monitoring of plasma levels of LOR in laboratories.
Acknowledgements
The authors thank Torrent pharmaceuticals, Ahmedabad for providing MET succinate as gift sample for this research work. The authors also thank Institutional Animal Ethics Committee (IAEC) for giving approval for pharmacokinetic study.
Conflict of interest
The authors declare that there is no conflict of interests.
Financial support and sponsorship
Nil.
References
- Thomsen WJ, Grottick AJ, Menzaghis F, Reyes-Saldana H, Espitia S, Yuskin D, et al. Lorcaserin, a novel selective human 5-hydroxytryptamine 2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 2008; 325:577-87.
- https://www.tib.eu/en/search/id/BLCP%3ACN073458973/A-Chiral-LC-MS-MS-Method-for-the-Separation-and/.
- Sharma BK. Instrumental Methods of Chemical Analysis. Meerut: Goel Publication House; 2005. p.133-61.
- Beckett AH, Stenlake JB. UV-visible spectrophotometry-Practical Pharmaceutical Chemistry. 4th ed. Delhi: CBS Publishers; 2001. p. 285-97.
- Sharma YR. Ultraviolet and Visible Spectroscopy in Elementary Organic Spectroscopy. 1st ed. New Delhi: S Chand and Company Ltd.; 2004. p. 9-60.
- Jeffrey G.H, Basett J, Mendham J, and Denny R.C. Introduction-Vogel’s Textbook of Quantitative Chemical Analysis. 5th ed. Europe: Longman Publication; 1997. p. 3-8.
- http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf.
- Lalit VS, Bhagwat NP, Sharad VU, Pradeepkumar VW, Laxman HS. Bioanalytical method validation and its pharmaceutical application- A Review. Pharm Anal Acta 2014;5:1-7.
- https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Multidisciplinary/M10/ICH_M10_Concept_paper_final_7Oct2016.pdf.
- http://www.who.int/medicines/areas/quality_safety/quality_assurance/QualityAssurancePharmVol2.pdf.
- https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf.
- United States Pharmacopoeia/National Formulary, 25th ed. Rockville, Maryland: Pharmacopeial Convention, 2007. p. 1475.
- United States Pharmacopoeia/National Formulary, 24th ed. Rockville, MD: Pharmacopeial Convention, 2000. p. 2149-2152.
- Sandy L. HPLC by Open Learning. London: John Wiley and Sons; 1991.
- Whitmire M, Ammerman J, DelisioP, Killmer J, Kyle D. LC-MS/MS bioanalysis method development, validation and sample analysis: points to consider when conducting nonclinical and clinical studies in accordance with current regulatory guidances. J Anal Bioanal Tech 2011;6:1-10.
- Savoie N, Booth BP, Bradley T. White Paper- the 2nd Calibration and Validation Group workshop on recent issues in good laboratory practice bio analysis. Bioanalysis 2009;5:19-30.
- Sumit C, Saahil A, Tanvi S. Bioanalytical method development and validation of Ibuprofen using RP-HPLC. Am J Pharm Tech Res 2012;6:2249-3387.
- Sunil Kumar RT, Chitra K. Bio analytical method development by HPLC-a review. Int J Med Chem Anal 2012;1:1-8.
- Kim H, Chang KY, Park CH, Jang MS, Lee JA, Lee HJ, et al. Determination of glimepiride in human plasma by LC-MS-MS and comparison of sample preparation methods for glimepiride. Chromatographia 2004;60:93-98.
- Shah VP, Midha KK, Dighe S, McGliveray IJ, Skelly JP, Jacobi TA, et al. Analytical methods validation-bioavailability, bioequivalence and pharmacokinetic studies. J Pharm Sci 1992;81:309-12.
- Lough WJ, Wainer IW. HPLC Fundamental Principles and Practices. Detroit, Michigan: Blackie Academic and Professional; 1991.p. 52-67.
- Meyer Veronica R. Practical High Performance Liquid Chromatography. 2nd ed. London: John Wiley and Sons; 1993. p. 26-258.
- Sethi PD. High Performance Liquid Chromatography: Quantitative Analytical Pharmaceutical Formulations.1st ed. New Delhi: CBS publishers; 2001. p. 3-40.
- Raj K, Alvia A, Rani PM, Hima S, Kiran KR. Method development and validation of tradozone by RP-HPLC. Scholars Research Library 2016;6:241-50.
- Shah JM. Development & Validation of HPLC Method for Analysis of Some Antihypertensive Agents in their Pharmaceutical Dosage Forms. J Pharm Sci Res 2010;8:459-64.
- Shalini P, Sarvesh P, Kona SS, Yogendra S, Varun J. Development and validation of HPLC method for analysis of some antihypertensive agents in their pharmaceutical dosage forms. J Pharm Sci Res 2010;2:459-64.
- Samala S, Tatipamula SR, Veeresham C. Determination of glimepiride in rat serum by RP-HPLC method. Am J Anal Chem 2011; 2: 152-157.
- Thammera RK, Shitut NR, Pasikanti KK, Menon C, Vinu A, Venkata V, et al. Determination of rosuvastatin in rat plasma by HPLC-validation and its application to pharmacokinetic studies. Biomed Chromatogr 2006;20:881-87.
- Talari R , Varshosaz J, Mostafavi A, Nokhodchi A. Development and validation of a novel RP-HPLC method for pharmacokinetic studies of gliclazide in rat. Farmacia 2011;59:388-95.
- http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=5948D39B8E21621FAE417DE71279EB1F?doi=10.1.1.174.2423&rep=rep1&type=pdf.
- https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.