- *Corresponding Author:
- Seema Pattewar
Department of Pharmacy, Banasthali University, Vanasthali-304 022, India
E-mail: tsdpatil99@gmail.com
| Date of Submission | 24 April 2017 |
| Date of Revision | 22 July 2017 |
| Date of Acceptance | 23 February 2018 |
| Indian J Pharm Sci 2018;80(2):350-358 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of present study was to formulate and evaluate self-microemulsifying drug delivery system containing piroxicam and to use the ability of porous magnesium alumina metasilicate as a solid carrier for self-micro emulsifying drug delivery system. It was developed to resolve the problems of piroxicam such as low water solubility, low bioavailability and gastrointestinal irritation. Self-micro emulsifying drug delivery system containing varying proportions of Capmul MCM, Cremophor EL and Transcutol-P were prepared and optimized using response surface methodology of Design-Expert® software version 10 stat-ease. Liquid self-microemulsifying drug delivery systems were subjected to in vitro evaluation, including self-emulsification efficiency study, droplet size, zeta potential measurement and in vitro drug release studies. Solid self-micro emulsifying drug delivery system was prepared by adding liquid self-micro emulsifying drug delivery system with Neusilin US2 and filled in hard gelatin capsule. The optimized formulation consists of 28.26 % of Capmul MCM, 44.16 % of Cremophor EL and 27.58 % of Transcutol-P. The results showed that the drug release profile of piroxicam from the self-microemulsifying drug delivery formulations was higher than the pure piroxicam powder. The release of piroxicam was rapid and complete. Solid self-micro emulsifying drug delivery systems after filled in hard gelatin capsule, predicted to be a promising technique to deliver a liquid formulation in solid dosage form.
Keywords
Bioavailability; SMEDDS, piroxicam, solubility, zeta potential
Though a drug can be administered by several ways, the oral route of administration is the most obvious route of administration preferred by almost every patient. The oral route is the most economical and simplest route of administration that does not require use of any device and therefore self-medication is possible. About 40 % of new drugs have poor solubility in water and ultimately leads to poor oral bioavailability [1,2]. Since dissolution is the rate limiting step, formulation design can be a proper approach to enhance absorption and ultimately bioavailability of these drugs [3,4]. Many formulation approaches like complexation [5], solid dispersions [6] and nanosuspensions [7] are employed to solve this problem, but these approaches have some limitations like less stability, laborious and expensive methods.
The self-microemulsifying drug delivery system (SMEDDS) is known as one of the most useful approach to overcome problems of drug with low solubility and poor oral absorption. SMEDDS comprises isotropic mixtures of natural or synthetic oils, surfactants and co-surfactants. These systems spontaneously emulsify when exposed to gastrointestinal fluids to form oil in water microemulsion with nanometric droplet size. SMEDDS enhances in vitro dissolution and improves the in vivo absorption of lipophilic drugs [8,9]. SMEDDS is system with droplet size ranging from 20- 200 nm [10,11]. The solubilisation capacity for was to investigate SMEDDS for piroxicam. In the present study, a stable SMEDDS formulation for different drugs in SMEDDS is different due to various intermolecular forces between drug and SMEDDS components [12]. The small-sized droplets of SMEDDS offer an increase in dissolution rate and bioavailability of drugs [13,14]. Other important advantages of SMEDDS include ease of manufacturing and scale-up [15].
But, SMEDDS possesses several shortcomings, in manufacturing and clinical practices such as potential risk of drug precipitation and migration of some of SMEDDS components into the capsule shells. To overcome these drawbacks, solid SMEDDS (S-SMEDDS) was investigated as an alternative. S-SMEDDS was developed by incorporating SMEDDS into inert solid excipients. Solidification methods were adsorption to solid carriers, spray drying, freeze drying, rotary evaporation, melt extrusion-spheronization and melt granulation. Among these methods, adsorption to solid carriers is a simple and easy technique, offering a stable free-flowing S-SMEDDS [16]. Low density porous carriers with large surface area i.e. Neusilin UFL2, Neusilin US2 were used in order to improve dissolution rate of drugs such as carvedilol and indomethacin [17].
According to Biopharmaceutical Classification System, piroxicam is a class II drug with low solubility and high permeability. Piroxicam is a non-steroidal antiinflammatory drug with a long half-life. Piroxicam is used to treat pain associated with rheumatoid arthritis, osteoarthritis, gout and in different musculoskeletal disorders. Piroxicam blocks cyclooxygenase and ultimately inflammation, pain and fever are reduced [18].
The aim of this study piroxicam was developed and evaluated. The SMEDDS consisting of Capmul MCM, Cremophor EL, Transcutol P was characterized for the particle size, self-emulsifying ability and zeta potential. After development of SMEDDS it was converted into S-SMEDDS by adsorption technique using carriers like Neusilin US2 and filled in hard gelatin capsule.
Materials and Methods
Piroxicam was obtained as a gift sample from Apex Healthcare Limited, Ankleshwar, Gujarat, India. Capmul MCM (medium chain mono and diglyceride), Captex-300 (glyceryl tricaprylate/tricaprate), were obtained as gift samples from Intas Pharmaceuticals Limited, Ahmedabad, Gujarat. Cremophor-EL (macro glycerol ricinoleate), Transcutol P (diethylene glycol monoethyl ether), Labrafil were gift samples from Dr. Reddy's Laboratories Ltd., Hyderabad, India. Cremophor RH-40 (macrogolglycerol hydroxystearate) was a gift from Trans Chem Corporation, Bhiwandi, India. Neusilin US2 (magnesium aluminometasilicate) was obtained as a gift sample from Gangwal Chemicals Pvt. Ltd., Mumbai, India. Tween 80, Tween 20, oleic acid, Brij-35, sunflower oil, sesame oil, polyethylene glycol 400, propylene glycol were purchased from S. D. Fine-Chem Limited, Mumbai, India.
Drug-excipient compatibility studies
The Fourier-transform infrared spectroscopy (FTIR) spectra of drug, excipients, SMEDDS and selfmicroemulsifying mouth dissolving film formulation were recorded. Briefly, solid sample was compressed along with about 100 mg dried potassium bromide into a disc. For liquid sample, few drops of the sample were dripped onto NaCl or KBr aperture plate and sandwiched it under another aperture plate, such that no gas bubbles were trapped. The sample allowed formation of a thin liquid membrane between the two aperture plates. Thereafter, sample was scanned for absorbance over the range from 4000 to 400 (cm-1) wave numbers. The obtained spectrum was then compared with standard group frequencies of piroxicam.
Solubility study of piroxicam
Piroxicam solubility was determined in oil, surfactant, and co-surfactant by shake-flask method. Oleic acid, Capmul MCM, Labrafil, Captex, sunflower oil, sesame oil were used as oils for solubility study. It was carried out by solubilizing an excess quantity of drug in 2 ml of the vehicle. Mixture was then vortexed and kept for 72 h at 25° in an orbital shaking incubator (Dolphin) for solubilisation. After equilibrium, it was centrifuged at 10 000 rpm for 15 min. Supernatant liquid was diluted with 0.1 M methanolic HCl and drug was quantified at 334 nm [19] by using UV spectrophotometer by placing a blank. The blank was formulated by mixing respective oil, surfactant or co-surfactant in 0.1 M HCl in methanol with same dilution as for the sample [20].
Surfactant selection
Piroxicam solubility was carried out in Tween 80, Cremophor EL, Tween 20, Brij 35 and Cremophor RH-40 as per above mentioned procedure. Thereafter the surfactants were selected by their emulsification study, water uptake capacity and percent transmittance of the selected oil phase. For water-uptake study, selected oil and various surfactants were mixed in ratio of 1:4 and vortexed to form homogenous mixture using a cyclomixer (model CM 101, Remi Instruments, Ltd., India). Oil-surfactant mixture (1 ml) was placed in test tubes and water was added dropwise till the system became turbid and the quantity of water uptake was noted. For emulsification study and percent transmittance study, surfactant of 200 μl was mixed with 200 μl of oil, which was selected and then 50 μl of this was diluted to 50 ml with water. After 2 h transmittance was taken at 650 nm against water as the blank [21].
Co-surfactant selection
Piroxicam solubility was then carried out in different co-surfactant such as propylene glycol, Transcutol P, PEG-400 by following the above-mentioned procedure. For this study surfactant 40 μl was mixed with cosurfactant 20 μl (S:coS mix 2:1) and oil phase 60 μl was added to mixture (oil:Smix, 1:1). Then mixture was heated to 45 to 50° in water bath for proper mixing, from this mixture 50 μl was diluted up to 50 ml doubledistilled water. After 2 h, transmittance was taken at 650 nm [22].
Construction of pseudo-ternary phase diagrams
It was constructed by titration of homogeneous liquid mixture of oil, surfactant and co-surfactant with water phase at 37° [23]. The ratio of surfactant to co-surfactant was taken as 1:1, 2:1. Oil and Smix were mixed properly in different weight ratios (1:9 to 9:1) [24]. Then each mixture titrated with distilled water until they exhibited turbidity. Only transparent mixtures were considered as microemulsions. Phase diagram plot was constructed using CHEMIX school software version 3.0 developed by Arne Standnes, Lakeland, USA.
Formulation of liquid SMEDDS (L-SMEDDS)
Different SMEDDS formulations were formulated on phase diagram plot. Oil (Capmul MCM), surfactant (Cremophor EL) and co-surfactant (Transcutol P) were selected for L-SMEDDS. The L-SMEDDS was prepared as reported in literature [25]. Piroxicam (10 mg) was transferred into a screw-capped glass vial containing selected oil, surfactant and co-surfactant mixture (S-Cos mix in 2:1 ratio) and stirred with vortex to obtain homogenous solution. The prepared SMEDDS was stored in room temperature in sealed transparent vials until used [26].
Experimental design
Optimization of the piroxicam SMEDDS were done using 2 level factorial design. From the preliminary study, oil, surfactant and co-surfactant were selected for L-SMEDDS formulation. Quantities of Capmul MCM (X1) and Cremophor EL(X2) were selected as the two factors for optimization. Two levels for each factor were used to construct experimental design. Levels for Capmul MCM (25.88 and 30.88 %) and Cremophor EL (38.82 and 52.23 %) were selected from preliminary study. Drug content (Y1) and percent transmittance (Y2) was selected as a desire to response for optimization. Four experiments were planned as per 22 factorial designs. Response surface diagram was constructed. Formulation was optimized by response surface diagram obtained from Design-Expert® software version 10- Stat-Ease.
Characterization of L-SMEDDS
Precipitation was evaluated visually after 24 h. L-SMEDDS were categorized after 24 h as clear or turbid and stable or unstable. Those transparent formulations exhibiting no precipitation were used for further study [27]. Self-emulsification efficiency was carried out using USP dissolution apparatus II [28]. Each formulation (1 g) was mixed with distilled water (500 ml) maintained at 37° with paddle speed 50 rpm. The self-emulsification performance of each SMEDDS was observed visually using grading systems [29]. Formulations those passed self-emulsification efficiency test were selected for further evaluation. The SMEDDS was evaluated by thermodynamic stability study tests viz. freeze thaw cycles and centrifugation [30]. Three cycles of formulations subjected to the freezing at –4° for 24 h in deep freeze and followed by thawing at 40° for 24 h in hot air oven and visually inspected for phase separation. The SMEDDS was centrifuged at 5000 rpm for 30 min in centrifuge (Remi instruments, India). Piroxicam SMEDDS were diluted with water (1:100) and the emulsion was observed visually for precipitation. Percent transmittance was measured at 650 nm (FTIR-8400S, Shimadzu, Japan). Globule size and polydispersity index (PDI) of reconstituted piroxicam SMEDDS was studied using Malvern Zetaseizer. (Nano ZS, Malvern Instruments, United Kingdom). The zeta potential was determined by using Malvern Zetasizer. The samples were diluted with a ratio 1:100 (V/V) with distilled water. The percent drug content of formulations was determined from the calibration curve of piroxicam in 0.1 M methanolic HCl and assay of drug was done by UV/ Vis spectrophotometer at wavelength 334 nm. The viscosity of optimized formulation was evaluated by Brookfield Viscometer (DV-E, Brookfield Engineering Laboratories Inc., Middleboro, MA).
S-SMEDDS of piroxicam were prepared by adsorbing technique by using solid adsorbent. Piroxicam-loaded SMEDDS were added drop-wise over highly porous Neusilin US2 (magnesium aluminometasilicate) in ratio 1:1 by weight. Mixture was mixed properly after each addition. The granular mass obtained was passed through sieve with mesh no. 120 to get free flowing powder. For one capsule 160 mg of Neusilin US2 was mixed with 160 mg of L-SMEDDS and filled in hard gelatin capsule size ‘0’ [21]. S-SMEDDS formulations were evaluated for angle of repose, Carr’s compressibility index and Hausner’s ratio [31-33].
In vitro dissolution studies
Drug releases of formulation were studied in 900 ml of 0.1 N HCl using USP dissolution II (paddle type) apparatus at 50 rpm and 37±0.5°. A quantity of S-SMEDDS, L-SMEDDS, piroxicam powder equivalent to 10 mg of piroxicam were filled in size “0” hard gelatin capsule and taken into vessel containing 900 ml of dissolution media. Five milliliter aliquots were taken out at time intervals such as 5, 10, 15, 25, 30, 35, 40, 45, 60, 90 and 120 min from medium and replace with same 0.1 N HCl solutions and were analysed at the wavelength 335 nm.
Stability studies
Stability study was performed as per ICH Q1A (R2) stability guidelines [31]. For that purpose accurately weighed S-SMEDDS equivalent to one dose was filled capsule size ‘0’. Samples were placed at 30±2°/75± 5 % RH. The samples were analysed for percent drug content and percent drug released for 3 mo.
Results and Discussion
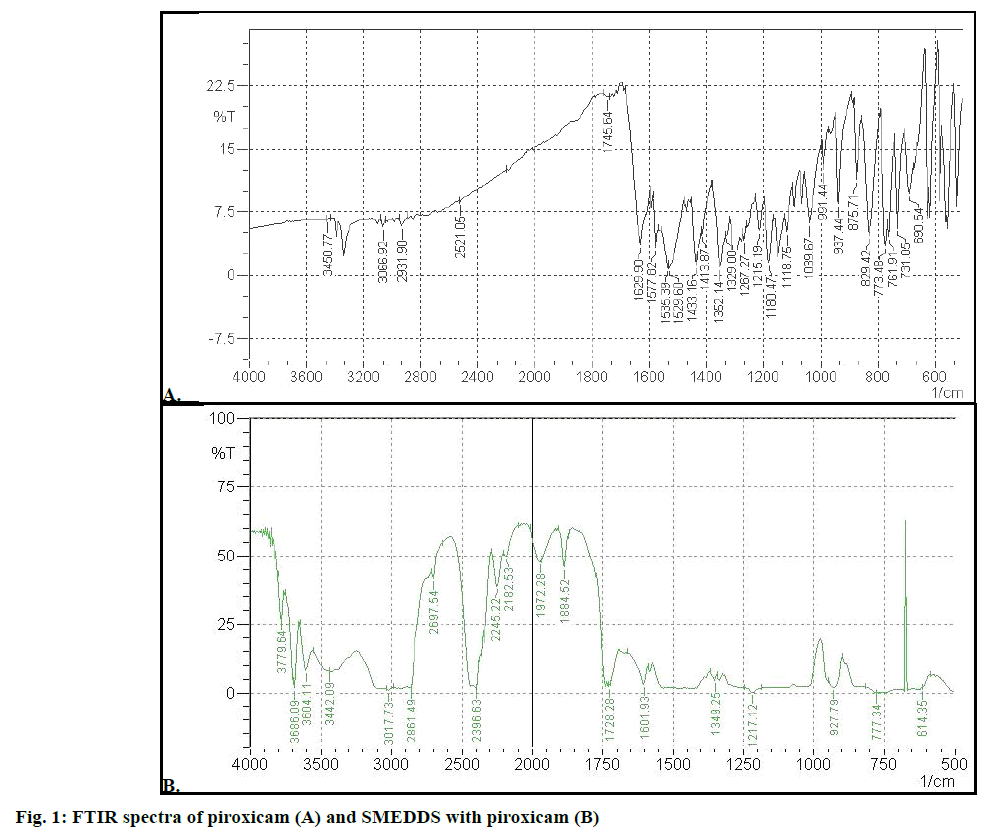
Figure 1A showed FTIR spectra of piroxicam. It has been reported that piroxicam has two inter convertible crystalline forms, namely the needle and cubic forms. The IR 1629.90 cm–1 was assigned to the stretching of amide carbonyl groups of cubic form, suggesting that the cubic form of piroxicam was used. Main characteristic peak of piroxicam were 3338 cm–1 for secondary amine N-H stretching, 3450 cm–1 for O-H stretch and 1180.47cm–1 for S=O asymmetric stretch. SMEDDS formulation (Figure 1B) gave peaks at 1728.28 cm–1 for C=O amide carbonyl, 3442.09 cm–1 for N-H stretch, 3604.11 for O-H stretch and 1217.12 for S=O asymmetric stretch. The N-H group of amide in piroxicam formed hydrogen bond with carboxyl group of SMEDDS excipients (soluble and dilute form for piroxicam) and this reduced electron density on nitrogen. This lead to shifting of C=O stretch of amide carbonyl. The IR spectra of formulation (SMEDDS with drug) indicates absence of remarkable changes in the location of the characteristic infrared absorption bands of the drug in formulation suggesting that there is no interaction between piroxicam and excipients.
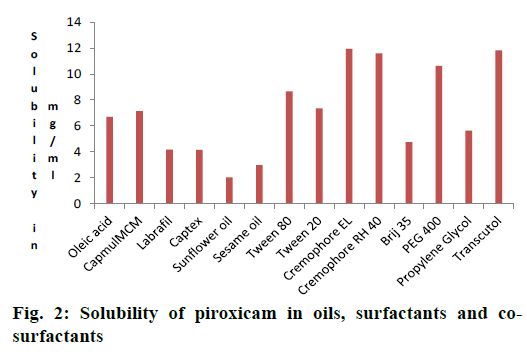
Results of solubility of piroxicam in various oils were shown in Figure 2. It was observed that the Capmul MCM possessed higher ester content, so drug showed higher solubility in Capmul MCM. Hence Capmul MCM was selected as oil phase.
Non-ionic surfactants are believed to be safer than the ionic surfactants [34]. They are expected produce reversible change in intestinal mucosal permeability [35]. Those having HLB 4.3 to 16 were screened. The final surfactant was selected by their emulsifying ability and percent transmittance. Cremophor RH-40 (94.87 %) and Cremophor EL (99.22 %) showed better percent transmittance, indicating good emulsification ability as showed in Table 1. Piroxicam showed better solubility in these surfactants and they were used for further study (Figure 2).
| Oil | Surfactant | Surfactant+co-surfactant | Water uptake capacity (ml) | Emulsification study | Percent transmittance | Remark |
|---|---|---|---|---|---|---|
| Capmul MCM | CR-EL | - | 0.75 | A | 99.22 | Pass |
| Capmul MCM | CR-RH 40 | - | 0.35 | B | 94.87 | Fair |
| Capmul MCM | - | CR EL+PEG 400 | 0.6 | B | 94.12 | Fair |
| Capmul MCM | - | CR RH 40+PEG 400 | 0.3 | C | 84.33 | Rejected |
| Capmul MCM | - | CREL+Transcutol | 0.8 | A | 98.80 | Pass |
| Capmul MCM | - | CR RH 40+Transcutol | 0.3 | B | 93.23 | Fair |
*Where, CR EL: Cremophor EL, PEG: Propylene glycol. A. Clear, rapidly forming micro emulsion in less than 1 min. B. White, rapidly forming emulsion in less than 2 min. C. White emulsion forming in more than 3 min
Table 1: Selection of surfactant and co surfactant
Co-surfactants were screened for improving the emulsification ability of selected surfactant. The percent transmittance and emulsification time were carried out using PEG 400 and Transcutol P as cosurfactants in combination with surfactant and oil are given in Table 1. Cremophor-EL showed very good ability to emulsify as compare to Cremophor RH-40. Among all the co-surfactants screened, Transcutol P showed highest percent transmittance (98.80 %) when used along with Cremophor EL. Cremophor EL having higher HLB i.e. 15. High HLB value surfactants lead to more rapid dispersion and finer emulsion droplet size on addition to aqueous phase [36]. Piroxicam was more soluble in Capmul MCM (7.1372 mg/ml), Cremophor EL [36,37] (11.94 mg/ml) and Transcutol P (11.81 mg/ml).
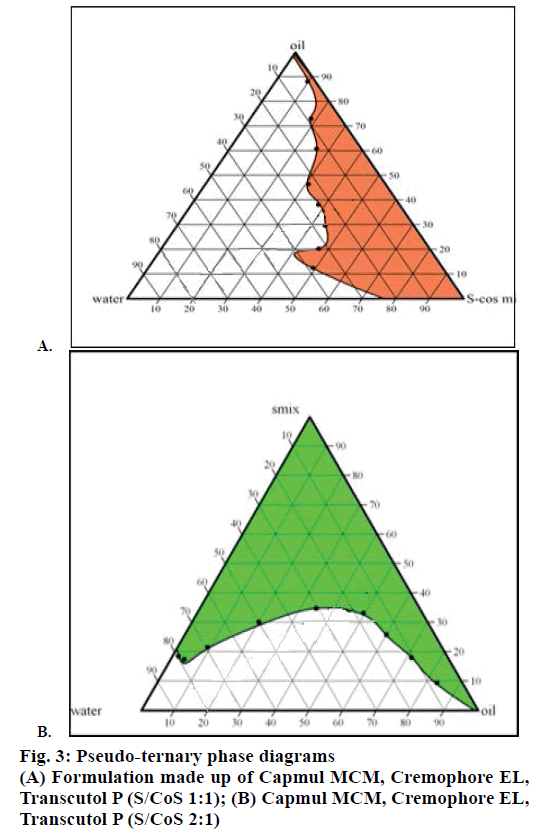
Solubility study was used to identify the area for stable and clear formulations in 1:1, 2:1 S-Cos mix ratio. The quantities of three components i.e. oil, surfactant and co-surfactant were selected from pseudo-ternary phase diagrams (Figure 3). It was observed that micro-emulsion formation area was increased with an increase in S-Cos mix and was highest at S-Cos mix 2:1. So Smix in 2:1 ratio was selected for formulation of SMEDDS.
Different batches of the SMEDDS were prepared by using pseudo-ternary phase diagrams. Formulation batches were prepared containing 10 mg of piroxicam. Optimization of the SMEDDS was done using 2 level factorial designs (Table 2). From the preliminary study Capmul MCM was selected as oil, Cremophor EL as a surfactant and Transcutol P as a co-surfactant. Quantities of Capmul MCM (X1), Cremophor EL (X2) were selected as the two factors for optimization. Full factorial statistical experimental design requires 4 runs. Y1 and Y2 ranges were 97 to 99.90 % and 94 to 99.88 %, respectively. Responses observed in 4 prepared formulation were simultaneously fitted to models using Design-Expert® 10. It was observed that best fitted model was linear. For linear model standard deviation (SD) was 0.65, R2 value was 0.9102, coefficient of variation (CV %) was 0.66, mean 98.13 and PRESS value was 6.76. These values were significant for linear model. A possitive value represents an effect that favours the optimization, while a negative value indicate an inverse relationship between the factor and the response. Y1 = 110.82–0.25 X1–0.12304 X2; Y2 = 118.21–0.479 X1–0.195 X2.
| Runs | Batch | Quantity of transcutol (%) | Independent variables | Dependent variables | ||||
|---|---|---|---|---|---|---|---|---|
| Observed value | Predicted value | |||||||
| X1 | X2 | Y1 | Y2 | Y1 | Y2 | |||
| 1 | F1 | 30.3 | 30.88 | 38.82 | 98 | 94.22 | 98.33 | 95.85 |
| 2 | F2 | 35.30 | 25.88 | 38.82 | 99.9 | 99.88 | 99.58 | 95.25 |
| 3 | F3 | 21.89 | 25.88 | 52.23 | 97.6 | 94 | 97.92 | 95.63 |
| 4 | F4 | 16.89 | 30.88 | 52.23 | 97 | 94.87 | 96.67 | 93.24 |
Where, X1: Quantity of capmul MCM %, X2: Quantity of cremophore EL %, Y1: Drug content % , Y2: % transmittance. Each formulation contains 10 mg of piroxicam
Table 2: Full factorial design experiment with result for formulation of SMEDDS
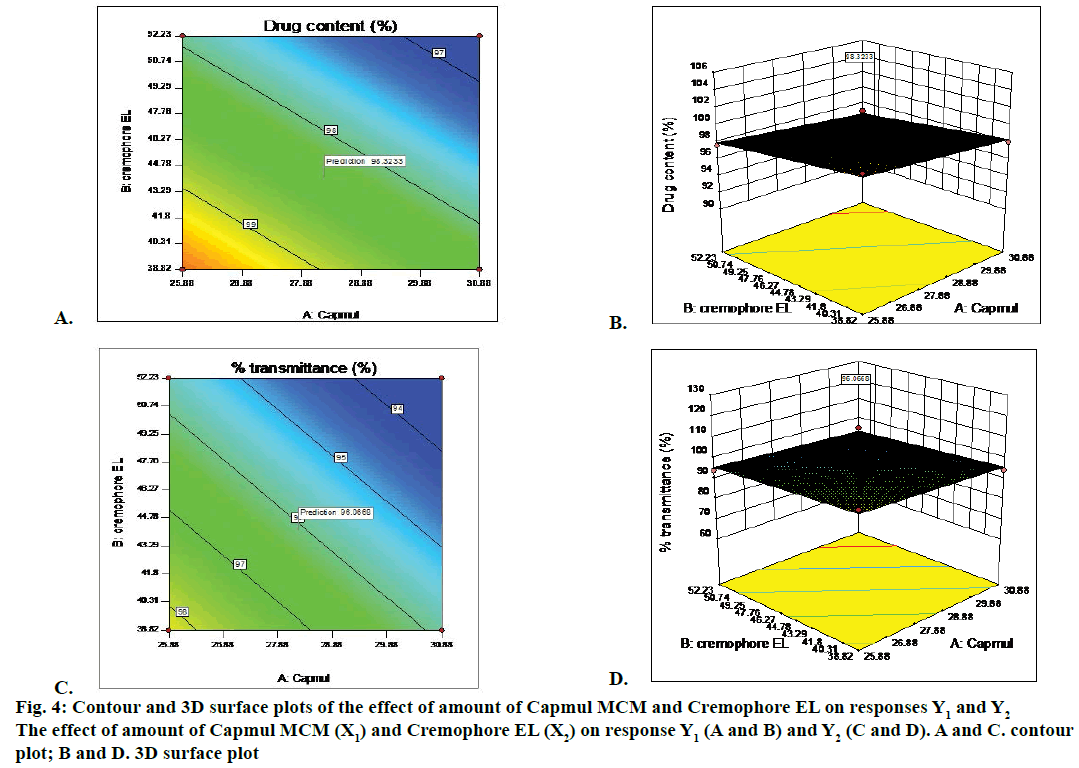
Two dimension contour plots and 3D surface plots were prepared for all responses as showed in Figure 4 for responses Y1 and Y2, respectively. These plots were known to study the interaction effect of the factor on the response properties. The percent relative error for batches was in between –0.3413 to +1.7. Experimental values and predicted values showed good agreement with each other. This confirmed the validity of the equations.
Thus, Chemix and response methodology was then used to predict the levels of the factor, amount of Capmul MCM (X1) and amount of Cremophor EL (X2) required to obtain an optimized formulation. A new formulation was prepared containing Capmul MCM 28.26 %, Cremophore EL 44.16 % and Transcutol P 27.58 % according to solution given by Design- Expert® 10. Each batch contained 10 mg of piroxicam in 150 mg of SMEDDS. The optimized formulation of piroxicam-loaded SMEDDS was transparent and stable. For self-emulsification efficiency study, distilled water was used to check the dispersion and self-emulsification. The optimized batch showed ‘A’ grade. The optimized batch passed freeze thaw cycles and centrifugation test. Phase separation, cracking and creaming was not observed during this study. Hence the batch was proceeds for further study.
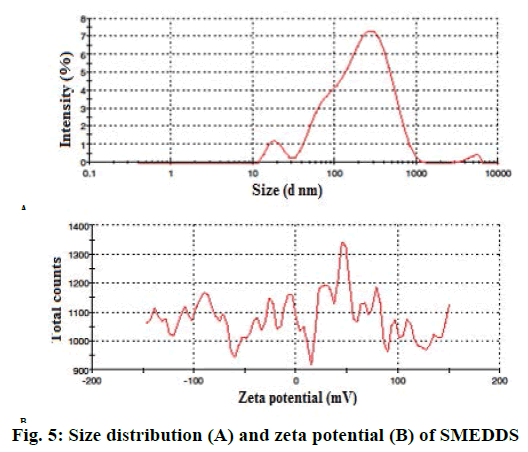
Percent transmittance was 96.06 % for optimized batch as per Table 3.The droplet size of optimized batch was found to be 138.8 nm as showed in Figure 5A, which could be due to the low concentration of oil. PDI value was found as 0.519 for SMEDDS. Zeta potential of optimized batch was showed in Figure 5B. Zeta potential governs the degree of repulsion between adjacent or similarly charged and dispersed droplet, it shows the practical application in the stability. The negative charge on SMEDDS was due to esters and fatty acids in Capmul MCM, which was present in formulations as oil phase. The negative zeta potential values of the formulations confirmed SMEDDS stability. Drug content for optimized formulations were determined using UV/Vis spectrophotometric analysis employing a standard calibration curve. The optimized batch showed 98.32±0.008 % of drug as per Table 3.
| Parameter | Optimized batch |
|---|---|
| Precipitation assessment | No precipitation |
| Assessment of self-emulsification study: grade | “A” Grade |
| Drug content | 98.32±0.008165 % |
| % Transmittance | 96.06 % |
| Emulsification time | 11 s |
Table 3: Characterization of liquid SMEDDS
Viscosity of the L-SMEDDS formulations was found as 0.8872 cps. S-SMEDDS prepared by adsorption of liquid SMEDDS using Neusilin US2. A 1:1 proportion of Neusilin US2:L-SMEDDS was sufficient to obtain a free-flowing powder. S-SMEDDS prepared with 1:1 (adsorbent:L-SMEDDS of piroxicam) showed good flow properties with Carr’s index as 13 i.e. between 12 and 15, Hausner’s ratio as 1.21 within 1.2-1.25 and angle of repose 25.9 between 25-30.
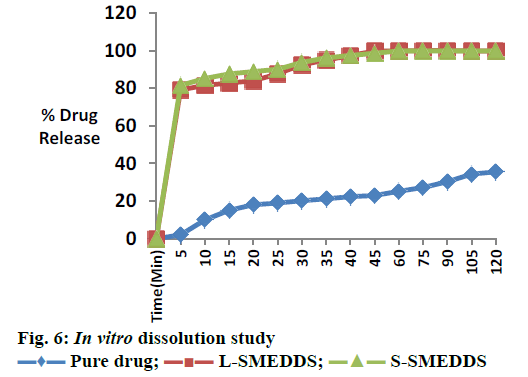
For L-SMEDDS, in vitro dissolution studies showed 80.65 % drug release in 5 min, 99.72 % within 45 min. For S-SMEDDS batch, in vitro dissolution studies showed 81.48 % in 5 min, 98.51 % in 45 min. Plain drug showed very less release 35.51 % after 120 min in 0.1 N HCL as showed in Figure 6. A stability study was performed and there was insignificant difference in percent drug content and percent drug release after 3 mo. Initially slight reduction in drug release was observed, however 98.51 % drug release was found in 45 min after 3 mo (Table 4). Optimization of formulation was performed using 2 factors, 2 level designs. The quantity of Capmul MCM and Cremophor EL showed significant effect on the percent drug content and percent transmittance of micro emulsion. Response methodology was then used to predict the levels of X1 and X2 required to obtain an optimized formulation. A new formulation was prepared with Capmul MCM 28.26 %, Cremophore EL 44.16 % and Transcutol P 27.58 % according to solution obtained from Design Expert 10. S-SMEDDS were prepared with adsorbents, Neusilin US2. Formulations were evaluated for in vitro drug release studies. It showed improvement in drug release rate compared to pure drug. The improved in vitro dissolution from the S-SMEDDS is an indication of improvement in solubility, dissolution rate of the pure drug. Thus S-SMEDDS is a promising system for piroxicam.
| Time (Month) |
% Drug content | % Drug release after 5 min | % Drug release after 45 min |
|---|---|---|---|
| 0 | 98.32 | 81.48 | 98.51 |
| 1 | 99.03 | 78 | 98.51 |
| 2 | 98.45 | 79.05 | 98 |
| 3 | 98.36 | 79.05 | 98.51 |
Table 4: Stability studies of optimized formulation at 30±2°/75±5 % RH
Acknowledgements
The authors are grateful to Apex Healthcare Limited, Gujarat, India for providing piroxicam drug. The authors are also grateful to Intas Pharmaceuticals Limited, Ahmedabad, Trans Chem Corporation, Bhiwandi, Dr. Reddy's Laboratories Ltd., Hyderabad, Gangwal Chemicals Pvt. Ltd., Mumbai for providing the gift samples of oils, surfactants, co-surfactants and adsorbents.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Obitte NC, Rohan LC, Adeyeye CM, Parniak MA, Esimone CO. The utility of self-emulsifying oil formulation to improve the poor solubility of the anti HIV drug CSIC. AIDS Res Ther 2013;10:14.
- Yang M, Gong W, Wang Y, Shan L, Li Y, Gao C. Bioavailability Improvement Strategies for Poorly Water-Soluble Drugs Based on the Supersaturation Mechanism: An Update. J Pharm Pharm Sci 2016;19:208-25.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 2012;64:4-17.
- Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci 2006;29:278-87.
- Chaudhary VB. Cyclodextrin Inclusion Complex to Enhance Solubility of Poorly Water Soluble Drugs: A Review. Int J Pharm Sci Res 2013;4(1);68-76.
- Sridhar I, Doshi A, Joshi B, Wankhede V, Doshi J. Solid Dispersions: an Approach to Enhance Solubility of poorly Water Soluble Drug. Int J Sci Innov Res 2013;2(3):685-94.
- Shid RL, Dhole SN, Kulkarni N, Shid LS. Nanosuspension: A Review. Int J Pharm Sci Rev Res 2013;22:98-106.
- Singh SK, Verma PRP, Razdan B. Development and characterization of a lovastatin loaded self-microemulsifying drug delivery system. Pharm Dev Technol 2010;15:469-83.
- Hong JY, Kim JK, Song YK, Park JS, Kim CK. A new self-emulsifying formulation Itraconazole with improved dissolution and oral absorption. J Control Release 2006;110:332-8.
- Mou D, Chen H, Du D, Mao C, Wan J, Xu H, et al. Hydrogel-thickened system for topical delivery of lipophilic drugs. Int J Pharm 2008;353:270-76.
- Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov 2007;6:231-48.
- Li S, Madan P, Lin S. Effect of ionization of drug on drug solubilization in SMEDDS prepared using Capmul MCM and caprylic acid. Asian J Pharma Sci 2017;12:73-82.
- Nazzal S, Smalyukh OD, Mansoor AK. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. Int J Pharm 2002;235:247-85.
- Wang YP, Gan Y, Zhang XX. Novel gastroretentive sustained-release tablet of tacrolimus based on self-microemulsifying mixture: in vitro evaluation and in vivo bioavailability test. Acta Pharmacol Sin 2011;32:1294-302.
- Gupta S, Kesarla R, Omri A. Formulation Strategies to Improve the Bioavailability of Poorly Absorbed Drugs with Special Emphasis on Self-Emulsifying Systems. ISRN Pharm 2013;848043:16.
- Singh B, Bandopadhyay S, Kapil R, Singh R, Katare O. Self-emulsifying drug delivery systems (SEDDS): formulation development, characterization, and applications. Crit Rev Ther Drug Carrier Syst 2009;26:427-521.
- Bahl D, Bogner RH. Amorphization of indomethacin by co-grinding with Neusilin US2: amorphization kinetics, physical stability and mechanism. Pharm Res 2006;23:2317-25.
- Dahl SL, Ward JR. Pharmacology, clinical efficacy, and adverse effects of piroxicam, a new nonsteroidal anti-inflammatory agent. Pharmacotherapy 1982;2(2):80-90.
- Dhage MA, Chhabra GS, Banerjee SK. Development and validation of UV-spectrophotometric method for piroxicam in bulk and pharmaceutical formulation. J Chem Pharm Res2011;3:765-69.
- Kallakunta VR, Bandari S, Jukanti R, Veerareddy PR. Oral self-emulsifying powder of lercanidipine hydrochloride: formulation and evaluation. Powder Technol 2012;221:375-82.
- Date AA, Nagarsenker M. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoximeproxetil. Int J Pharm2007;329:166-72.
- Gupta S, Chavan S, Sawant K. Self nanoemulsifying drug delivery system for adefovirdipivoxil: Design, characterization, in vitro and ex vivo evaluation. Colloids Surf A: Physicochem Eng Asp 2011;392:145-55.
- Shen H, Zhong M. Preparation and evaluation of self-microemulsifying drug delivery systems (SMEDDS) containing atorvastatin. J Pharm Pharmacol 2006;58:1183-91.
- Liu W, Tian R, Hu W, Jia Y, Jiang H, Zhang J. Preparation and evaluation of selfmicroemulsifying drug delivery system of baicalein. Fitoterapia 2012;83:1532-39.
- Quan Q, Kim DW, Marasini N, Kim DH, Kim JK, Kim JO. Physicochemical characterization and in vivo evaluation of solid self-nanoemulsifying drug delivery system for oral administration of docetaxel. J Microencapsul 2013;30:307-14.
- Zanchetta B, Chaud MV, Santana MHA. Design and development of self-emulsifying drug delivery systems novel cardioactive N-acylhydrazone compound. Int J Nanosci Nanotechnol 2016;4:1-12.
- Kyatanwar AU, Jadhav KR, Kadam VJ. Self-micro-emulsifying drug delivery system (SMEDDS): Review. J Pharm Res 2010;3:75-83.
- Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of Ramipril nanoemulsion formulation. Eur J Pharm Biopharm 2007;66:227-43.
- Gurram AK, Deshpande PB, Kar SS, Nayak UY, Udupa N, Reddy MS. Role of Components in the Formation of Self-microemulsifying Drug Delivery Systems. Indian J Pharm Sci 2015;77:249-57.
- Singh AK, Chaurasiya A, Awasthi A, Mishra G, Asati D, Khar RK, et al. Oral Bioavailability Enhancement of Exemestane from Self-Microemulsifying Drug Delivery System (SMEDDS). AAPS PharmSciTech 2009;10:906-16.
- Prajapat MD, Patel NJ, Bariya A, Butani S. Formulation and evaluation of self-emulsifying drug delivery system for nimodipine, a BCS class II drug. J Drug Deliv Sci Technol 2017;39:59.
- Shah RB, Tawakkul MA, Khan MA. Comparative Evaluation of Flow for Pharmaceutical Powders and Granules. AAPS PharmSciTech 2008;9:250-58.
- Yeom DW, Son HY, Kim JH, Kim SR, Lee SG, Song SH, et al. Development of a solidified self-microemulsifying drug delivery system (S-SMEDDS) for atorvastatin calcium with improved dissolution and bioavailability. Int J Pharm 2016;506:302-11.
- Nazzal S, Smalyukh II, Lavrentovich OD, Khan MA. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. Int J Pharm 2002;235:247-65.
- Swenson ES, Milisen WB, Curatolo W. Intestinal permeability enhancement: efficacy, acute local toxicity and reversibility. Pharm Res 1994;11:1132-42.
- Doney AB, Saroj S, Sabitha M. Mechanism of solubility of liquisolid formulation in non-volatile solvent: A review. Int J Pharm Pharm Sci 2012;4(3):710-15.
- Wadhwa J, Nair A, Kumria R. Emulsion forming drug delivery system for lipophilic drugs. Acta Pol Pharm 2012;69(2):179-91.
 Pure drug;
Pure drug;  L-SMEDDS;
L-SMEDDS;  S-SMEDDS
S-SMEDDS



