- *Corresponding Author:
- P. P. Mane Indira Institute of Pharmacy, Sadavali (Deorukh), Sangmeshwar, Ratnagiri-415 804 E-mail: prashantmane01@gmail.com
| Date of Submission | 20 October 2012 |
| Date of Revision | 08 February 2014 |
| Date of Acceptance | 12 February 2014 |
| Indian J Pharm Sci 2014;76(2):166-169 |
Abstract
Nebivolol, a cardioselective β-blocker undergoes extensive metabolism in the liver after its oral administration resulting in low bioavailability. Oral administration of nebivolol also causes gastrointestinal disturbances characterised by stomach ache. To overcome these short comings, mucoadhesive buccal films of nebivolol were prepared using different concentrations of hydroxypropyl methylcellulose and hydroxyl ethylcellulose in the ratios of 2:1, 4:1 and 6:1 and hydroxypropyl methylcellulose and methylcellulose in the ratio of 2:2, 4:3 and 6:4 by solvent casting technique. All the prepared films were found to be smooth, elegant and uniform in thickness and weight. Among the three polymer combinations used, 6:4 (BFN 6 ) showed increased in vitro residence time, which appeared to be mainly due to mucoadhesive nature of hydroxylpropyl methylcellulose and methylcellulose. Evaluation of the films showed uniform dispersion of the drug throughout the formulation (96.21±0.71 to 97.02±0.12%). In vitro drug release studies showed better results at the end of 8 h. The release profile of all the formulations was subjected to kinetic analyses, which suggested that the drug was released by diffusion mechanism following super case-II transport.
Keywords
Nebivolol, buccal films, mucoadhesion, in vitro drug release, diffusion
The buccal route has been a subject of growing interest since many years because of its numerous advantages. It is quite well established that absorption of drugs from oral mucosa allows direct entry of the drug in to the systemic circulation, thereby avoiding the first pass hepatic metabolism as well as degradation of the drug in the gastrointestinal tract, both of which are associated with oral administration [1]. The buccal route provides additional advantages such as allowing excellent accessibility, reasonable patient acceptance and compliance. In order to optimise drug delivery to, or via, the buccal cavity, the use of adhesive dosage forms has been investigated [2]. The oral cavity has a moist environment; the membrane that lines the oral cavity is covered with mucus, which is derived mainly from minor salivary glands and is constantly bathed in saliva, an aqueous substance rich in inorganic salts, proteins and bacteria [3].
Recently, interest has been expressed on the delivery of drug to or via mucous membrane by the use of mucoadhesive materials. Several mucoadhesive formulations are under development and drug delivery via buccal mucosa is a novel route of drug administration. The buccal film has been increasingly used for administration because of advantages that the drug is directly available to the systemic circulation, avoidance of first pass metabolism, rapid onset of action, prolonged drug release and also easy removal of film from the site of application.
Nebivolol is a cardioselactive β-blocker used in the management of hypertension [4,5]. Nebivolol undergoes extensive metabolism in the liver after its oral administration, which results in very poor bioavailability (approximately 12%). Oral administration of nebivolol also reported to cause gastrointestinal disturbances and abdominal or stomachache. In an attempt to improve the bioavailability, efficacy and to minimise the side effects associated with oral administration, we have prepared mucoadhesive buccal films of nebivolol.
Nebivolol was a gift sample from Micro Labs Ltd. Bangalore. Hydroxylpropyl methylcellulose (HPMC), hydroxyl ethylcellulose (HEC) and methylcellulose (MC) were purchased from S. D. Fine-Chem, Mumbai and all other chemicals used were of analytical grade.
Nebivolol mucoadhesive buccal patches were prepared using HPMC, HEC and MC in the various concentration ratios of HPMC and HEC, and HPMC and MC (Table 1) by solvent casting technique [6]. Accurately weighed quantity of HPMC and HEC was soaked in ethanol for 24 h, calculated amount of nebivolol was dissolved in the polymeric solution and propylene glycol was added gradually with continuous stirring. Then 5 ml resultant mixture was poured into each fabricated glass ring placed on aluminium foil in a Petri dish, and then the Petri dish was kept aside for drying at room temperature for 24 h. The dried polymeric films are cut into circular films of 10 mm diameter for further evaluation. The same films were prepared by using HPMC and MC in different concentration ratio.
| Ingredients (%w/v) | Formulation code | |||||
|---|---|---|---|---|---|---|
| BFN1 | BFN2 | BFN3 | BFN4 | BFN5 | BFN6 | |
| HPMC | 2 | 4 | 6 | 2 | 4 | 6 |
| HEC | 1 | 1 | 1 | ‑ | ‑ | ‑ |
| MC | ‑ | ‑ | ‑ | 2 | 3 | 4 |
| Propylene glycol* | 30 | 30 | 30 | 30 | 30 | 30 |
*Percent of polymer weight. Each patch contains 5 mg of nebivolol, HPMC=hydroxylpropyl methylcellulose , HEC=hydroxyl ethylcellulose, MC=methylcellulose.
Table 1: Formulation details of nebivolol buccal films
To determine the weight uniformity, three films of 10 mm diameter each from every batch were weighed individually using Shimadzu digital balance and the average weight was calculated [7]. The thickness uniformity was determined by measuring thickness of the films using a screw gauge with a least count of 0.01 mm at three different spots of the films and the average thickness was calculated [8]. Flexibility of films can be measured quantitatively in terms of folding endurance [9], which can be determined by repeatedly folding a small strip of the films at the same place till it broke. The number of times films could be folded at the same place without breaking gives the value of folding endurance and the procedure was repeated three times. The results are shown in Table 2.
| FC | Wt (mg) | TN (mm) | FE (No.) | SI (%) | SpH | MS (g) | RT (h) | DCU (%) |
|---|---|---|---|---|---|---|---|---|
| BFN1 | 7.7±0.6 | 0.13±0.01 | 347±7 | 40.2±1.1 | 6.61±0.11 | 3.2±0.05 | 2.9±0.37 | 97.0±0.1 |
| BFN2 | 12.1±0.3 | 0.18±0.01 | 327±5 | 47.8±1.6 | 6.37±0.05 | 3.6±0.2 | 3.5±0.03 | 97.2±0.1 |
| BFN3 | 17.7±1.5 | 0.24±0.02 | 312±5 | 54.5±1.1 | 6.91±0.12 | 3.9±0.05 | 4.5±0.06 | 97.2±0.2 |
| BFN4 | 13.5±0.5 | 0.22±0.01 | 373±6 | 34.1±1.9 | 5.70±0.1 | 4.2±0.10 | 4.3±0.04 | 97.4±1.1 |
| BFN5 | 15.0±0.3 | 0.26±0.02 | 345±4 | 44.8±0.6 | 6.7±0.26 | 5.2±0.03 | 4.5±0.04 | 97.1±0.3 |
| BFN6 | 18.0±0.4 | 0.29±0.02 | 299±1 | 50.5±0.9 | 6.66±0.15 | 5.8±0.02 | 5.4±0.03 | 96.2±0.7 |
Nebivolol buccal films bearing different FC=Formulation codes of BFN1, BFN2, BFN3, BFN4, BFN5 and BFN6 were evaluated for Wt=weight variation, TN=thickness, FE=folding endurance, SI=swelling index, S pH=surface pH, MS=mucoadhesive strength, in vitro RT=residence time and percent DCN=Drug content uniformity, All values are mean±standard deviation of three determinations
Table 2: Evaluation of nebivolol buccal films
Swelling index [10] was determined using the following method. A buccal film of 10 mm diameter was weighed on a preweighed cover slip, the initial weight of the film was recorded (W0) and then it was kept in a Petri dish containing 5 ml of pH 6.8 phosphate buffer. The cover slip was removed at time intervals of 0.5, 1, 2, 3, 4, 5, 6, 7, 8 h, excess water was carefully removed and the films were re-weighed (Wt). Then the percentage swelling was calculated by the following formula %S = (Wt−W0/W0)×100.
The experiment was repeated three times. Surface pH [11] was determined by allowing the film to come in contact with 1 ml of phosphate buffer for 1-3 min then the surface pH was measured using a pen pH meter and the procedure was repeated three times.
Drug content uniformity was carried out to know the complete and uniform dispersion of the drug throughout the film. In this test, the film of 10 mm diameter was dissolved in methanol and the absorbance of the solution (after suitable dilution) was measured at 282 nm [12] using UV/Vis spectrophotometer (Shimadzu UV-1700) and finally the percent drug content was calculated with the help of calibration curve. The procedure was repeated three times.
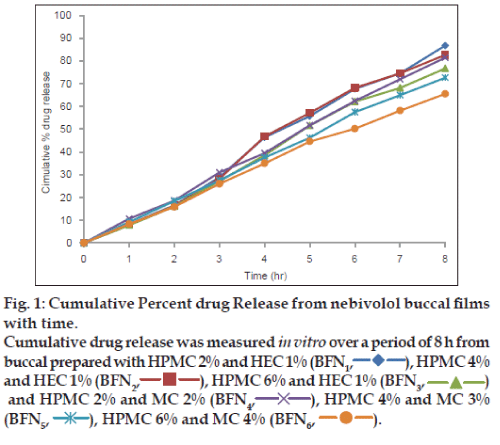
In vitro drug release from buccal films was studied by standard cylindrical tube method using Sigma dialysis membrane. The membrane was tied to one end of an open cylinder, which acted as the donor compartment, the buccal film was placed inside this compartment and it was in contact with the receptor compartment containing 100 ml 6.8 pH phosphate buffer. The diffusion medium was stirred continuously using magnetic stirrer and the temperature was maintained at 37±0.5°. Five millilitre samples were withdrawn from the receptor compartment at periodic intervals and the same was replaced by equal volume of fresh buffer solution. The samples were analysed for drug content spectrophotometrically at 280 nm, the amount of drug released was calculated with the help of standard calibration curve and then cumulative percentage drug release was calculated and plotted (fig. 1). The in vitro release data was subjected to various kinetic equations and were plotted for various plots.
Ex vivo mucoadhesive strength of the prepared films was determined using the following method [13]. Fresh sheep buccal mucosa was obtained from a local slaughterhouse; the mucosal membrane was separated by removing the underlining fat and loose tissues. The membrane was washed with distilled water and then with isotonic phosphate buffer solution of 6.8 pH at 37°. Bioadhesive strength of the film ware measured on a physical balance modified to suit the purpose.
In vitro residence time [13] was determined using a locally modified USP disintegration test apparatus. A 3-cm long segment of sheep buccal mucosa was glued to the surface of a glass slab, vertically attached to the apparatus. The mucoadhesive film was hydrated from one surface using 15 μl isotonic phosphate buffer and the hydrated surface was brought into contact with the mucosal membrane. The glass slab was vertically fixed to the apparatus and allowed to move up and down in 800 ml isotonic phosphate buffer pH 6.8 maintained at 37°, so that the film was completely immersed in the buffer solution at the lowest point and was out at the highest point. The time taken for complete erosion or detachment of the films from the mucosal surface was recorded (mean of triplicate determinations).
In the present research work, nebivolol mucoadhesive buccal films were prepared using different combinations of different concentration ratio of HPMC, HEC and MC by solvent casting technique to improve the efficacy of drug by improving its bioavailability. The prepared films were evaluated for various parameters and the results are given in Table 2 and are discussed in the following sections.
The thickness of the films prepared with various polymer combination ratios was found to be in the range of 0.126-0.29 mm, suggesting that the films were thin enough and they would not cause any inconvenience after their application to the buccal cavity. The surface pH of all the films was found to be in the range of pH 5.7-6.9, a range that is nearer to the salivary pH, hence it can be safely assumed that the films when applied to any to the mucus membrane of the buccal cavity would not cause any irritation.
Folding endurance of the prepared film was measured to be 373 times for BFN4 and the mucoadhesive strength was found to be 5.81 mg for BFN6, the values for these parameters are high enough to indicate that the films were flexible and would not detach easily. The films when applied could be retained for longer period of time at the site of application and this conclusion is well supported by the long in vitro residence time of 5.40 h obtained for BFN6. The buccal films were evaluated for drug content uniformity, which indicated that the drug was uniformly dispersed in the range of 96.21-97.43%.
Drug release from the films was studied for a period of 8 h and the release profiles were subjected to Higuchi diffusion Eqn. (Q=Kt1/2) and Peppas exponential Eqn. (Q=Ktn) to understand the drug release mechanism (Table 3). In both cases, the plots were found to be fairly linear and the linearity was well supported by regression coefficient values very nearer to one. Slope values of the Peppas Eqn are more than one in all the cases suggesting that the drug-release mechanism is by diffusion following super case-II transport. In conclusion the present investigation reports the development of nebivolol mucoadhesive buccal film containing varying concentration of HPMC, HEC and MC. The films were prepared using solvent casting technique. All the prepared formulations were found to possess good mucoadhesive strength, nonirritant and release the drug completely by a diffusion mechanism.
| Formulation No | Regression coefficients | Slope | |||
|---|---|---|---|---|---|
| Zero order | First order | Higuchi diffusion plots | Peppas exponential plots | ||
| BFN1 | 0.993 | 0.933 | 0.896 | 0.835 | 1.657 |
| BFN2 | 0.987 | 0.966 | 0.898 | 0.839 | 1.664 |
| BFN3 | 0.994 | 0.969 | 0.900 | 0.835 | 1.615 |
| BFN4 | 0.998 | 0.949 | 0.909 | 0.784 | 1.545 |
| BFN5 | 0.998 | 0.974 | 0.916 | 0.795 | 1.526 |
| BFN6 | 0.996 | 0.987 | 0.923 | 0.806 | 1.508 |
Drug release kinetics of various nebivololbuccal films bearing different formulation nos BFN1, BFN2, BFN3, BFN4, BFN5 and BFN6 were studied
Table 3: Release kinetics of nebivolol buccal films
Acknowledgements
The authors are thankful to Micro Labs Ltd., Bangalore for providing gift samples of nebivolol. The authors are also thankful to the principal HKES’s college of Pharmacy, Gulbarga for providing laboratory facilities to carry out the research work.
References
- John DS. Drug delivery using buccal-adhesive systems. Adv Drug DelivRev1993;ll:253-70.
- Michael JR, Bernadette KD, Ian GT. The oral cavity as a site for systemic drug delivery. Adv Drug Deliv Rev 1994;13:1-22.
- Bing Li, Robinson JR. In: Swarbrick J, editor. Drug Delivery to the Oral Cavity. USA: CRC Press; 2005. p. 41-66.
- Sweetman SC. Martindale: The Complete Drug Reference. 35th ed.London: Pharmaceutical Press; 2007. p.1211.
- Available from: http://www.drug.com/nebivolol: medline plus druginformation.html.
- Satishbabu BK, Shrinivasan BP. Preparation and evaluation of buccoadhesive film of atenolol. Indian J Pharm Sci 2008;20:175-9.
- Rasool BK, Khan S. In vitroevaluation of miconazolemucoadhesivebuccal films. Int J App Pharm 2010;2:23-6.
- Singh S, Gangwar S, Garg G, Garg V, Sharma PK. Formulation and evaluation of rapidly disintegrating film of levocetrizine hydrochloride. Der Pharm Lett 2010;2:434-9.
- Koland M, Sandeep VP, Charyulu NR. Fast dissolving sublingual films of ondansetron hydrochloride: Effect of additives on in vitro drug release and mucosal permeation. J Young Pharm 2010;2:216-22.
- Noha AN, Fatma AI, Nabila AB, Lobna MM. Mucoadhesivebuccal patches of miconazole nitrate: In vitro/in vivo performance and effect of ageing. Int J Pharm 2003;264:1-14.
- Vinod R, Ashok KP, Rao SB, Kulkarni SV, Shankar MS. Design and evaluation of miconazole nitrate buccalmucoadhesive patches. J Pharm Res 2010;3:1338-41.
- Mishra P, Shah K, Gupta A. Spectrophotometric methods for simultaneous estimation of nebivolol hydrochloride and amlodipine besylate in tablets. Int J Pharm PharmSci 2009;1:55-61.
- Giradkar KP, Channawar MA, Kajale AD, Sridhar E, Kamble RS, Bakde BV, et al. Design, development andin vitroevaluation ofbioadhesive dosage form for buccal route. Int J Pharm Res Dev 2010;2:1-20.