- *Corresponding Author:
- R. K. Joshi
Department of Phytochemistry,
Indian Council of Medical Research-National Institute of Traditional Medicine,
Belagavi, Karnataka 590010,India
E-mail: joshirk_natprod@yahoo.com
| Date of Received | 23 February 2020 |
| Date of Revision | 23 February 2021 |
| Date of Acceptance | 25 July 2021 |
| Indian J Pharm Sci 2021;83(4):750-757 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In the traditional system of medicine Ocimum sanctum Linn. is used to cure diseases like dysentery, bronchitis, malaria, bronchial asthma, diarrhea, arthritis, skin diseases, painful eye diseases, chronic fever. The essential oil obtained by using hydrodistillation of the leaves of Ocimum sanctum Linn. (purple variety) is collected throughout 1 y and analyzed employing gas chromatography equipped with flame ionization detector and gas chromatography connected with mass spectrometry. The yield of essential oil varied in the range from 0.14 to 0.21 % (w/w). 23 to 39 constituents were identified which represented 95.39±0.38 % of the total oil. The most abundant compound was identified as methyl eugenol with an average of 69.88±0.09 % with varying quantities from 55.48±0.36 to 76.78±0.54 %. The other constituents were beta-caryophyllene 10.40±0.06 %, alpha-ylangene 4.18±0.04 %, gamma-muurolene 3.64±0.04 % and borneol 2.02±0.01 %. The oil was found to be rich in phenylpropanoid (71.98±0.27 %) type constituents (average of 12 mo). A high amount of methyl eugenol (76.78±0.54 %) was observed during November while low (55.48±0.36 %) in April. It was noticed that when the plant was matured in April the accumulation of sesquiterpenes increased and phenylpropanoids decreased.
Keywords
Ocimum sanctum Linn., methyl eugenol, beta-caryophyllene, alpha-ylangene, gamma-muurolene, borneol, gas chromatography-mass spectrometry
Ocimum sanctum L. (O. sanctum) (purple variety) of the family Lamiaceae is an erect, softly hairy, aromatic herb. Two types of O. sanctum come across within cultivation; with green leaves known as Sri or Lakshmi Tulsi and with purple leaves known as Krishna Tulsi [1]. The plant is commonly cultivated in temple premises and households as a sacred plant [2]. In the traditional system of medicine O. sanctum is used to cure diseases like dysentery, bronchitis, malaria, bronchial asthma, diarrhea, arthritis, skin diseases, painful eye diseases, chronic fever, etc., [3]. It has also been demonstrated to have anticancer, antifertility, antifungal, antidiabetic, anti-microbial, cardioprotective, hepatoprotective, analgesic, antiemetic, antispasmodic, adaptogenic and diaphoretic actions [4]. The dried powdered leaves mixed with mustard oil in the form of a paste are useful to cure pyorrhea, foul smell and other tooth troubles. The seeds are used in genito-urinary system disorders [4,5]. Various pharmacological activities viz., anticancer [6], radioprotective [7,8], anticarcinogenic [7], antioxidant [7,9], anticandidal [10], antileishmanial, immunomodulatory [11], immunotherapeutic [12], antimicrobial [9,13], antifertility [14], anti-inflammatory [15] and neuroprotective [16] properties of this plant have also been reported. The main constituent of the essential oil of O. sanctum from different regions of the world is represented in Table 1 [17-31]. The present investigation was planned to evaluate the influence of the environmental factors on the essential oil composition of the leaves of O. sanctum growing in North West Karnataka, India.
Materials and Methods
Plant material:
The leaves of O. sanctum were collected from three plants of a single population grown in properly maintained medicinal plant garden of Indian Council of Medical Research-National Institute of Traditional Medicine (ICMR-NITM), Belagavi and the collection was taken from similar plants throughout a year between May 2014 and April 2015 from an altitude of ~800 m from Belagavi district (N 15.88668; E 74.52353), Karnataka, India. The plant was identified by a Taxonomist, Dr. Harsha Hegde, at ICMR-NITM, Belagavi, where the herbarium specimen has been deposited (RMRC‑531).
Isolation of essential oil:
The fresh plant material (100 g) was subjected to hydrodistillation by using a Clevenger-type apparatus for 3 h. The essential oil was collected and dehydrated over anhydrous sodium sulfate and stored in sealed vials at -4° until analysis. The oil yield per month collected from essential oil of leaves is shown in Table 1.
| Country | Major compound |
|---|---|
| Australia | Methyl chavicol (87 %), camphor (4 %) and β-caryophyllene (5 %) [17] |
| Brazil | Eugenol (79.0-82.7 %), β-caryophyllene (7.9-9.8 %), β-elemene (5.0-7.6 %) and germacrene A (1.7-4.7 %) [18] |
| Cuba | Eugenol (34.3 %), β-elemene (18.0 %) and β-caryophyllene (23.1 %) [19] |
| India | Methyl eugenol (92.4 %), eugenol (2.4 %) [8]; methyl eugenol (82.9 %), β-caryophyllene (4.1 %), borneol (2.4 %), germacrene D (2.3 %) [20]; methyl eugenol (72.41 %), β-caryophyllene (12.00 %), isoeugenol (6.31 %), β-elemene (2.48 %) [21]; methyl eugenol (75.25 %), β-elemene (2.84 %), (E)-cinnamyl acetate (3.44 %), β-caryophyllene (6.37 %) [22]; eugenol (0.6-46.2 %), β-elemene (4.3-16.3 %), methyl eugenol (67.8 %), (E)-caryophyllene (17.1-27.6 %) [23]; methyl chavicol (44.63 %), linalool (21.84 %), carvone (6.31 %) and D-limonene (4.39 %) [24] |
| Nigeria | Methyl eugenol (44.7 %), isocaryophyllene (16.8 %) and β-elemene (8.8 %) [25] |
| Poland | β-Bisabolene (13.0-20.4 %), linalool (0.6-19.2 %), 1,8-cineole 8.9-33.0 %), methyl chavicol (1.9-12.5 %) and (E)-α-bisabolene (4.1-4.9 %) [26] |
| North Alabama | Eugenol (25.3±3.1 %-51.5±1.6 %), β-caryophyllene (2.8±0.2-25.4±1.4 %), trans-β-guaiene (2.6±0.3-19.2±1.4 %), α-cadinene (0.1±0.1-10.8±0.4) [27] |
| Iran | Methyl chavicol (27.64-38.96 %), linalool (5.43-12.13 %), epi-α-cadinol (5.02-11.5 %) [28] |
| Malaysia | Methyl eugenol (36.47-76.27 %), β-caryophyllene (8.52-53.63 %), β-elemene (1.59-6.35 %) [29] |
| Mississippi | Eugenol (8–43 %), methyl chavicol (15–27 %) [30] |
| Romania No methyl eugenol | Linalool (38.60 %), eugenol (11.82 %), tau-cadinol (10.20 %), germacrene D (6.08 %) [31] |
Table 1: Major Chemical Constituents of Various Plant Parts of O. sanctum Reported From Various Countries
| Months | Jan | Feb | Mar | Apr | May | Jun | July | Aug | Sept | Oct | Nov | Dec | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | RI | Content % | Mean±SEM | *Identification | |||||||||||
| a-Pinene | 929 | t | 0.71±0.02 | t | 0.71±0.02 | t | t | t | t | t | 0.10±0.01 | t | t | 0.51±0.01 | a, b, c |
| Camphene | 939 | 0.19±0.01 | 0.85±0.02 | - | 0.95±0.03 | t | - | - | - | 0.12±0.02 | 0.13±0.02 | 0.10±0.02 | 0.16±0.01 | 0.36±0 | a, b |
| Sabinene | 958 | 0.31±0.01 | 0.18±0 | - | 0.22±0.01 | - | t | t | - | 0.21±0.01 | - | - | 0.21±0.01 | 0.23±0 | a, b |
| ß-Pinene | 962 | 0.10±0.01 | 0.54±0.01 | - | 0.61±0.1 | - | t | t | - | - | - | - | t | 0.42±0.01 | a, b |
| Myrcene | 973 | 0.29±0.01 | t | - | t | - | - | - | - | - | 0.10±0.02 | t | t | 0.20±0.01 | a, b |
| Phellandrene | 986 | - | - | t | t | - | - | - | - | 0.11±0 | - | 0.13±0.01 | - | 0.12±0 | a, b, c |
| a-Terpinene | 997 | t | t | - | 0.35±0.02 | - | - | - | - | t | t | 0.10±0.01 | t | 0.22±0.01 | a, b |
| O-Cymene | 1000 | 0.21±0.02 | t | t | 0.12±0.01 | - | - | t | t | 0.11±0.01 | t | t | 0.19±0 | 0.15±0.01 | a, b, c |
| 1,8-Cineole | 1009 | 0.13±0.02 | 0.31±0.01 | t | 0.14±0.01 | 0.10±0.01 | - | t | t | 0.32±0.02 | t | 0.10±0.02 | t | 0.18±0 | a, b |
| (Z)-ß-Ocimene | 1017 | - | - | t | - | - | - | - | t | 0.12±0.02 | 0.10±0.01 | t | t | 0.11±0 | a, b, c |
| (E)-ß-Ocimene | 1029 | - | - | - | - | - | - | - | - | 0.21±0.02 | - | - | - | 0.21±0.02 | a, b |
| ?-Terpinene | 1039 | t | t | t | t | t | t | t | t | t | t | t | t | t | a, b |
| Cis-Sabinene hydrate | 1044 | - | t | t | - | - | - | t | - | t | 0.20±0.01 | t | - | 0.20±0.01 | a, b, c |
| Terpinolene | 1071 | 0.11±0 | 0.15±0.01 | - | t | - | t | 0.12±0.01 | t | 0.21±0.02 | t | 0.31±0.03 | t | 0.18±0 | a, b, c |
| Trans-Sabinene hydrate | 1076 | - | - | t | - | - | - | t | - | t | t | - | - | t | a, b, c |
| Linalool | 1079 | 0.39±0.03 | 0.94±0.04 | 0.57±0.01 | 0.76±0.02 | t | t | t | t | 0.10±0.01 | t | t | 0.30±0.01 | 0.51±0.01 | a, b |
| Camphor | 1116 | 0.11±0.02 | 0.13±0 | t | 0.23±0.01 | t | t | t | t | 0.13±0 | 0.12±0.01 | 0.10±0.01 | t | 0.14±0 | a, b, c |
| Borneol | 1151 | 2.10±0.07 | 2.44±0.05 | 2.95±0.04 | 2.71±0.07 | 0.83±0.04 | 0.91±0.02 | 2.35±0.08 | 2.39±0.09 | 1.79±0.05 | 1.59±0.06 | 2.00±0.1 | 2.24±0.03 | 2.02±0.01 | a, b |
| Terpinen-4-ol | 1166 | 0.10±0.01 | t | t | t | t | t | t | t | t | t | 0.10±0.01 | t | 0.10±0.01 | a, b |
| Eugenol | 1372 | 2.03±0.04 | 1.46±0.02 | 1.63±0.02 | 1.31±0.01 | 1.06±0.03 | 0.84±0.01 | 0.95±0.02 | 1.03±0.08 | 1.11±0.07 | 1.24±0.02 | 1.21±0.04 | 1.48±0.03 | 1.28±0.02 | a, b, c |
| a-Ylangene | 1394 | 3.80±0.15 | 4.13±0.1 | 2.06±0.09 | 3.16±0.09 | 2.62±0.29 | 3.94±0.07 | 6.34±0.11 | 6.40±0.26 | 5.79±0.1 | 4.71±0.16 | 3.33±0.19 | 3.83±0.09 | 4.18±0.04 | a, b |
| Methyl eugenol | 1435 | 66.20±1.12 | 61.28±0.26 | 73.92±0.2 | 55.48±0.36 | 76.16±0.36 | 73.98±0.43 | 69.31±0.64 | 71.61±0.73 | 68.53±0.57 | 74.79±0.63 | 76.78±0.54 | 70.50±0.29 | 69.88±0.09 | a, b, c |
| ß-Isocomene | 1444 | 0.10±0.01 | t | t | - | - | t | t | - | t | t | - | t | 0.10±0.01 | a, b, c |
| ?-Caryophyllene | 1475 | 10.63±0.43 | 10.47±0.29 | 8.21±0.16 | 20.32±0.2 | 11.10±0.49 | 12.22±0.09 | 9.31±0.16 | 8.20±0.12 | 9.40±0.15 | 8.11±0.22 | 7.67±0.23 | 9.15±0.15 | 10.40±0.06 | a, b, c |
| ß-Gurjunene | 1485 | 0.21±0.02 | 0.21±0.01 | 0.15±0.01 | 0.18±0.01 | 0.10±0 | 0.10±0.01 | 0.13±0.01 | 0.10±0.01 | 0.21±0.01 | 0.21±0.02 | t | 0.15±0 | 0.16±0 | a, b, c |
| a-Humulene | 1511 | 0.60±0.03 | 0.70±0.01 | 0.59±0.01 | 1.22±0.02 | 0.85±0.04 | 0.82±0.02 | 0.55±0.02 | 0.51±0.02 | 0.49±0.03 | 0.50±0.03 | 0.39±0.04 | 0.54±0.03 | 0.65±0.01 | a, b, c |
| ?-Muurolene | 1542 | 4.66±0.17 | 5.57±0.08 | 2.55±0.15 | 3.98±0.04 | 1.30±0.03 | 2.11±0.06 | 4.60±0.19 | 4.33±0.37 | 4.21±0.18 | 3.56±0.12 | 3.03±0.04 | 3.74±0.05 | 3.64±0.04 | a, b, c |
| Epi-Cubebol | 1557 | 0.10±0 | 0.64±0.02 | t | 0.10±0.01 | 0.10±0.01 | - | 0.11±0.01 | 0.41±0.15 | 0.20±0.01 | 0.10±0.01 | 0.11±0.02 | t | 0.21±0.01 | a, b |
| a-Selinene | 1559 | 0.11±0.02 | 0.10±0.01 | 0.10±0.01 | t | - | - | 0.10±0.01 | 0.10±0.01 | 0.10±0.02 | 0.10±0.01 | 0.20±0.02 | t | 0.11±0.01 | a, b, c |
| Trans-ß-Guaiene | 1564 | t | t | - | - | - | - | - | - | 0.51±0.02 | 0.11±0.01 | - | - | 0.31±0.01 | a, b, c |
| (Z)-a-Bisabolene | 1569 | 0.10±0.01 | t | - | t | 0.10±0.01 | t | 0.45±0.02 | t | t | 0.40±0.03 | t | t | 0.26±0.01 | a, b |
| Cubebol | 1579 | 0.48±0.01 | 0.32±0.01 | - | t | 0.11±0.01 | t | 0.10±0.02 | 0.41±0.04 | 0.50±0.03 | 0.61±0.02 | 0.41±0.01 | 0.53±0.01 | 0.39±0.01 | a, b |
| d-Cadinene | 1590 | 0.21±0.02 | 0.55±0.02 | 0.11±0.01 | 0.37±0.01 | 0.10±0.01 | 0.16±0.01 | 0.18±0.01 | t | 0.31±0.02 | t | t | 0.47±0.01 | 0.27±0 | a, b |
| Caryophyllene oxide | 1646 | 1.17±0.01 | 0.97±0.01 | 2.05±0.03 | 1.50±0.09 | 0.68±0.03 | 0.69±0.01 | 0.43±0.01 | 0.33±0.01 | 0.53±0.01 | 0.61±0.01 | 0.76±0.02 | 0.57±0.01 | 0.86±0.01 | a, b |
| 1-epi-Cubenol | 1699 | 0.11±0.01 | t | t | t | - | t | t | - | t | t | 0.10±0 | 0.21±0.01 | 0.14±0 | a, b, c |
| ?-Eudesmol | 1702 | - | - | - | - | - | - | t | - | 0.10±0.01 | 0.10±0 | 0.11±0.02 | 0.10±0.01 | 0.10±0 | a, b |
| Epi-a-Cadinol | 1711 | 0.21±0.01 | t | 0.11±0.02 | t | t | - | t | t | t | 0.1±0.01 | 0.13±0.02 | t | 0.14±0 | a, b |
| Cubenol | 1716 | 0.10±0.01 | - | - | - | - | - | - | - | - | t | 0.20±0.01 | - | 0.15±0 | a, b |
| a-Eudesmol | 1721 | t | - | t | - | - | t | t | 0.11±0.02 | 0.18±0.04 | t | 0.23±0.01 | 0.17±0.01 | a, b | |
| a-Cadinol | 1724 | - | t | 0.12±0.01 | 0.11±0.01 | - | - | t | t | t | 0.11±0.01 | t | t | 0.11±0 | a, b |
| 7-epi-a- Eudesmol | 1736 | 0.10±0.02 | 0.10±0.01 | t | t | 0.10±0.01 | t | 0.11±0 | t | t | t | t | t | 0.10±0 | a, b |
| Khusinol | 1754 | t | t | t | t | t | t | t | t | t | t | t | t | t | a, b |
| Monoterpene hydrocarbons | 1.21 ±0.01 | 2.43 ±0.03 | t | 2.95 ±0.05 | t | t | 0.12 ±0.01 | t | 1.0 9±0.02 | 0.44 ±0.01 | 0.64 ±0.05 | 0.5 ±0.02 | 1.18± 0.36 | ||
| Oxygenated monoterpenes | 2.82 ±0.07 | 3.81 ±0.09 | 3.53 ±0.03 | 3.85 ±0.1 | 0.94 ±0.05 | 0.91 ±0.02 | 2.35 ±0.08 | 2.39 ±0.09 | 2.33 ±0.04 | 1.91 ±0.05 | 2.30 ±0.12 | 2.54 ±0.04 | 2.47 ±0.28 | ||
| Sesquiterpene hydrocarbons | 20.42 ±0.31 | 21.73 ±0.29 | 13.76 ±0.23 | 29.24 ±0.14 | 16.17 ±0.66 | 19.35 ±0.08 | 21.66 ±0.3 | 19.62 ±0.5 | 21.03 ±0.25 | 17.70 ±0.2 | 14.63 ±0.18 | 17.89 ±0.2 | 19.43 ±1.17 | ||
| Oxygenated sesquiterpenes | 2.27 ±0.03 | 2.03 ±0.04 | 2.28 ±0.01 | 1.71 ±0.09 | 0.99 ±0.02 | 0.69 ±0.01 | 0.75 ±0 | 1.15 ±0.17 | 1.45 ±0.03 | 1.76 ±0.01 | 1.83 ±0.5 | 1.64 ±0 | 1.55 ±0.16 | ||
| Phenyl propanoate | 68.23 ±1.13 | 62.74 ±0.27 | 75.55 ±0.21 | 56.80 ±0.37 | 77.22 ±0.39 | 74.81 ±0.44 | 70.26 ±0.66 | 72.64 ±0.74 | 69.64 ±0.51 | 76.04 ±0.61 | 77.99 ±0.53 | 71.98 ±0.27 | 71.16 ±1.81 | ||
| Total identified | 94.95 ±1.49 | 92.73 ±0.57 | 95.12 ±0.42 | 94.54 ±0.47 | 95.32 ±0.21 | 95.76 ±0.49 | 95.13 ±0.96 | 95.79 ±1.05 | 95.53 ±0.52 | 97.84 ±0.9 | 97.38 ±0.49 | 94.61 ±0.19 | 95.39 ±0.38 | ||
| Essential oil yield % (w/w) | 0.18 | 0.16 | 0.16 | 0.16 | 0.19 | 0.21 | 0.15 | 0.14 | 0.18 | 0.19 | 0.18 | 0.18 | |||
*a=RI relative to C8?C25 n-alkanes on BP-1 column, b=MS NIST and Wiley library and the literature, t trace (<0.1 %), c=coinjection of commercial samples (=98 % purity). Values indicate mean±SEM of three injections of sample
Table 2: Composition of the Essential Oil of O. sanctum Leaf Oil Collected From May 2014 TO April 2015
Chromatographic analysis:
The chemical composition of the essential oil (essential oil in an equal ratio of n-hexane:dichloromethane made 1 % solution) was analyzed by using a gas chromatography (GC) (Varian 450 fitted with a fused silica capillary column BP-1 (100 % dimethyl polysiloxane; SGE Analytical Science), 30 m×0.25 mm inner diameter, 0.25 μm film thickness) under the experimental conditions reported earlier [32-34]. The oven temperature was programmed from 60-220° at 3°/min, using nitrogen as a carrier gas. The injector and the flame ionization detector (FID) temperature were set at 230° and 240°, respectively. Gas chromatography-mass spectrometry (GC-MS) analysis was employed by Thermo Scientific Trace Ultra GC interfaced with a Thermo Scientific ITQ 1100 mass spectrometer fitted with a BP-1 (100 % dimethylpolysiloxane; SGE Analytical Science) fused silica capillary column (30 m×0.25 mm; 0.25 μm film thickness). The oven temperature range was programmed from 60-220° at 3°/min and helium was used as carrier gas at 1.2 ml/min for analysis. The injector temperature was set at 230° and the injection volume was 0.1 μl in n-hexane, with a split ratio of 1:50. Mass spectra were taken at 70 eV with a mass range of mass to charge ratio (m/z) 40-450 and other parameters used were those reported earlier [35-37]. Identification of constituents was done based on retention index (RI, determined [linear temperature programmed analysis was used with the formula RI=100× [(tRi–v)–(tRZ–v)/(tR(Z+1)–v)–(tRZ–v)+Z] where tRi=retention time of sample peak; v=column void time; tRZ=retention time of n-alkane peak eluting immediately before sample peak; tR(Z+1)=retention time of n-alkane peak eluting immediately after sample peak; Z=carbon number of n-alkane peak eluting immediately before sample peak [38] concerning homologous series of n-alkanes C8-C25 under identical experimental conditions on BP-1 column), mass spectral (MS) library search NIST 08 MS Library (Version 2.0 f; Thermo Fisher Scientific Austria) and Wiley MS 9th Edition (Thermo Fisher Scientific Austria) and by comparing with the MS literature data [39] and co-injection of commercial samples from Sigma-Aldrich, India (≥98 % purity). The relative quantities of individual components were calculated based on the GC peak area (FID response) without using a correction factor. The statistical analysis of each sample injected in triplicate was performed by using the Microsoft excel worksheet.
Results and Discussion
Variation in yield of essential oil was observed. The yield of essential oil of O. sanctum leaves varied throughout the examined period of 1 y in the range from 0.14-0.21 % w/w. The maximum yield was observed during June while the minimum one in August (Table 1).
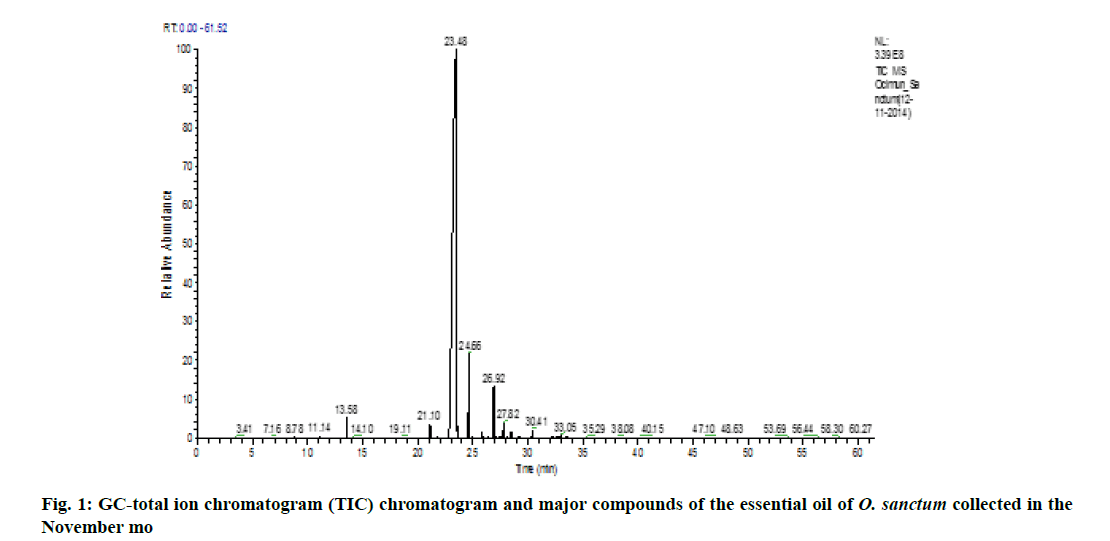
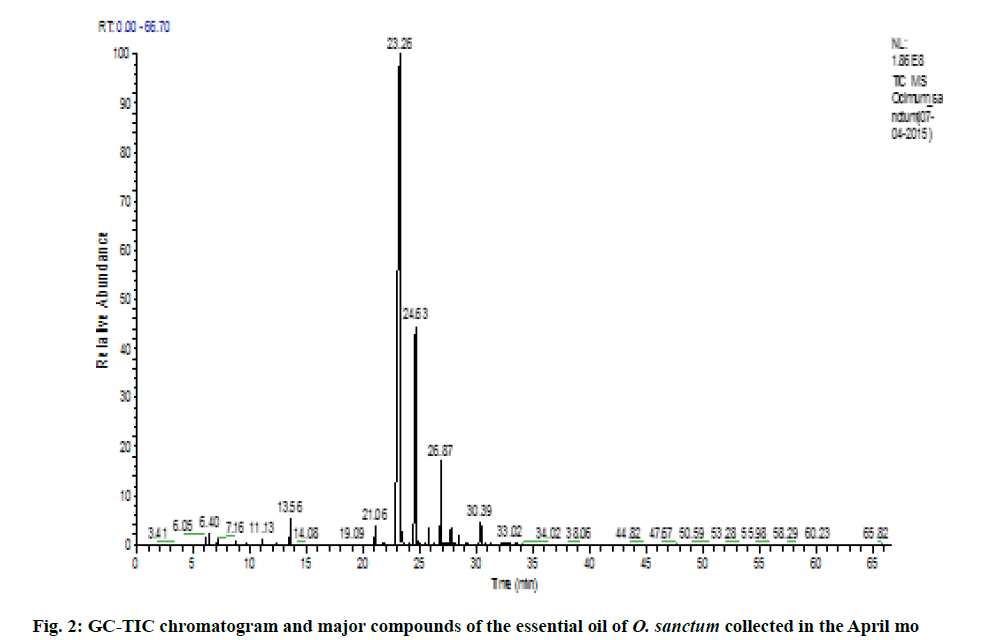
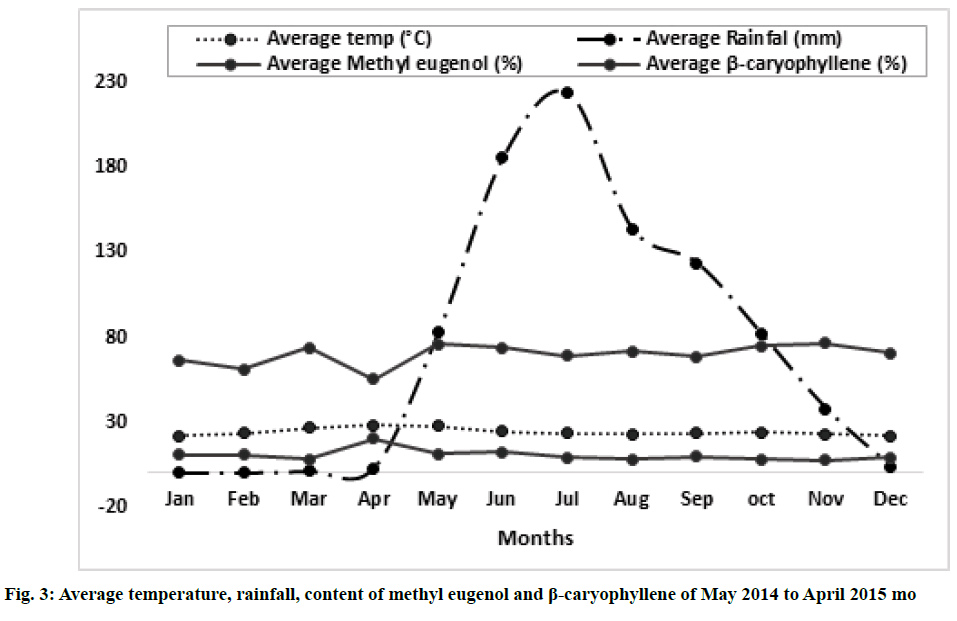
Variation of essential oil content was identified. The chemical composition of the leaves essential oil of O. sanctum at different months of the studied year (May 2014 to April 2015) was analyzed by GC-MS according to their mass spectra and their relative retention indices determined on a non-polar, stationary phase capillary column. The percentage of each compound in three replicates is calculated as mean±standard error of mean (SEM) and represented in Table 2. 23 to 39 constituents comprising 95.39±0.38 % of the total oil were identified. The main compound was identified as methyl eugenol (69.88±0.09 %). The highest content of methyl eugenol (76.78±0.54 %) was observed in November (fig. 1) and the lowest in April (fig. 2). The second major compound identified as β-caryophyllene (10.40±0.06 %) with its highest level in April (20.32±0.20 %) and lowest content in November (7.67±0.23 %). The highest variation was observed in two major compounds viz., methyl eugenol and β-caryophyllene. During April, the quantity of methyl eugenol was decreased (55.48±0.36 %) and β-caryophyllene was increased (20.32±0.20 %) and in contrast, methyl eugenol had its greatest values in November (76.78±0.54 µ) and again this month the lowest level of β-caryophyllene (7.67±0.23 %) was observed). Apart from April and November, the levels of methyl eugenol and β-caryophyllene were observed more than 60 % and 8 %, respectively. It was observed that environmental factors such as temperature and rainfall (fig. 3) could not influence much in the chemical composition of the essential oil of O. sanctum except in April. The other constituents detected in fluctuating quantity throughout the examined period are shown in Table 2. The essential oils collected throughout a year were found to be phenylpropanoids rich constituents (71.98±0.27 %), followed by sesquiterpene hydrocarbons (17.89±0.20 %), oxygenated monoterpenes (2.54±0.04 %), oxygenated sesquiterpenes (1.64±0.0 %) and monoterpene hydrocarbons (0.56±0.02 %) (Table 2).
In the present study, significant variation was observed in taking consideration of two compounds viz., methyl eugenol and β-caryophyllene. In the month of April it was noticed that the plant was fully in ripened conditions with matured seeds, it may be because of the low level of methyl eugenol and higher content of β-caryophyllene. Methyl eugenol is a phenylpropene and phenylpropanoids contribute to all aspects of plant responses towards biotic and abiotic stimuli. They are not only indicators of plant stress responses upon variation of light or mineral treatment but were also key mediators of the plants resistance towards pests [40]. The decreasing amount of methyl eugenol could be due to the elevation of temperature as stress. On the other hand, sesquiterpenes are biogenetically derived from E, E-Farnesyl pyrophosphate following an initial cyclization and subsequent oxidative modifications [41]. According to Chen et al. [41], the accumulation patterns of sesquiterpenes were strongly influenced by the development process and the highest amount during the maturity of the plant. A similar pattern was observed in April during this study when the content of phenylpropanoid decreased and sesquiterpenes increased.
This study revealed that a high fluctuation of the quantity of methyl eugenol and β-caryophyllene was observed in April and May. The remaining months show somehow constant production of the major compounds methyl eugenol and β-caryophyllene and other minor compounds of the leaves of the plant O. sanctum grown in the Belagavi and nearby areas. Environmental conditions like temperature and rainfall did not much affect the production of essential oil constituents.
Acknowledgements:
The authors are grateful to the Indian Council of Medical Research (ICMR), New Delhi, India for providing the necessary facilities and thankful to Mr. Mahesh B. Wagharwadi, Multi-Tasking Staff (MTS), Department of Phytochemistry, ICMR-NITM, Belagavi for the collection, processing and extraction of the oil.
Conflicts of interest:
The authors declared no conflict of interest.
References
- Pandey BP. Economic Botany. S. Chand Publishing, Chand and Company Ltd.: New Delhi; 1990.
- Yadav SR, Sardesai MM. Flora of Kolhapur District. Rajhuns Printing Press, Kolhapur; 2002.
- Shasany AK. The Holy basil (Ocimum sanctum L.) and its genome. Indian J Hist Sci 2016;51(2):343-50.
- Prakash PA, Gupta N. Therapeutic uses of Ocimum sanctum Linn. (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol 2005;49(2):125-31.
- Bhasin M. Ocimum-Taxonomy, medicinal potentialities and economic value of essential oil. J Biosph 2012;1:48-50.
- Manaharan T, Thirugnanasampandan R, Jayakumar R, Kanthimathi MS, Ramya G, Ramnath MG. Purified essential oil from Ocimum sanctum Linn. triggers the apoptotic mechanism in human breast cancer cells. Pharmacogn Mag 2016;12:S327-31.
- Devi PU. Radioprotective, anticarcinogenic and antioxidant properties of the Indian holy basil, Ocimum sanctum (Tulasi). Indian J Exp Biol 2001;39:185-190.
- Baliga MS, Rao S, Rai MP, D'souza P. Radio protective effects of the Ayurvedic medicinal plant Ocimum sanctum Linn. (Holy Basil): a memoir. J Cancer Res Ther 2016;12(1):20-7.
- Joshi RK. Chemical composition, in vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Indian J Pharm Sci 2013;75(4):457.
- Zaidi KU, Shah F, Parmar R, Thawani V. Anticandidal synergistic activity of Ocimum sanctum and fluconazole of azole resistance strains of clinical isolates. J Mycol Med 2018;28(2):289-93.
- Bhalla G, Kaur S, Kaur J, Kaur R, Raina P. Antileishmanial and immunomodulatory potential of Ocimum sanctum Linn. and Cocos nucifera Linn. in murine visceral leishmaniasis. J Parasit Dis 2017;41(1):76-85.
- Mukherjee R, Dash PK, Ram GC. Immunotherapeutic potential of Ocimum sanctum (L) in bovine subclinical mastitis. Res Vet Sci 2005;79(1):37-43.
- Ahirwar P, Shashikiran ND, Sundarraj RK, Singhla S, Thakur RA, Maran S. A clinical trial comparing antimicrobial efficacy of “essential oil of Ocimum sanctum” with triple antibiotic paste as an intracanal medicament in primary molars. J Indian Soc Pedod Prev Dent 2018;36(2):191-7.
- Poli V, Challa C. A comparative study of eugenol and Ocimum sanctum Linn. leaf extract on the antifertility effect in female albino rats. J Chin Med Assoc 2019;82(3):231-4.
- Singh S, Majumdar DK. Evaluation of antiinflammatory activity of fatty acids of Ocimum sanctum fixed oil. Indian J Exp Biol 1997;35(4):380-3.
- Hening P, Auriva MB, Wijayanti N, Kusindarta DL, Wihadmadyatami H. The neuroprotective effect of Ocimum sanctum Linn. ethanolic extract on human embryonic kidney-293 cells as in vitro model of neurodegenerative disease. Vet World 2018;11(9):1237-43.
- Brophy JJ, Goldsack RJ, Clarkson JR. The essential oil of Ocimum tenuiflorum L. (Lamiaceae) growing in Northern Australia. J Essent Oil Res 1993;5(4):459-61.
- Machado MI, de Vasconcelos Silva MG, Matos FJ, Craveiro AA, Alencar JW. Volatile constituents from leaves and inflorescence oil of Ocimum tenuiflorum L. f. (syn. O. sanctum L.) grown in Northeastern Brazil. J Essent Oil Res 1999;11(3):324-6.
- Pino JA, Rosado A, Rodriguez M, Garcia D. Composition of the essential oil of Ocimum tenuiflorum L. grown in Cuba. J Essent Oil Res 1998;10(4):437-8.
- Joshi RK, Hoti SL. Chemical composition of the essential oil of Ocimum tenuiflorum L. (Krishna Tulsi) from North West Karnataka, India. Plant Sci Today 2014;1(3):99-102.
- Bhattacharya AK, Kaul PN, Rajeswara Rao BR. Essential oils of Ocimum gratissimum L. and Ocimum tenuiflorum L.(syn. Ocimum sanctum L.) grown in Andhra Pradesh. Indian Perfumer 1996;40(3):73-5.
- Kothari SK, Bhattacharya AK, Ramesh S, Garg SN, Khanuja SP. Volatile constituents in oil from different plant parts of methyl eugenol-rich Ocimum tenuiflorum Lf (syn. O. sanctum L.) grown in South India. J Essent Oil Res 2005;17(6):656-8.
- Awasthi PK, Dixit SC. Chemical Compositions of Ocimum sanctum Shyama and Ocimum sanctum Rama Oils from the Plains of Northern India. J Essent Oil Bear Plants 2007;10(4):292-6.
- Khan A, Ahmad A, Akhtar F, Yousuf S, Xess I, Khan LA, et al. Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res Microbiol 2010;161(10):816-23.
- Gbolade AA, Lockwood GB. Toxicity of Ocimum sanctum L. essential oil to Aedes aegypti larvae and its chemical composition. J Essent Oil Bear Plants 2008;11(2):148-53.
- Kicel A, Kurowska A, Kalemba D. Composition of the essential oil of Ocimum sanctum L. grown in Poland during vegetation. J Essent Oil Res 2005;17(2):217-9.
- Sims CA, Juliani HR, Mentreddy SR, Simon JE. Essential oils in holy basil (Ocimum tenuiflorum L.) as influenced by planting dates and harvest times in North Alabama. J Med Act Plants 2014;2(3):33-41.
- HeroAbadi F, Milani Kalkhorani N, Rezaee MB. Essential oil Analysis of Fresh Aerial part of Iranian Ocimum sanctum L. by Hydro & Steam Distillation. J Med Plants By-product 2014;3(2):171-5.
- Vani SR, Cheng SF, Chuah CH. Comparative study of volatile compounds from genus Ocimum. Am J Appl Sci 2009;6(3):523-8.
- Zheljazkov VD, Cantrell CL, Tekwani B, Khan SI. Content, composition and bioactivity of the essential oils of three basil genotypes as a function of harvesting. J Agric Food Chem 2008;56(2):380-5.
- Stefan M, Zamfirache MM, Padurariu C, Truta E, Gostin I. The composition and antibacterial activity of essential oils in three Ocimum species growing in Romania. Cent Eur J Biol 2013;8(6):600-8.
- Joshi RK. Volatile constituents of Emilia sonchifolia from India. Nat Prod Commun 2018;13(10): 1355-6.
- Joshi RK. Chemical composition of Blumea virens roots from India. Chem Nat Compd 2018;54(3):584-5.
- Sharma S, Rasal VP, Joshi RK, Patil PA. In vivo evaluation of antiasthmatic activity of the essential oil of Zanthoxylum armatum. Indian J Pharm Sci 2018;80(2):383-90.
- Joshi RK. Terpenoids of Blumea oxyodonta essential oil. Chem Nat Compd 2018;54(2):377-9.
- Sharma S, Rasal VP, Patil PA, Joshi RK. Mentha arvensis essential oil suppressed airway changes induced by histamine and ovalbumin in experimental animals. Nat Prod Res 2018;32(4):468-72.
- Joshi RK. Chemical disparity in the oil from leaves of Cinnamomum zeylanicum Blume. Flavour Fragr J 2019;34(6):443-9.
- Joshi RK. Leucas aspera (Willd.) Link Essential oil from India: β-caryophyllene and 1-octen-3-ol chemotypes. J Chromatogr Sci 2016;54(3):295-8.
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, IL: Allured publishing corporation; 2007.
- La Camera S, Gouzerh G, Dhondt S, Hoffmann L, Fritig B, Legrand M, et al. Metabolic reprogramming in plant innate immunity: the contributions of phenylpropanoid and oxylipin pathways. Immunol Rev 2004;198(1):267-84.
- Chen Y, Wu YG, Xu Y, Zhang JF, Song XQ, Zhu GP, et al.Dynamic accumulation of sesquiterpenes in essential oil of Pogostemon cablin. Rev Bras Farmacogn 2014;24:626-34.