- *Corresponding Author:
- B. N. Suhagia
Department of Quality Assurance, L. M. College of Pharmacy, Navrangpura, Ahmedabad–380 009, India
E-mail: patelhary@rediffmail.com
| Date of Submission | 13 January 2005 |
| Date of Revision | 24 May 2005 |
| Date of Acceptance | 16 March 2006 |
| Indian J Pharm Sci., 2006, 68 (2): 242-245 |
Abstract
A simple and sensitive spectrophotometric method has been developed for determination of azithromycin in its pharmaceutical dosage forms. In the proposed method, azithromycin is oxidized with potassium permanganate to liberate formaldehyde, which is determined in situ using acetyl acetone, in the presence of ammonium acetate. A yellow coloured chromogen was obtained, having an absorption maxima at 412 nm. The method is found to be linear in the concentration range of 10-75 µg/ml, with regression coefficient of 0.9978. Various reaction parameters such as concentration of potassium permanganate and reagent, time required for oxidation, and maximum colour intensity were optimized. The method was validated, and can be used successfully to assay azithromycin in its pharmaceutical dosage forms viz. tablets, capsules, and injections.
Azithromycin [1] is a macrolide antibiotic which acts on Gram positive bacteria and Gram negative bacteria. Chemically2-3 , it is (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-2-ethyl-3,4,10-trihydroxy-3, 5, 6, 8, 10, 12, 14 –heptamethyl - 11 - [[3, 4, 6 – trideoxy - 3 - (dimethylamino)-b-D-xylo – hexo pyranosyl] oxy]-1-oxa-6azacyclopentadecan-15-one dihydrate. It is used in respiratory tract infections [2] like pharyngitis, pneumonia, chronic bronchitis, and bronchopneumonia. The recommended dosage for azithromycin is 100-500 mg per day. Azithromycin [3] is official in the United States Pharmacopoeia, and it is assayed by the high performance liquid chromatographic method. Literature survey reveals that azithromycin is estimated in pharmaceuticals and biological fluids by spectrophotometric [4], HPLC [5-9], and microbiological methods [10].
In the present investigation, an attempt has been made to develop a simple, accurate, and reproducible spectrophotometric method for estimation of azithromycin in pharmaceutical formulations. In the proposed method, azithromycin is oxidized with potassium permanganate (excess potassium permanganate is decolourized with oxalic acid) to liberate formaldehyde. The librated Formaldehyde is determined in situ, using acetyl acetone, in the presence of ammonium acetate, which gives a yellow coloured chromogen with absorption maxima at 412 nm. The proposed method was successfully applied for determination of azithromycin in its pharmaceutical formulations.
A double beam Shimadzu 160A UV/vis spectrophotometer having two matched quartz cells, with 1 cm light path, was employed for spectral measurement. A thermostatically controlled water bath (Remi Instruments, Mumbai) was used to control temperature of the reaction mixture. Azithromycin BP working standard was procured as a gift sample from Torrent Pharmaceuticals Ltd., Ahmedabad. Acetyl acetone (freshly distilled, ExcelaR), ammonium acetate, formaldehyde solution, glacial acetic acid, and oxalic acid, were all procured from S. D. Fine Chem. Pvt. Ltd., Mumbai. Double distilled water was used throughout the investigation.
Ammonium acetate- acetyl acetone reagent was prepared by dissolving ammonium acetate (30 g) in water (50 ml). Acetyl acetone solution (1.0 ml) was added, and the final volume was adjusted to 100 ml with water, and stored in a refrigerator. Freshly prepared reagent was used in the study.
Azithromycin (250 mg) was accurately weighed and transferred to a 100 ml volumetric flask. It was dissolved in glacial acetic acid (20 ml, 3 M), and diluted to 100 ml with distilled water. An aliquot (5.0 ml), was further diluted with water in 50 ml volumetric flask, to obtain the final concentration of 250 μg/ml.
In a 10 ml volumetric flask, standard azithromycin solution
(2.0 ml) and glacial acetic acid solution (1.0 ml) were pipetted successively. Potassium permanganate solution
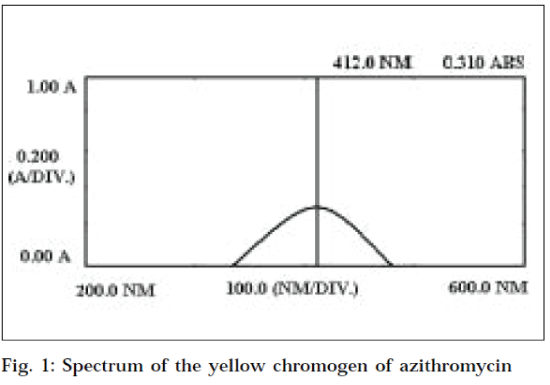
(0.25 % w/v, 0.025 ml) was added. The reaction flask was heated on a water bath at 37° for 10 min. Excess of potassium permanganate was neutralized with oxalic acid (10 % w/v). The reagent solution (2.0 ml) was added to it, and mixed thoroughly. The reaction flask was heated on a water bath at 37º for 1 min, cooled, and the volume was adjusted upto the mark with distilled water. Absorbance of the coloured solution was scanned on Shimadzu UVvisible spectrophotometer from 600 nm to 200 nm, against reagent blank. Maximum absorbance was obtained at 412 nm (Fig. 1).
Standard solution of azithromycin (0.4, 0.8, 1.0, 2.0, 3.0 ml, 250 μg/ml) was pipetted out into a series of 10 ml volumetric flasks, colour was developed as described above, and absorbance was measured at 412 nm. The Beer’s law is obeyed in the concentration range of 10-75 μg/ml of azithromycin.
Twenty tablets/capsules were weighed accurately, and powdered/emptied. The powder equivalent to 25 mg azithromycin was dissolved in glacial acetic acid (20 ml, 3 M), sonicated for 15 min, and filtered through Whatman No. 41 filter paper. The residues were washed thoroughly with distilled water. The filtrate and washing were combined in a 100 ml volumetric flask, and diluted to mark with the same solvent, to produce the final concentration of 250 μg/ml. The solution (2.0 ml) was analyzed as above. The amount of azithromycin was computed from the calibration curve (Table 1).
| Parameters | Values |
|---|---|
| Wavelength for measurement (nm) | 412 |
| Beer’s Law limit (µg/ml) | 10-75 |
| Molar absoptivity(lit/mole·cm) | 8.7184×103 |
| Sandell’s sensitivity(µg/ml/cm2/0.001 abs. unit) | 8.60×10-2 |
| Regression equation (Ya) | |
| Slope (b) | 0.0100 |
| Intercept (a) | 0.0233 |
| Correlation coefficient (r)b | 0.9978 |
| Precision | |
| Intra day precision (%)b | 0.70-2.85 |
| Inter day precision (%)b | 1.04-3.2 |
| Relative standard deviation (%)c | 0.1180 |
| Recovery (%) | 98.27-101.71 |
ameans Y=a+bC, where ‘C’ is concentration in μg/ml and Y is absorbance unit, bmeans five replicate samples, c mean five replicate samples.
Table 1: Optical characteristics of the proposed method
Injection powder, equivalent to 25 mg of azithromycin was accurately weighed and dissolved in glacial acetic acid (20 ml, 3 M), sonicated for 15 min, filtered through Whatman No. 41 filter paper, and analyzed as above. The amount of azithromycin was computed from the calibration curve (Table 1).
It was known that alpha-amino alcohol, in which the amine group is primary or secondary, liberates formaldehyde on periodate oxidation.11 The liberated formaldehyde produced yellow coloured chromogen, 3, 5- diacetyl-1, 4dihydrolutidine, on reaction with acetyl acetoneammonium acetate reagent. The coloured solution exhibits a wavelength of maximum absorption at 412 nm. Azithromycin has number of hydroxyl moieties. Therefore, the above principle was used to analyze azithromycin in its dosage forms.
In the proposed method, azithromycin is oxidized with potassium permanganate (excess potassium permanganate is decolourized with oxalic acid), to liberate formaldehyde. The liberated formaldehyde was treated with acetyl acetone - ammonium acetate reagent. A yellow coloured chromogen was obtained, having maximum absorption at 412 nm (Fig. 1). The colour is found to be stable for at least 2 h.
In the proposed method, various parameters such as concentration of potassium permanganate and reagent, time required for oxidation, and maximum colour intensity were studied, and optimized to obtain maximum colour intensity. The optical characteristics of azithromycin such as Beer’s law limit, Sandell’s sensitivity, and molar extinction coefficient were determined. The linear regression equation for determination of azithromycin is y=0.010x+0.0233, with co-relation coefficient 0.9978. The RSD was found to be 0.12-3.20% (Table 1).
For recovery study, known amounts of pure drug was added to the previously analyzed pharmaceutical preparations, the mixtures were analyzed by proposed method, and the percent recovery was calculated, which was found to be 98.3-101.7% for azithromycin. The analysis was carried out in triplicate for three pharmaceutical dosage forms i.e. tablets, capsules, and injections. The results of analysis of pharmaceutical dosage forms are shown in Table 2. The good recovery confirmed the accuracy and the specificity of the proposed method, and the lack of interference from the common excipients, film coating materials and colorant/ preservatives, used in the manufacture of tablets/capsules/ injections. This method is particularly useful for routine in-process quality control for its pharmaceutical preparations i.e. tablets, capsules, and injections.
| Formulation | Label claim (mg)/ dosage form | % Amount found by proposed method Mean ± S.D.d | % Recovery by proposed methodd |
|---|---|---|---|
| Tablet -1 | 250 | 97.28±0.25 | 100.37±0.95 |
| Tablet -2 | 100 | 98.92±0.78 | 101.71±0.93 |
| Capsule | 250 | 98.73±0.54 | 98.27±0.88 |
| Injection | 500 | 101.70±0.62 | 100.41±1.22 |
dmeans three replicate samples, Tablet-1 stands for tablet of Bal pharma Ltd., Mumbai (brand name-Aziwin, strength-250 mg), tablet-2 stands for tablet of Protec Ltd., Mumbai (brand name-Azee, strength-100 mg) and capsule stands for capsule of Sarabhai Chemicals Ltd., Vadodara (brand name-Azisara, strength-250 mg) and injection stands for injection of Alembic Chem. Works, Vadodara (brand name-Azithral, strength-500 mg).
Table 2: Analysis of pharmaceutical formulations
Acknowledgements
The authors are thankful to Torrent Pharmaceuticals Ltd., Ahmedabad for supplying gift sample of azithromycin.
References
- Budavari, S. Eds., In; The Merck Index, 12th Edn., Merck and Co. Inc., Whitehouse Station, N.J.,1996, 948.
- Reynolds, J.E.F., Eds., In; Martindale: The Extra Pharmacopoeia, 32th Edn., The Pharmaceutical Press, London, 1999, 155.
- United States Pharmacopoeia, XXIV, NF XIX, The USP convention, Inc, Rockville, MD, 2000, 185.
- Sivasubramanian, L., Mervin, M.A., Jayashankar, L., Ramu, P. and Raja, T.K., Indian J. Pharm. Sci., 2004, 66, 249.
- Guan Q.M., Li, L.X. and Zhang, Q.L., YaowuFenxiZahi, 1998, 18, 300.
- Ghone, A.K., Mehendra, R.P. and Tipis, H.P., Indian Drugs, 2000, 32, 65.
- Fouda, H.G. and Schneider, R.P., Ther. Drug Monit., 1995, 17, 179.
- Reidel, K.D., Wildreuer, A. and Zimmermann, T., J. Chromatogr., 1992, 576, 358.
- Shedpard, R.M., Duthu, G.S. and Ferraina, R.A., J. Chromatogr.Biomed. Appl., 1991, 565, 321.
- Girard, A.E., Girard, D. and English, A.R., Antimicrob. AgentsChemother.,1987, 31, 1948.
- Micolet, B.H. and Shinn, L.A., J. Amer. Chem. Soc., 1939, 61, 1614.