K. Sirisha1, G. Achaiah2 and A. Raghu Ram Rao2*
1Medicinal Chemistry Research Division, Vaagdevi College of Pharmacy, Ramnagar, Warangal-506 001, India
2Medicinal Chemistry Research Division, University College of Pharmaceutical Sciences, Kakatiya University, Warangal-506 009, India
- *Corresponding Author:
- A. Raghu Ram Rao
Medicinal Chemistry Research Division, University College of Pharmaceutical Sciences, Kakatiya University, Warangal-506 009, India
E-mail: raghumed@kakatiya.ac.in
| Date of Submission | 06 January 2014 |
| Date of Decision | 19 September 2014 |
| Date of Acceptance | 21 September 2014 |
| Indian J Pharm Sci 2014;76(6):519-528 |
§Part of the work presented at “Challenges in Bioorganic and Organic Medicinal Chemistry: 13th Tetrahedron Symposium”, held at Amsterdam June 26-29, 2012, Abstr. No. P3. 62.
DOI: 10.4103/0250-474X.147237
Abstract
A series of new 10-(alkylamino)-8-methyl-2,6-dihydroimidazo[1,2-c]pyrimido[5,4-e]pyrimidine-5(3H)-thiones (4a-g) were subjected to molecular property prediction (drug-likeness, lipophilicity and solubility parameters) using Osiris Property Explorer, ALOGPS 2.1, Molinspiration and ACD/Chemsketch 12.0 software programmes. The calculated drug-related properties of the designed molecules were similar to those found in most marketed drugs. Amongst the proposed analogues, four promising candidates were chosen (4a-d) for synthesis on the basis of Lipinski's 'Rule of Five' and drug-likeness scores. The significant biological activity of the test compounds in two in vitro modes (isolated guinea pig tracheal chain preparation, isolated guinea pig ileum) supports the promise and accuracy of the prediction. Among them, 4a was the most potent antihistaminic (IC 50 value of 30.2 μM; standard, chlorpheniramine maleate showed an IC 50 of 14.1 μM).
Keywords
Antihistaminic, asthma, bronchodilator, drug-likeness, pyrimidine
Asthma is primarily a complex, chronic inflammatory disease of the airways, which affects more than 300 million people worldwide, making it a serious global health problem [1]. It is characterized by recurrent reverse obstruction of the airways, airway inflammation and bronchial hyper responsiveness to stimuli which are not in themselves noxious and which do not affect non-asthmatic subjects [2]. Bronchial asthma results from a complex interplay between genetic and environmental factors [3]. An asthmatic subject suffers from intermittent attacks of dyspnoea (difficulty in breathing), wheezing and cough. Epidemiological studies show that its occurrence has been on a rise in children and young adults since early 1970’s, with the greatest incidence in more industrialized and urbanized societies, worldwide [4,5]. Nearly 7-10% of the world population suffers from bronchial asthma and India alone has an estimated burden of more than 15 million patients [6].
Currently bronchodilators (anticholinergics, selective β2-adrenergic agonists, methylxanthines, antihistaminics) and antiinflammatory agents (inhaled steroids, leukotriene antagonists, and mast cell stabilizers) are used in the treatment of asthma [7]. Despite the availability of a wide range of drugs, asthma control is poor globally and especially in developing countries due to the under utilization of these agents in view of their cost and adverse drug reaction (ADR) profiles [8-10]. Moreover, the relief offered by these drugs is not only symptomatic but short-lived. Poorly controlled asthma is associated with significant morbidity, mortality and consequent socioeconomic issues [11]. Hence, there is a dire need to search for new, effective and safer remedies to treat bronchial asthma.
While reviewing the recent perspectives in the design of antiasthmatic agents, we observed that different angularly fused heterocyclic ring systems like imidazoquinolines, imidazonaphthyridines, thienopyrimidines, triazolothienopyrimidines, benzimidazoloquinazolines, imidazoquinazolines, b e n z i m i d a z o l o p y r i d o p y r i m i d i n e s , imidazothienopyrimidines and triazinoquinazolines are potentially useful compounds [12,13]. Among them, pyrimidopyrimidines, being annelated uracils, attracted considerable interest in recent years. Numerous reports have been patented [14] and have delineated the antiallergic, antiviral, antibacterial, antioxidant and hepatoprotective properties of fused pyrimidines [15,16]. Furthermore, imidazo [1,2-c]pyrimidines are also important structural moieties which are known to exhibit antiinflammatory, antiallergic, bronchodilatory, antimycobacterial and antiviral activities [17,18]. Thus, with an effort to capitalize the pharmacological potential of the above heterocyclic nuclei and to synthesize biologically potent compounds, an attempt has been made to bring such important molecular moieties into a single molecular framework and evaluate them for their possible bronchodilatory and H1-antihistaminic activities, and this communication is a part of such continued efforts.
In modern drug discovery the potential of a new compound is often investigated initially with virtual tools. The likelihood of a compound to exhibit useful therapeutic activity (sometimes called its ‘drug-likeness’) is predicted from its molecular structure [19,20]. Prediction of bioavailability and bioavailability related properties, such as solubility, lipophilicity are important before actual synthesis. This could be the best way in order to avoid wastage of expensive chemicals, precious time and above all the likely environmental issues. In the present investigation, seven analogues were designed (4a-g) and were subjected to drug-like molecular properties prediction using Osiris Property Explorer [21], ALOGPS 2.1 [22], Molinspiration [23] and ACD/Chemsketch 12.0 [24] softwares to select the compounds for further synthesis and pharmacological evaluation.
Materials and Methods
Drug likeness and molecular property prediction
The drug-related properties for some blockbuster drugs [25] and newly designed compounds 4a-g were calculated by Molinspiration, Osiris Property Explorer, ALOGPs 2.1 and ACD/Chemsketch software programmes. Molinspiration was used to find the number of rotatable bonds, H-bond acceptors, H-bond donors and PSA. Molar refractivity was calculated using ACD/Chemsketch software. Lipophilicity (log P), solubility (log S), molecular weight, toxicity risks (mutagenicity, tumorigenicity, irritating effects, reproductive effects) and the values of drug-likeness and drug score were calculated by Osiris. ALOGPs predicted similar values for log P and log S as Osiris.
Chemistry
Melting points were determined in open capillaries using Toshniwal electrical melting point apparatus (Toshniwal Instruments, Amjer, India) and are uncorrected. IR spectra were recorded on a Perkin-Elmer FTIR 240-C spectrophotometer using KBr optics. 1H-NMR spectra were recorded in CDCl3 or DMSO-d6 with tetramethylsilane (TMS) as an internal standard on a Bruker 80 MHz FT-NMR (300 MHz) spectrometer (Bruker Bioscience, USA) and the chemical shifts are reported as δ (ppm). Mass spectra were recorded on a GC-MS QP-1100 Shimadzu instrument (70 eV; Shimadzu, Kyoto, Japan). Elemental analyses (C, H, N) of the compounds were obtained from Perkin Elmer 240B analyzer (Perkin Elmer, USA) and were within ±0.4 of the theoretical value. Reaction courses and product mixtures were routinely monitored by thin-layer chromatography (TLC) on precoated silica gel 60 F254< aluminum plates (Merck, Germany). Column chromatography was performed on silica gel (70–230 mesh ASTM, Merck).
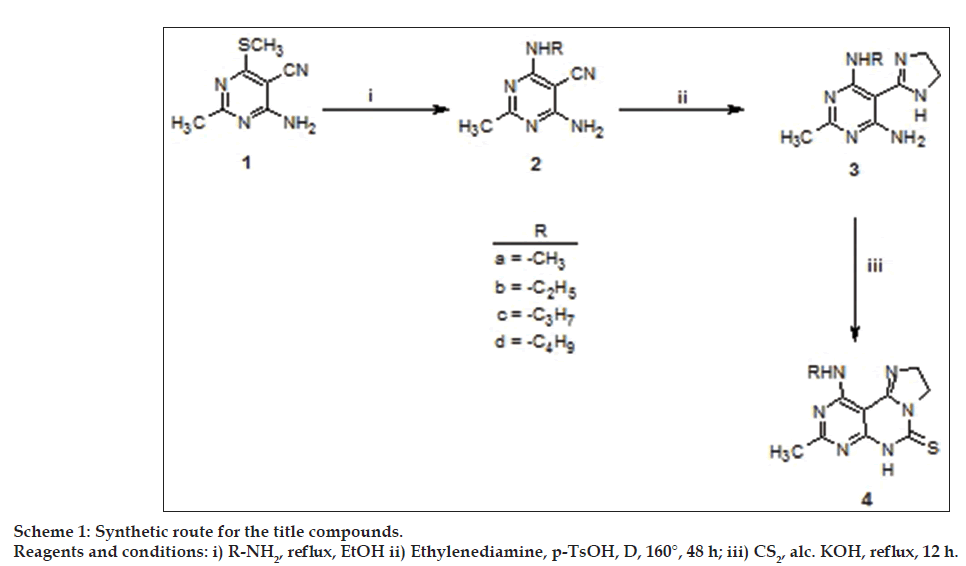
The synthetic route for the title compounds is shown in Scheme 1. The yields of compounds are not optimized. A reaction of malononitrile with carbondisulfide and dimethyl sulfate in the presence of potassium hydroxide and dimethyl formamide afforded di(methylthio) methylene malononitrile (92%) which when treated with acetamidine hydrochloride in the presence of sodium ethoxide, yielded 4-amino-2-methyl-6- (methylsulfanyl)pyrimidine-5-carbonitrile 1 in 64% yield (mp: 258-260°) [26].
General procedure for the synthesis of 6-(alkylamino)-4-amino-2-methylpyrimidine-5-carbonitriles (2a-d)
A mixture of 4-amino-2-methyl-6-(methylsulfanyl) pyrimidine-5-carbonitrile (1, 1.8 g, 0.01 mol) and 1 ml of 40% solution of an appropriate aliphatic amine in water were heated under reflux in 10 ml alcohol, until the evolution of methylmercaptan ceased, as detected with sodium nitroprusside solution (methylmercaptan turns filter paper dipped in sodium nitroprusside pink). The reaction mixture was cooled, solid thus obtained was filtered and washed with ice cold alcohol, dried and recrystallized from alcohol to yield compounds 2a-d, respectively.
4 - A m i n o - 2 - m e t h y l - 6 - ( m e t h y l a m i n o ) pyrimidine-5-carbonitrile (2a)
% Yield: 88; mp: 242-245°, IR (KBr) v (cm-1): 3373 (NH), 2221 (C≡N), 1683 (C=N). 1H NMR (CDCl3) δ (ppm): 2.13 (s, 3H, -CH3), 2.47 (d, 3H, J=5.4 Hz, -NHCH3), 3.71 (s, 2H, -NH2), 5.59 (s, 1H, -NHCH3). MS m/z: 163 [M+], Anal. calcd. (%) for C7H9N5: C, 51.52; H, 5.56; N, 42.92. Found: C, 51.49; H, 5.55; N, 42.95.
4-Amino-6-(ethylamino)-2-methylpyrimidine-5- carbonitrile (2b)
% Yield: 85; mp: 247-249°, IR (KBr) v (cm-1): 3345 (NH), 2289 (C≡N), 1677 (C=N). 1H NMR (CDCl3) δ (ppm): 2.10 (s, 3H, - CH3), 2.39 (t, 3H, J=7.1 Hz, - NHCH2CH3), 2.52 (m, 2H, - NHCH2CH3), 3.65 (s, 2H, -NH2), 5.49 (s, 1H, -NHCH2-). MS m/z: 177 [M+], Anal. calcd. (%) for C8H11N5: C, 54.22; H, 6.26; N, 39.52. Found: C, 54.25; H, 6.24; N, 39.56.
4 - A m i n o - 2 - m e t h y l - 6 - ( p r o p y l a m i n o ) pyrimidine-5-carbonitrile (2c)
% Yield: 79; mp: 250-252°, IR (KBr) v (cm-1): 3362 (NH), 2257 (C≡N), 1690 (C=N). 1H NMR (CDCl3) δ (ppm): 2.11 (s, 3H, -CH3), 2.21 (t, 3H, J=7.3 Hz, -NHCH2CH2CH3), 2.45 (m, 2H, -NHCH2CH2CH3), 2.54 (m, 2H, - NHCH2CH2CH3), 3.59 (s, 2H, -NH2), 5.36 (s, 1H, -NHCH2-). MS m/z: 191 [M+], Anal. calcd. (%) for C9H13N5: C, 56.53; H, 6.85; N, 36.62. Found: C, 56.55; H, 6.82; N, 36.58.
4-Amino-6-(butylamino)-2-methylpyrimidine-5-carbonitrile (2d)
% Yield: 75; mp: 255-256°, IR (KBr) v (cm-1): 3359 (NH), 2265 (C≡N), 1687 (C=N). 1H NMR (CDCl3) δ (ppm): 2.08 (s, 3H, -CH3), 2.15 (t, 3H, J=7.35 Hz, -NHCH2CH2CH2CH3), 2.38 (m, 4H, -NHCH2CH2CH2CH3), 2.48 (m, 2H, -NHCH2CH2CH2CH3), 3.42 (s, 2H, -NH2), 5.32 (s, 1H, -NHCH2-). MS m/z: 205 [M+], Anal. calcd. (%) for C10H15N5: C, 58.51; H, 7.37; N, 34.12. Found: C, 58.48; H, 7.34; N, 34.16.
General procedure for the synthesis of N-alkyl-5- (4,5-dihydro-1H-imidazol-2-yl)-2-methylpyrimidine-4,6-diamine (3a-d)
An equimolar mixture of compound 2a (0.02 mol) and ethylene diamine (0.02 mol) in p-toluenesulfonic acid (0.22 mol) was heated under reflux at 160° for 48 h. The reaction mixture was made alkaline with an aqueous sodium carbonate solution (10%) and extracted with chloroform. Evaporation of the solvent gave a residue, which was purified by column chromatography to afford compound 3a. Compounds 3b-d were prepared similarly.
5 - ( 4 , 5 - D i h y d r o - 1H- i m i d a z o l - 2 - y l ) -N, 2 - dimethylpyrimidine-4,6-diamine (3a)
% Yield: 64, mp: 272-274°, IR (KBr) v (cm-1): 3391 (NH), 2924 (CH), 1682 (C=N). 1H NMR (DMSO) δ (ppm): 1.81 (s, 3H, CH3), 2.06 (d, 3H, J=5.1 Hz, -NHCH3), 2.48 (t, 4H, J=6.9 Hz, -NCH2CH2N-), 3.07 (s, 1H, -NH), 3.57 (s, 1H, -NHCH3), 3.88 (s, 2H, -NH2). MS m/z: 206 [M+], Anal. calcd. (%) for C9H14N6: C, 52.41; H, 6.84; N, 40.75. Found: C, 52.45; H, 6.79; N, 40.78.
5-(4,5-Dihydro-1H-imidazol-2-yl)-N-ethyl-2- methylpyrimidine-4,6-diamine (3b)
% Yield: 58, mp: 280-282°, IR (KBr) v (cm-1): 3387 (NH), 2945 (CH), 1666 (C=N). 1H NMR (DMSO) δ (ppm): 1.87 (s, 3H, -CH3), 1.99 (t, 3H, J=7.3 Hz, -NHCH2CH3), 2.45 (m, 2H, NHCH2CH3), 2.54 (m, 4H, -NCH2CH2N-), 3.05 (s, 1H, -NH), 3.51 (s, 1H, - NHCH2-), 3.76 (s, 2H, - NH2). MS m/z: 220 [M+], Anal. calcd. (%) for C10H16N6: C, 54.53; H, 7.32; N, 38.15. Found: C, 54.55; H, 7.29; N, 38.19.
5-(4,5-Dihydro-1H-imidazol-2-yl)-2-methyl-Npropylpyrimidine- 4,6-diamine (3c)
% Yield: 47, mp: 289-292°, IR (KBr) v (cm-1): 3395 (NH), 2958 (CH), 1674 (C=N). 1H NMR (DMSO) δ (ppm): 1.78 (s, 3H, -CH3), 1.92 (t, 3H, J=7.2 Hz, -NHCH2CH2CH3), 2.35 (m, 2H, -NHCH2CH2CH3), 2.48 (m, 2H, -NHCH2CH2CH3), 2.55 (m, 4H, -NCH2CH2N-), 2.98 (s, 1H, -NH), 3.45 (s, 1H, - NHCH2-), 3.65 (s, 2H, - NH2). MS m/z: 234 [M+], Anal. calcd. (%) for C11H18N6: C, 56.39; H, 7.74; N, 35.87. Found: C, 56.42; H, 7.72; N, 35.85.
N-Butyl-5-(4,5-dihydro-1H-imidazol-2-yl)-2- methylpyrimidine-4,6-diamine (3d)
% Yield: 50, mp: 296-298°, IR (KBr) v (cm-1): 3372 (NH), 2932 (CH), 1690 (C=N). 1H NMR (DMSO) δ (ppm): 1.80 (s, 3H, -CH3), 1.87 (t, 3H, J=7.3 Hz, -NHCH2CH2CH2CH3), 2.08 (m, 4H, -NHCH2CH2CH2CH3), 2.44 (m, 2H, -NHCH2CH2CH2CH3), 2.53 (m, 4H, -NCH2CH2N-), 2.89 (s, 1H, -NH), 3.27 (s, 1H, -NHCH2-), 3.45 (s, 2H, -NH2). MS m/z: 248 [M+], Anal. calcd. (%) for C12H20N6: C, 58.04; H, 8.12; N, 33.84. Found: C, 58.06; H, 8.15; N, 33.81.
General procedure for the synthesis of 10-(alkylamino)-8-methyl-2,6-dihydroimidazo [1,2-c] pyrimido [5,4-e]pyrimidine-5(3H)-thiones (4a-d)
5 - ( 4 , 5 - D i h y d r o - 1H- i m i d a z o l - 2 - y l ) - N , 2 - dimethylpyrimidine-4,6-diamine (3a) (0.01 mol) was heated under reflux with a mixture of alcoholic KOH solution (10 ml, 0.01 mol) and carbondisulfide (8 ml) for 12 h. Excess of carbondisulfide was distilled off and the residue was neutralized with dilute hydrochloric acid. The crude product of 4a so obtained was filtered, washed with water, dried and recrystallized from DMF. Compounds 4b, 4c and 4d were prepared similarly.
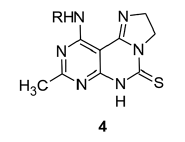
8 - M e t h y l - 1 0 - ( m e t h y l a m i n o ) - 2 , 6 - dihydroimidazo [1,2-c]pyrimido [5,4-e]pyrimidine- 5(3H)-thione (4a)
% Yield: 52, mp: 310° (decomp.), IR (KBr) v (cm-1): 3430 (NH), 2960 (CH), 1654 (C=N), 1141 (C=S). 1H NMR (DMSO) δ (ppm): 1.25 (s, 3H, CH3), 2.58 (d, 3H, J=5.6 Hz, -NHCH3), 3.48 and 3.95 (each t, 2H, J=10.7 Hz, -NCH2CH2N-), 4.50 (m, 1H, -NHCH3), 12.35 (bs, 1H, S=C-NH). MS m/z: 248 [M+], Anal. calcd. (%) for C10H12N6S: C, 48.37; H, 4.87; N, 33.85. Found: C, 48.41; H, 4.85; N, 33.82.
10-(Ethylamino)-8-methyl-2,6-dihydroimidazo [1,2-c] pyrimido [5,4-e]pyrimidine-5(3H)-thione (4b)
% Yield: 55, mp: 314° (decomp.), IR (KBr) v (cm-1): 3439 (NH), 2972 (CH), 1665 (C=N), 1145 (C=S). 1H NMR (DMSO) δ (ppm): 1.33 (s, 3H, CH3), 2.47 (t, 3H, J=6.9 Hz, -NHCH2CH3), 3.52 (m, 2H, - NHCH2CH3), 3.60 and 4.05 (each t, 2H, J=9.2 Hz, -NCH2CH2N-), 4.41 (s, 1H, -NHCH2-), 12.21 (bs, 1H, S=C-NH). MS m/z: 262 [M+], Anal. calcd. (%) for C11H14N6S: C, 50.36; H, 5.38; N, 32.05. Found: C, 50.4; H, 5.36; N, 32.02.
8 - M e t h y l - 1 0 - ( p r o p y l a m i n o ) - 2 , 6 - dihydroimidazo [1,2-c]pyrimido [5,4-e]pyrimidine- 5(3H)-thione (4c)
% Yield: 48, mp: 320° (decomp.), IR (KBr) v (cm-1): 3435 (NH), 2965 (CH), 1662 (C=N), 1151 (C=S). 1H NMR (DMSO) δ (ppm): 1.29 (s, 3H, CH3), 2.35 (t, 3H, J=7.3 Hz, -NHCH2CH2CH3), 2.51 (m, 2H, -NHCH2CH2CH3), 3.45 (m, 2H, -NHCH2CH2CH3), 3.54 and 4.08 (each t, 2H, J=10.4 Hz, -NCH2CH2N-), 4.35 (s, 1H, -NHCH2-), 12.4 (bs, 1H, S=C-NH). MS m/z: 276 [M+], Anal. calcd. (%) for C12H16N6S: C, 52.15; H, 5.84; N, 30.41. Found: C, 52.12; H, 5.87; N, 30.42.
10-(Butylamino)-8-methyl-2,6-dihydroimidazo [1,2-c] pyrimido [5,4-e]pyrimidine-5(3H)-thione (4d)
% Yield: 50, mp: 326° (decomp.), IR (KBr) v (cm-1): 3427 (NH), 2970 (CH), 1660 (C=N), 1137 (C=S). 1H NMR (DMSO) δ (ppm): 1.20 (s, 3H, CH3), 1.88 (t, 3H, J=7.1 Hz,-NHCH2CH2CH2CH3), 2.48 (m, 4H, -NHCH2CH2CH2CH3), 3.35 (m, 2H, -NHCH2CH2CH2CH3), 3.57 and 4.04 (each t, 2H, J=9.8 Hz,-NCH2CH2N-), 4.28 (s,1H, -NHCH2-), 12.31 (bs, 1H, S=C-NH). MS m/z: 290 [M+], Anal. calcd. (%) for C13H18N6S: C, 53.77; H, 6.25; N, 28.94. Found: C, 53.74; H, 6.28; N, 28.92.
Isolated guinea pig tracheal chain preparation
Albino guinea pigs of either sex weighing 250- 300 g were obtained from National Institute of Nutrition, Hyderabad and housed in wire-mesh cages maintained at 23±2°, 12 h light and dark cycles. The animals were allowed to acclimatize to the environment for 3-4 days and supplied with a standard pellet diet and water ad libitum. The experimental protocols were approved by the Institutional Animal Ethical Committee, Kakatiya University, Warangal, India.
Overnight fasted animals were sacrificed by a head blow and exsanguination. The trachea was excised and transferred to a petri dish containing oxygenated Krebs-Henseleit solution (KHS) consisting of in mM, NaCl, 113.0; KCl, 4.8; CaCl2, 2.5; NaHCO3, 25; KH2PO4, 1.2; MgSO4, 1.2; glucose 5.7 maintained at pH 7.4. (This buffer usually gives pH of 7.4 at 37° when bubbled with carbogen (95:5, oxygen: carbondioxide mixture).
It was cut transversely between the segments of the cartilage, so as to give a number of individual rings of trachea. About 5-6 of the rings were tied together in series with silk threads and mounted in an organ bath containing KHS, continuously aerated and maintained at 37±1°. One end of the tracheal chain was attached to an S-shaped aerator tube and other attached to an isotonic frontal writing lever to smoked drum. Tissue was allowed to equilibrate for 45 min under a load of 0.5 g, during which the bath solution was replaced every 5 min. Contact time of 30 s. and 15 min time cycle was followed for recording the response of histamine. The consecutive concentrations of histamine were added every 2 min (range 0.1–1000 μM); and the graph of percentage of maximum contractile response (Emax) on ordinate and logarithm of molar concentration of histamine on abscissa was plotted to record dose response curve of histamine, in absence and in presence of test compounds 4a-d (200 and 400 μg/ml) [27].
Isolated guinea pig ileum preparation
Overnight fasted guinea pig was sacrificed and ileum was mounted in an organ bath containing Tyrode solution (NaCl 8.0, KCl 0.2, CaCl2 0.2, MgCl2 0.1, NaHCO3 1.0, NaH2PO4 0.05 and glucose 1.0 g/l), which was continuously aerated and maintained at 37±1º. One end of ileum was attached to an S-shaped aerator tube and other attached to isotonic frontal writing lever to smoked drum. The tissue was allowed to equilibrate for 30 min under a load of 0.5 g. Contact time of 30 s and 15 min time cycle was followed for recording the response of histamine. After obtaining a dose response curve of histamine on ileum, the test compounds 4a-d (200 and 400 μg/ml) were added to the reservoir and same doses of histamine were repeated. Percent maximum contractile response (Emax) on ordinate and logarithm of molar concentration of histamine on abscissa was plotted to obtain dose response curves of histamine, in the absence and the presence of test compounds [28].
Results and Discussion
Molecular properties such as membrane permeability, hydrophobicity and bioavailability are associated with some basic molecular descriptors such as log P (partition coefficient), log S (solubility), molecular weight, number of hydrogen bond acceptors and donors in a molecule. Lipinski’s rule of five [29] is widely used as filter to estimate molecular drug-likeness. According to the rule, a drug like molecule has log P≤5, molecular weight <500 g/mol, hydrogen bond acceptors ≤10, hydrogen bond donors ≤5 and molar refractivity between 40-130. Molecules violating more than one of these rules are not expected to be viable drug candidates.
The solubility parameter, log S, is another important parameter for determining drug likeness. The absorption of a compound is considerably influenced by its solubility. Generally, high log S values correspond to good absorption. Over 80% of the marketed drugs have log S >-4, which corresponds to the solubility of 0.1 mmol/l [21,30]. Molecular polar surface area (PSA) is a very useful parameter for the prediction of drug transport properties (PSA must be ≤140 Å2). It is used to estimate the percentage of absorption using the expression %ABS=109- 0.345PSA [31]. PSA and volume are inversely proportional to %ABS. Number of rotatable bonds dictates the conformational changes of molecules under study, which consequently decides binding of receptors or channels. They should be ≤10 to have good oral bio availability.
Tables 1 and 2 show the calculated drug-related properties of some blockbuster drugs [25] and newly designed compounds 4a-g, respectively. All of them showed no predicted toxicity, overall drug scores ranging from 36 to 92%, and optimal values for all other drug-related estimated properties.
| Generic drug | Therapeutic use | MW LogPLogS HBA HBD | MR (cm3) | nrotb | PSA (Å2) | % ABS | Drug likeness | Drug score (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole | Antipsychotic antidepressant | 448 | 4.58 | −5.14 | 5 | 1 | 120.3±0.3 | 7 | 44.80 | 93.54 | 4.88 | 48 |
| Atorvastatin | Cholesterol regulator | 558 | 5.55 | −6.92 | 7 | 4 | 155.2±0.5 | 12 | 111.78 | 70.43 | 1.03 | 22 |
| Azelastine | Asthma, Allergy | 381 | 4.49 | −4.82 | 4 | 0 | 109.9±0.5 | 3 | 38.13 | 95.84 | 5.52 | 57 |
| Clopidrogel | Antiplatelet agent | 321 | 2.95 | −3.22 | 3 | 0 | 85.4±0.3 | 4 | 29.54 | 98.80 | 1.33 | 74 |
| Duloxetine | Antidepressant, anxiolytic | 297 | 4.22 | −4.4 | 2 | 1 | 91.1±0.3 | 6 | 21.26 | 101.6 | 2.35 | 51 |
| Esomeprazole | Antiulcerant | 345 | 2.48 | −2.72 | 6 | 1 | 94.0±0.4 | 5 | 77.11 | 82.39 | 1.52 | 51 |
| Fluticasone | Chronic asthma | 444 | 2.12 | −4.42 | 4 | 2 | 106.9±0.4 | 3 | 74.59 | 83.26 | 4.33 | 66 |
| Montelukast | Asthma, seasonal allergies | 586 | 8.63 | −8.53 | 4 | 2 | 173.7±0.3 | 12 | 70.41 | 84.70 | 0.77 | 14 |
| Quetiapine | Antipsychotic | 383 | 2.80 | −2.46 | 5 | 1 | 110.1±0.5 | 6 | 48.83 | 92.15 | 2.97 | 80 |
| Rosuvastatin | Cholesterol regulator | 481 | 2.29 | −5.73 | 9 | 3 | 119.9±0.4 | 10 | 140.91 | 60.38 | 3.45 | 49 |
| Salbutamol | Asthma, lung diseases | 239 | 0.92 | −1.43 | 4 | 4 | 67.7±0.3 | 5 | 72.71 | 83.81 | 5.80 | 96 |
| Salmeterol | Chronic asthma | 415 | 4.36 | −3.62 | 5 | 4 | 121.8±0.3 | 16 | 81.94 | 80.73 | −8.28 | 32 |
| Terbutaline | Asthma, chronic bronchitis | 225 | 1.24 | −1.25 | 4 | 4 | 63.1±0.3 | 4 | 72.71 | 83.81 | 6.95 | 58 |
MW: molecular weight, P: partition coefficient, S: solubility, HBA: hydrogen bond acceptors, HBD: hydrogen bond donors, MR: molar refractivity, nrotb: number of rotatable bonds, PSA: polar surface area, ABS: absorption
Table 1: Drug Related Property Profiles Of Some Blockbuster Drugs, Calculated By Molinspiration, Osiris Property Explorer, Alogps 2.1 And Acd/Chemsketch Softwares
 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compd. | R | MW | LogP | LogS | HBA | HBD | MR (cm3) | nrotb | PSA (Å2) | % ABS | Drug likeness | Drug score (%) |
| 4a | −CH3 | 248 | 1.14 | −2.16 | 6 | 2 | 67.16±0.5 | 1 | 54.24 | 90.29 | 3.34 | 92 |
| 4b | −C2H5 | 262 | 1.58 | −2.46 | 6 | 2 | 71.77±0.5 | 2 | 53.72 | 90.47 | 3.26 | 91 |
| 4c | −C3H7 | 276 | 2.04 | −2.73 | 6 | 2 | 76.38±0.5 | 3 | 53.94 | 90.39 | 3.28 | 88 |
| 4d | −C4H9 | 290 | 2.51 | −3.0 | 6 | 2 | 80.99±0.5 | 4 | 53.94 | 90.39 | 1.4 | 78 |
| 4e | −C5H11 | 304 | 2.97 | −3.27 | 6 | 2 | 85.60±0.5 | 5 | 53.94 | 90.39 | −2.04 | 46 |
| 4f | −C6H13 | 318 | 3.43 | −3.54 | 6 | 2 | 90.20±0.5 | 6 | 53.94 | 90.39 | −6.46 | 39 |
| 4g | −C7H15 | 332 | 3.9 | −3.81 | 6 | 2 | 94.81±0.5 | 7 | 53.94 | 90.39 | −11.6 | 36 |
MW: molecular weight, P: partition coefficient, S: solubility, HBA: hydrogen bond acceptors, HBD: hydrogen bond donors, MR: molar refractivity, nrotb: number of rotatable bonds, PSA: polar surface area, ABS: absorption
Table 2: Drug‑Related Properties Of The Newly Designed Compounds 4a‑G, Calculated By Molinspiration, Osiris Property Explorer, Alogps 2.1 And Acd/Chemsketch Softwares
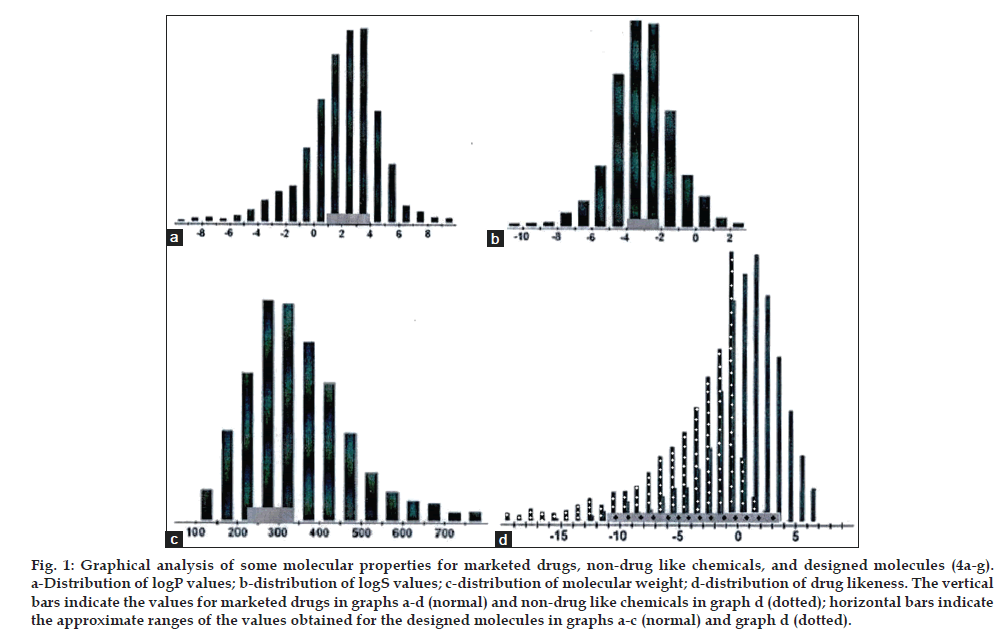
The values of log P ranged from 1.14 to 3.90 for all designed molecules, while the values of log S were between -2.16 and -3.81. Both of these set of values are well within the accepted ranges for drug like molecules, as described. The polar surface areas (PSA) of all 7 molecules are less than 55 Å2 (well below the “drug like” value of 140 Å2). The percentage of absorption (% ABS) calculated was found to be greater than 90 for all the molecules. They are small in size (molecular weights are less than 332 g/mol) also. All designed molecules have rotatable bonds between 1 and 7, 6 H-bond acceptors, and 2 H-bond donors. Compounds 4a-d showed positive drug-likeness values, ranging from 1.4 to 3.34 and compounds 4e-g gave negative values for drug-likeness between -2.04 and -11.60. Osiris calculations for 15000 nondrug like chemicals and 3300 marketed drugs found that about 80% of the marketed drugs had positive values of the drug likeness parameter, while almost all the nondrug like chemicals had negative values [21]. A positive value of drug likeness indicates that the molecule consists mostly of building blocks (fragments) that are commonly found in marketed drugs.
Fig. 1 shows the distributions of the logP, logS, molecular weight, and drug likeness values for marketed drugs, non-drug like chemicals, and compounds 4a-g designed in this study. Statistical relations (graphs) for marketed drugs and non-drug like chemicals were created using information from the Osiris Property Explorer website. The horizontal lines in the graphs indicate the approximate ranges of the values obtained for the designed molecules. As can be seen from fig. 1 the logP, logS, molecular weight, and drug likeness values for compounds 4a-g were comparable to those of the majority of marketed drugs. Maximum drug likeness values and drug scores were found to be 3.34 and 92% respectively for compound 4a. Next to it, compounds 4b-d were predicted to have drug likeness values between 3.26 and 1.4 and drug scores between 91 and 78%. Compounds 4e, 4f and 4g having pentyl, hexyl and heptyl substitution respectively on amino group in one of the pyrimidine moieties gave negative drug likeness values and low drug scores (<50%). Hence they could not be treated as potential candidates even though they complied with the Lipinski’s rule. It could be observed from the results that amongst the homologous series 4a-g, the drug likeness decreased with increase in bulkiness of the alkyl amino group in pyrimidine ring. On the basis of drug likeness model score, compounds 4a-d were predicted as potential therapeutic candidates and selected for synthesis.
Fig. 1: Graphical analysis of some molecular properties for marketed drugs, non-drug like chemicals, and designed molecules (4a-g). a-Distribution of logP values; b-distribution of logS values; c-distribution of molecular weight; d-distribution of drug likeness. The vertical bars indicate the values for marketed drugs in graphs a-d (normal) and non-drug like chemicals in graph d (dotted); horizontal bars indicate the approximate ranges of the values obtained for the designed molecules in graphs a-c (normal) and graph d (dotted).
Nucleophilic replacement of 6-methylthio moiety in 4-amino-2-methyl-6-(methylsulfanyl) pyrimidine- 5-carbonitrile 1 by different amines afforded the corresponding 6-alkylamino derivatives 2a-d (Scheme 1). The IR spectra of compound 2 showed characteristic band around 3350 cm-1 corresponding to the amino group. The incorporation of alkylamino group was further confirmed by the 1H NMR spectra with a singlet at around δ 5.45 ppm (exchangeable with D2O), corresponding to –NH group and by mass spectra.
Treatment of 6-(alkylamino)-4-amino-2- methylpyrimidine-5-carbonitriles 2a-d with ethylene diamine in presence of 4-toluene sulfonic acid, led to the formation of N-alkyl-5-(4,5-dihydro-1Himidazol- 2-yl)-2-methylpyrimidine-4,6-diamines 3a-d, respectively. Compound 3 was confirmed by the presence of a characteristic signal as multiplet around δ 2.52 ppm in 1H NMR spetrum, corresponding to the –CH2-CH2- of the dihydroimidazo ring system. Heating under reflux of compounds 3a-d with a mixture of alcoholic potassium hydroxide solution and carbon disulfide afforded the corresponding 10-(alkylamino)-8-methyl-2,6-dihydroimidazo [1,2-c] pyrimido [5,4-e]pyrimidine-5(3H)-thiones 4a-d. The title compounds 4 showed a band at around 3430 cm-1, corresponding to the amino group (IR) and the other IR signals were at around 2960 cm-1 (–CH stretch), 1650 cm-1 (–C=N stretch) and 1140 cm-1 (–C=S group). The 1H NMR spectrum showed a singlet at around δ 1.28 ppm corresponding to methyl protons at C-8. A signal appeared at around δ 2.35- 3.52 ppm corresponding to the alkyl protons at C-10. Also, two triplets appeared at around δ 3.55 ppm and δ 4.05 ppm, corresponding to >N–CH2- and =N-CH2- protons respectively, of the dihydroimidazo ring system. The aliphatic NH proton could be recorded at around δ 4.35 ppm and the cyclic NH proton could be recorded at around δ 12.35 ppm (D2O exchangeable). The mass spectra of all the compounds 4a-d have shown the molecular ion peaks [M+], which further confirmed their formation.
The purity of all the newly synthesized compounds was verified using TLC and elemental analysis (C, H, N). The structures of the synthesized compounds were assigned on the basis of spectral data. All the newly synthesized compounds were in full agreement with the proposed structures.
Compounds 4a-d were screened for their in vitro bronchodilatory activity on isolated guinea pig tracheal chain preparations [27]. Aminophylline (10 ng/ml) (Sigma Chemical Ltd. UK) was used as standard drug for comparison. They were also evaluated for in vitro H1-antihistaminic activity on isolated guinea pig ileum [28] using chlorpheniramine maleate (CPM) (10 nM) (Sigma Chemical Ltd. UK) as standard.
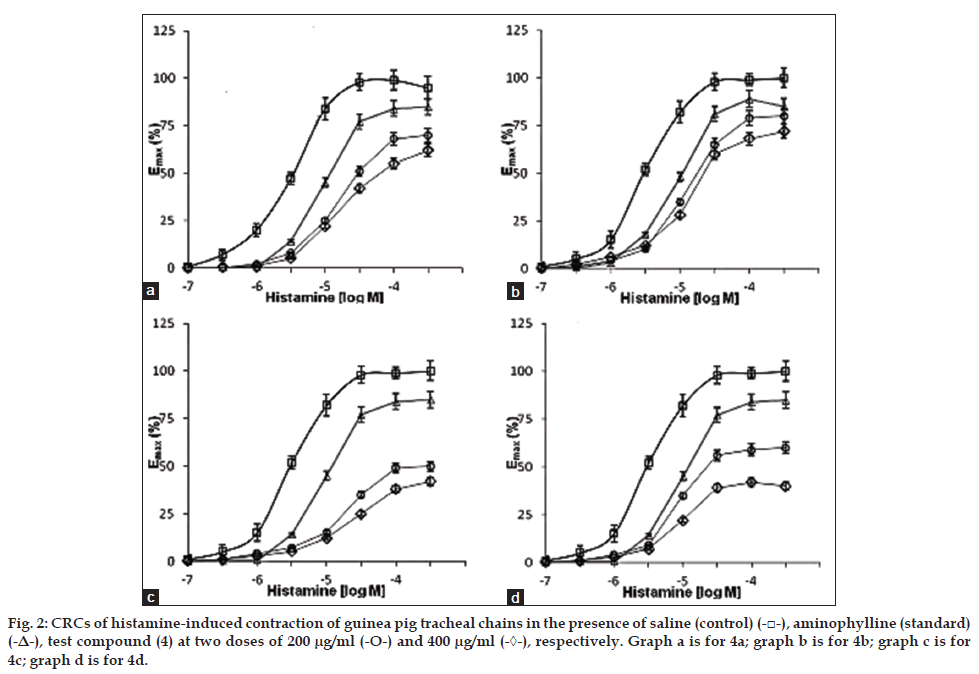
The inhibitory effect of the test compounds 4a-d on histamine H1 receptors was examined by producing the cumulative log concentration–response curve (CRC) of histamine acid phosphate (Hi-Media Laboratories Pvt. Ltd., India) induced contraction of guinea pig tracheal chains and ileum, 10 min after the exposure of tissue to each solutions: standard, two concentrations of test compounds 4a-d (200 and 400 μg/ml microsuspension in water), or saline (control). In both the experimental models, the CRCs of histamine obtained in the presence of both the concentrations of test compounds 4a-d and standard drugs, aminophylline and chlorpheniramine maleate showed clear rightward shift compared to control (figs. 2 and 3) indicating their antihistaminic activity. In the isolated guinea pig tracheal chain method, all the tested compounds 4a-d exhibited significant bronchodilatory activity, however less than the standard (aminophylline).
Fig. 2: CRCs of histamine-induced contraction of guinea pig tracheal chains in the presence of saline (control) (-□-), aminophylline (standard)
(-Δ-), test compound (4) at two doses of 200 μg/ml (-О-) and 400 μg/ml (-◊-), respectively. Graph a is for 4a; graph b is for 4b; graph c is for
4c; graph d is for 4d.
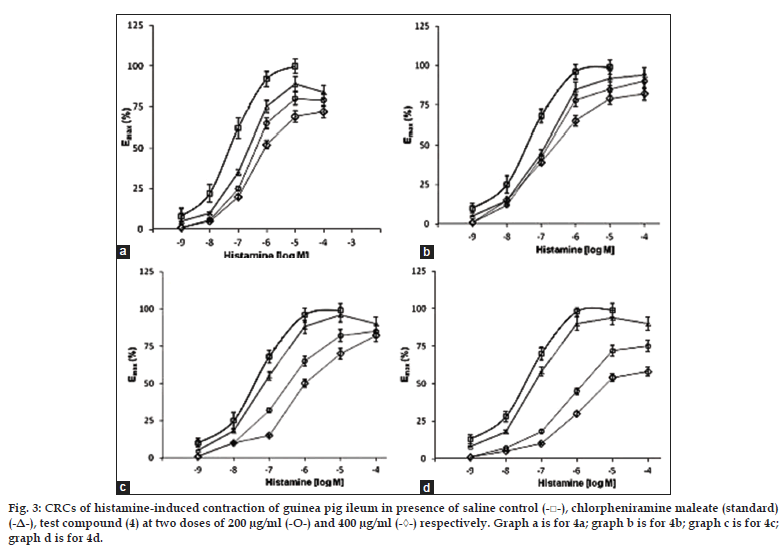
Fig. 3: CRCs of histamine-induced contraction of guinea pig ileum in presence of saline control (-□-), chlorpheniramine maleate (standard)
(-Δ-), test compound (4) at two doses of 200 μg/ml (-О-) and 400 μg/ml (-◊-) respectively. Graph a is for 4a; graph b is for 4b; graph c is for 4c;
graph d is for 4d.
In the isolated guinea pig ileum method, all the test compounds were also found to inhibit histamineinduced contractions of ileum. The concentration of the test compounds which inhibited 50% of response (median inhibitory concentration), IC50 was determined from the graph plotted of percent inhibition versus log dose and the results are presented in Table 3.
| Compound | R | IC50 (µM) |
|---|---|---|
| 4a | −CH3 | 30.2 |
| 4b | −C2H5 | 44.6 |
| 4c | −C3H7 | 62.7 |
| 4d | −C4H9 | 80.1 |
| CPM | _ | 14.1 |
CPM: Chlorpheniramine maleate
Table 3: In Vitro H1‑Antihistaminic Activity Of Test Compounds 4a‑D
Amongst the series tested, compound 4a was found to be relatively more potent with IC50 value of 30.241 μM compared to the standard, chlorpheniramine maleate (IC50=14.1 μM). Next to it, compound 4b was found to possess moderate antihistaminic activity (IC50=44.6 μM). Compounds 4c and 4d exhibited a weak antihistaminic activity with IC50 values of 62.7 and 80.1 μM, respectively. Thus, results in above two models suggest the antihistaminic (H1-antagonist) effect of compounds 4a-d.
Results obtained from each group were expressed as mean±SEM (n=6). The data was analyzed by one way ANOVA. P<0.05 were considered to be statistically significant.
In conclusion, a series of novel 2,6- d ihydroimidazo [1,2- c ] p y r imido [5,4- e ] pyrimidine-5(3H)-thiones were designed as possible bronchodilators and subjected for the prediction of molecular properties and drug-likeness by different software to identify their likely therapeutic potential. Amongst the series, only four compounds (4a-d) were selected on the basis of molecular properties and drug likeness score for oral bioavailability. They were synthesized and screened for antihistaminic and bronchodilatory activities by adopting standard protocols. All the test compounds exhibited bronchodilatory and H1-antihistaminic activities in in vitro evaluation. Further studies are in progress to determine the exact mechanism of these molecules, and to explore other possible isosteres.
Acknowledgements
We wish to thank the Principal, University College of Pharmaceutical Sciences, Kakatiya University, Warangal, for providing facilities and one of the authors (KS) is thankful to the All India Council for Technical Education (AICTE), New Delhi, India, for the award of GATE scholarship.
References
- Braman SS. The global burden of asthma. Chest 2006;130:4S-12.
- Busse WW, Lemanske RF. Asthma. N Engl J Med 2001;344:350-62.
- Factor P. Gene therapy for asthma. MolTher 2003;7:148-52.
- Bleecker ER. Early Life Influences and Interventions in Asthma. Introduction. J Allergy ClinImmunol 2000;105:S465.
- Pawankar R, Canonica GW, Holgate ST, Lockey RF. Allergic diseases and asthma: A major global health concern. CurrOpin Allergy ClinImmunol 2012;12:39-41.
- Kotwani A, Chhabra SK, Tayal V, Vijayan VK. Quality of asthma management in an urban community in Delhi, India. Indian J Med Res 2012;135:184-92.
- Nagai H. Recent research and developmental strategy of antiasthma drugs. PharmacolTher 2012;133:70-8.
- Aїt-Khaled N, Enarson DA, Bencharif N, Boulahdib F, Camara LM, Dagli E, et al. Treatment outcome of asthma after one year follow-up in health centres of several developing countries. Int J Tuberc Lung Dis 2006;10:911-6.
- Santhosh YL, Naveen MR. Medication adherence behavior in chronic diseases like asthma and diabetes mellitus. Int J Pharm PharmSci 2011;3:238-40.
- Sin DD, Tu JV. Underuse of inhaled steroid therapy in elderly patients with asthma. Chest 2001;119:720-5.
- Kwong KY, Morphew T, Scott L, Guterman JJ, Jones CA. Maintaining high degree of asthma control reduces future asthma related morbidity in inner city asthmatic children. Ann Allergy Asthma Immunol 2008;101:144-52.
- Kombu RS, Prasad MR, Harikrishna D, Prabhakar MC, Krishna DR, Rao AR. Synthesis and Bronchodilator Studies of Some Novel 6-Alkyl/ Aryl-1,2,4-Triazino [4,3-c]Quinazolines. Open Med Chem J 2008;2:101-11.
- Shireesha B, Narsiah B, Yakaiah T,Gayatri G, NarahariSastry G, Raghuram Rao A, et al. Synthesis and theoretical studies on energetics of novel N- and O- perfluoroalkyltriazole taggedthienopyrimidines - Their potential as adenosine receptor ligands. Eur J Med Chem 2010;45:1739-45.
- Ramsey AA. Herbicidal and growth-regulant compositions based on novel pyrimido [4,5-d] pyrimidinones. US Patent 3830812 A; CA998393A1, 1974.
- Kategaonkar AH, Sadaphal SA, Shelke KF, Shingate BB, Shingare MS. Microwave assisted synthesis of pyrimido [4, 5-d] pyrimidine derivatives in dry media. UkrainicaBioorganicaActa 2009;1:3-7.
- Cieplik J, Stolarczyk M, Pluta J, Gubrynowicz O, Bryndal I, Lis T, et al. Synthesis and antibacterial properties of pyrimidine derivatives. Acta Pol Pharm 2011;68:57-65.
- Yamamoto N, Takeshita K, Shichijo M, Kokubo T, Sato M, Nakashima K, et al. The orally available spleen tyrosine kinase inhibitor 2- [7-(3,4-dimethoxyphenyl)-imidazo [1,2-c]pyrimidin-5-ylamino] nicotinamidedihydrochloride (BAY 61-3606) blocks antigen-induced airway inflammation in rodents. J PharmacolExpTher 2003;306:1174-81.
- Chhabria MT, Jani MH. Design, synthesis and antimycobacterial activity of some novel imidazo [1,2-c]pyrimidines. Eur J Med Chem 2009;44:3837-44.
- Walters WP, Murcko MA. Prediction of ‘drug-likeness’. Adv Drug Deliv Rev 2002;54:255-71.
- Lajiness MS, Vieth M, Erickson J. Molecular properties that influence oral drug-like behavior. CurrOpin Drug DiscovDevel 2004;7:470-7.
- Available from: http://www.organic-chemistry.org/prog/peo/. [Last accessed on 2013 Dec 30].
- Available from: http://www.vcclab.org/lab/alogps/. [Last accessed on 2013 Dec 25].
- Available from: http://www.molinspiration.com/. [Last accessed on 2013 Dec 31].
- Available from: http://www.acdlabs.com/resources/freeware/chemsketch/. [Last accessed on 2014 Jan 02].
- Available from: http://www.businessinsider.com/10-best-selling-blockbuster-drugs-2012-6?op=1, [Last accessed on 2013 Jul 25].
- Available from: http://mastersearch.chemexper.com/cheminfo/servlet/org. dbcreator.MainServlet?sort=entry.catalogID and searchInfo=quicksearch and query=structure._structureID%3D4950955 and realQuery=structure._ structureID%3D4950955 and action=PowerSearch and target=entry and onclick=1 and format=ccd and searchValue=135158-59-7 and options=brandqtyoffer. [Last accessed on 2014 Sep 19].
- Castillo JC, de Beer EJ. The tracheal chain. J PharmacolExpTher 1947;90:104-9.
- Ghosh MN. Fundamentals of Experimental Pharmacology. In: Ghosh, MN editor.: Kolkata, India: Scientific Book Agency; 1984. p. 60-3.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 1997;23:3-25.
- Tyrchan C, Blomberg N, Engkvist O, Kogej T, Muresan S. Physicochemical property profiles of marketed drugs, clinical candidates and bioactive compounds. Bioorg Med ChemLett 2009;19:6943-7.
- Wang RX, Fu Y, Lai LH. A new atom-additive method for calculating partition co-efficients. J ChemInfComputSci 1997;37:615-21.