- *Corresponding Author:
- L. V. Ganji
Department of Pharmaceutical Chemistry, Prin. K. M. Kundnani College of Pharmacy, Plot No. 23, Jote Joy Building, Rambhau Salgaonkar Road, Cuff Parade, Colaba, Mumbai-400 005, India
E-mail: ganjilata@gmail.com
| Date of Submission | 29 March 2019 |
| Date of Revision | 13 August 2019 |
| Date of Acceptance | 07 November 2019 |
| Indian J Pharm Sci 2019;82(1):21-31 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Benzimidazole-2-thione derivatives are known to possess broad spectrum of biological activities, and the most prominent being the antiinflammatory activity. The synthesis of these compounds involve three steps, William’s reaction of 5(6)-(un)-substituted-1H-benzimidazol-2-thiols with chloroacetic acid in presence of sodium hydroxide and refluxing, which was further reacted with acetic anhydride in pyridine medium on a steam bath followed by hydrolysis with a N-mono substituted piperazine in ethanol medium under reflux conditions to get 5(6)-(un)-substituted(1H-benzimidazol-2-yl-thio)acetyl piperazine derivatives. Molecular docking experiments were carried out against cox-2 enzyme using GOLD software 5.1.0. Docking studies revealed important binding interactions of synthesized derivatives with cox-2 enzyme indicating non-selective interactions, which were in agreement with the in vivo antiinflammatory activity. Absorption, distribution, metabolism, and excretion properties of the synthesized derivatives were determined by QikProp. These derivatives were tested in the carrageenan-induced rat paw edema model to determine the antiinflammatory activity.
Keywords
Benzimidazole-2-thione, acetyl piperazine derivatives, molecular docking, ADME studies, in vivo antiinflammatory activity
Benzimidazole is one of the most promising heteroaryl moiety that yielded many successful drugs[1]. Benzimidazole moiety as well as its derivatives possessed a wide variety of pharmacological activities. Literature indicated that benzimidazole derivatives possessed antimicrobial, antiviral, antineoplastic, antihypertensive, and vasodilating activities[2]. Furthermore, it was reported that many compounds containing benzimidazole moiety possessed significant analgesic as well as antiinflammatory activity[3]. Benzimidazole moiety fulfilled the minimum structural requirements that are common for antiinflammatory compounds[4-6].
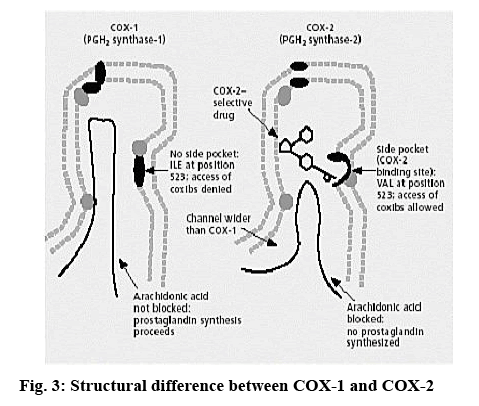
Literature survey revealed that among the various activities exhibited by substituted benzimidazoles such as 2-mercaptobenzimidazole derivatives or fused piperazine derivatives showed significant antiinflammatory activity. In recent years, a number of benzimidazole derivatives synthesized were found to exhibit antiinflammatory activity[3-12]. Non-steroidal antiinflammatory drugs (NSAIDs) are most widely used to treat inflammation, mild to moderate pain and fever. The most important mechanism of antiinflammatory action of NSAIDs is considered to be inhibition of prostaglandin synthesis at the site of injury. The antiinflammatory potency of different compounds roughly corresponds with their potency to inhibit cox enzyme. It is hypothesized that the undesirable side effects of NSAIDs are due to the inhibition of cox-1 whereas the beneficial effects are related to the inhibition of cox-2. The usages of NSAIDs are mainly restricted by their well-known and serious adverse gastrointestinal side effects such as gastro duodenal erosions and ulcerations[13-17]. NSAID-induced gastropathy are estimated to affect up to half of chronic NSAID users, with major world health implications[18].
Therefore, search for better and safer antiinflammatory agents continues.
In the present research work, new piperazine derivatives of (1H-benzimidazol-2-yl-thio)acetic acid were synthesized and molecular docking experiments were carried out against cox-1 and cox-2 enzymes using GOLD software 5.1.0 to investigate the binding mode of active compounds. Biological evaluation was carried out on the rat carrageenan-induced rat paw oedema model.
Materials and Methods
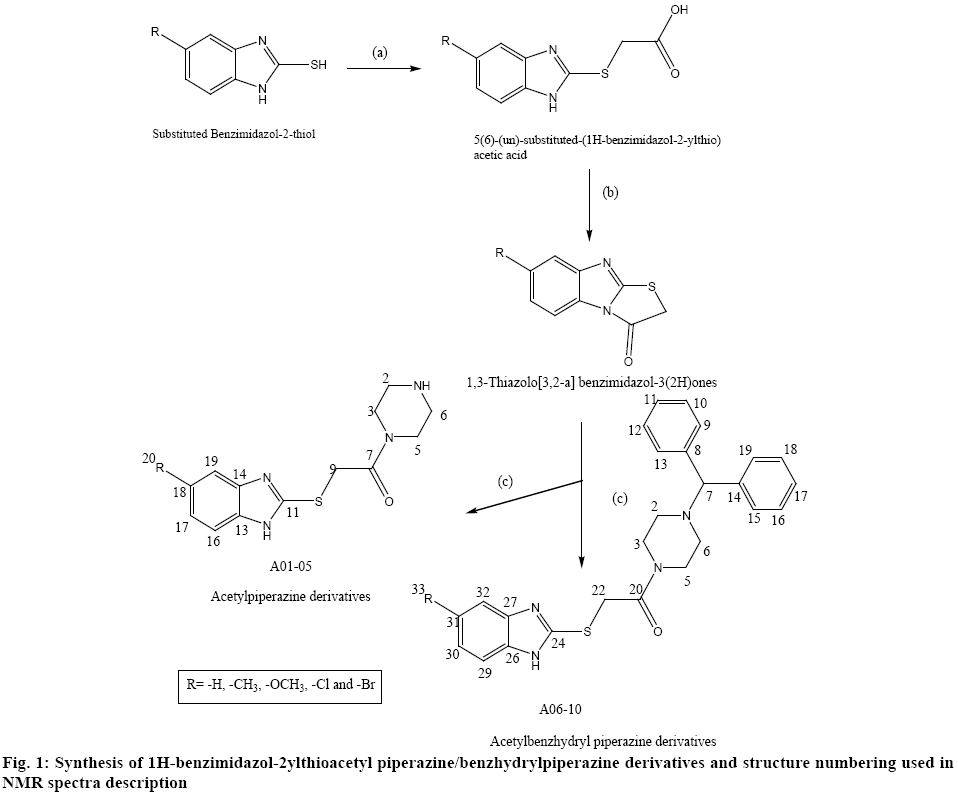
The physical constant and TLC of synthesized derivatives A01-10 were recorded and were found to be different from the starting material. The IR spectra of derivatives were recorded in KBr disc using FT-IR spectrophotometer (FTIR-8400) Shimadzu and the 1H-NMR spectra of derivatives were recorded in CDCl3 and DMSO-d6 using 300 MHz 1H-NMR Spectrometer (Jeol-300, Japan). The analysis of the NMR spectra was done taking into consideration the electronic effect of the respective substituent and the structure numbering used for the description of the 1H NMR spectra is given in figure 1. All chemicals used in the investigation are of laboratory grade and all the solvents which were used in the reactions were distilled prior to their usage.
Docking protocol:
Computational studies were carried out with the modeling package Discovery Studio 3.1 (Accelrys Inc., USA) running on a Red Hat Enterprise Linux WS Workstation. Docking studies were carried out with GOLD v 5.1.0 (CCDC, UK) running on a separate Red Hat Enterprise Linux WS Workstation. The crystal structure of the enzyme cox-2 in complex with naproxen (NPS5) figure 2 was taken from the Protein Data Bank (PDB code: 3NT1) and the structural difference between cox-1 and 2 was shown in figure 3. The enzyme is a dimer; however for docking studies only monomeric unit was used. All the water molecules were deleted and hydrogen was added at pH 7. The system was refined using the CHARMm force field with the backbone atoms tethered by a force constant of 10 kcal/mol/Å, to a gradient 0.1 kcal/mol/Å. The NPS5 structure was energy minimized using the Smart Minimizer method in DS3.1 that performs steepest descents and conjugate gradients with the CHARMm force field to a gradient 0.0001 kcal/mol/Å.
The following parameters in GOLD were permitted to change during the docking runs, (a) the dihedral angles of the inhibitors, (b) the inhibitors’ ring geometries (flipping ring corners), (c) the dihedral angles of enzyme OH and NH2 groups and (d) mappings of the H-bonds between the inhibitor and enzyme. At the start of a docking run, all these variables were randomized. Docking was carried out for 20 genetic algorithm (GA) runs, which was found sufficient to reproduce the binding pose of naproxen in 3NT1. Most of the other GA parameters like population size and the genetic operators were left at their default values. The binding site was defined as a sphere of 10 Å radius around the inhibitor. The docking protocol was validated by the reproduction of the binding pose of naproxen (NPS5) in 3NT1. This protocol was then used to dock the other inhibitors and synthesized analogues to cox-2 to determine their preferred binding orientations. The docked poses were scored using GoldScore. The GOLD fitness function is made up of four components protein-ligand hydrogen bond energy, protein-ligand van der Waals energy (external vdw), ligand internal vdw energy and ligand torsional strain energy. Poses were segregated on the basis of GoldScore. Hydrogen bonding interactions for all the ligands with the amino acid residues of the active site were observed.
Antiinflammatory activity:
The synthesized derivatives A01-10 were tested orally in the carrageenan-induced rat paw oedema model. Test compounds and standard drug were prepared as suspensions in 1 % w/v CMC (sodiumcarboxymethyl cellulose). Wistar rats were divided into 3 groups, group-I, which received 1 % CMC solution at 1 ml/kg orally+0.1 ml of 1 % (w/v) of carrageenan subcutaneously served as the control group, group-II received diclofenac sodium 30 mg/kg orally (1 h prior to carrageenan injection)+0.1 ml of 1 % (w/v) of carrageenan subcutaneously served as the standard group and group-III received test compounds 150 mg/kg orally (1 h prior to carrageenan injection)+0.1 ml of 1 % (w/v) of carrageenan subcutaneously was the test group. A digital plethysmometer was used to measure paw volumes. The study protocol was approved by the Institutional Animal Ethics Committee constituted as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals. The approved protocol number is 121308.
Absorption, distribution, metabolism, and excretion (ADME) studies:
In silico approaches are used in drug discovery to assess ADME properties of compounds at the early stages of discovery/development. QikProp v 3.3, Schrodinger, LLC, New York, NY, 2010, is a quick, accurate, easyto- use ADME prediction program. QikProp predicts physically significant descriptors and pharmaceutically relevant properties of organic molecules, either individually or in batches. In addition to predicting molecular properties, QikProp provides ranges for comparing the properties of a particular molecule with those of 95 % of known drugs. The synthesized analogues were used as test set ligands. The test set ligands were processed with the LigPrep-Software program v 2.5 to assign protonation states appropriate for a pH near to physiological pH, generates stereoisomers, converts 2D structures to 3D and generation of 100 conformations for each molecule using Macro Model torsion sampling with OPLS_2005 post-processing. After processing ligands, the Qikprop program started in normal mode. The completed operation gave CSV (comma-separatedvalue) output file that listed ADME descriptors and predicted properties. Software predicted the variety of pharmaceutically relevant properties such as octanol/ water and water/gas log Ps, log S, log BB, overall CNS activity, cell permeability, human serum albumin binding and log IC50 for K+ channel blockage.
General method for preparation of benzimidazolin- 2-thione:
A mixture of different substituted o-phenylenediamine (19 mmol), potassium hydroxide (12.34 g, 22 mmol), ethanol (200 ml) and water (30 ml) were placed in a 500 ml round bottom flask fitted with a water condenser. To this, carbon disulphide (16.75 g/13.28 ml, 22 mmol) was added. Reaction mixture was heated to reflux for 3 h. Reaction mixture was poured into 200 ml warm water (70°). Glacial acetic acid (50 %, 90 ml) was added drop wise to it with constant stirring till solid separates. The solid separated was filtered, washed with water and air dried. The crude product was purified by recrystallisation using methanol. The yields were in the range 84-92 %.
General procedure for synthesis of derivatives A01-A10:
5-substituted-(1H-benzimidazol-2yl-thio)acetic acid was synthesized by refluxing a solution of sodium hydroxide (0.012 mol) in ethanol (14 ml) and 5-substituted-1H-benzimidazole-2-thiol (0.0067 mol) for 1 h. After cooling, chloroacetic acid (0.0067 mol) was added and the reflux was continued for additional 1 to 5 h. Then, the reaction mixture was cooled, poured into ice cold water and acidified with dilute acetic acid. The crystallized product was filtered, carefully washed with water and re-crystallized using absolute methanol, and the yield was 59-75 %.
(1,3)-Thiazolo(3,2-a)benzimidazol-3-(2H)-one was prepared by refluxing 5-substituted-(1H-benzimidazol- 2yl-thio)acetic acid (0.0104 mol) and acetic anhydride (1.95 ml) in anhydrous pyridine (6.5 ml) for 10 min. Products were crystallized as pale orange sediment after cooling and, cold water (10 ml) was added in small portions by stirring, yield was 50-71 %.
Acetylpiperazine and acetylbenzhydryl derivatives were prepared by refluxing a mixture of 3.16 mmol [1,3]-thiazolo-[3,2-a]-benzimidazol-3(2H)-one and 3.79 mmol N-substituted piperazine in 12 ml ethyl alcohol for 3 h. The solvent was distilled under reduced pressure and the obtained oil product was washed with water several times in order to remove the excess of piperazine. By addition of ethanol to the oil, the compounds crystallized out, yield was 40-80 %.
2-[(2-Oxo-2-piperazin-1-ylethyl)thio]-1H-benzimid azole (A01), yield-58 %; melting point (mp)-95- 98 %; re-crystallized with ethanol; Rf-0.86; mobile phase, benzene:methanol (3:1); IR cm-1-1614.42 C=C stretching vibration of aromatic ring; 3007.02, 686.65 C-H stretching and bending vibrations of aromatic ring; 1643.35 C=N stretching vibration of aromatic ring; 3205.69, 1573.91 N-H stretching and bending vibrations of aromatic ring; 661.58 C-S stretching vibration; 2850.79, 1446.61 C-H stretching and bending vibrations; 1693.50 C-O stretching vibration of carbonyl; 1402.25 C-H stretching vibration of cyclic CH2; 1269.16 C-N stretching vibration; 3072.60 N-H stretching vibration; 300 MHz 1HNMRδ values (DMSO-d6): 2.5-2.8 (m, 4H, 2CH2-NH-CH2); 3.2-3.7 (m, 4H, 3CH2-N-5CH2); 4.4 (s, 2H, SCH2CO); 7.30- 7.40 (m, 2H-17CH, 18CH, Ar); 7.8 (m, 2H-16CH, 19CH, Ar) and 8.6(s, NH).
2-[(2-Oxo-2-piperazin-1-ylethyl)thio]-5-methyl-1Hbenzimidazole (A02), yield-60 %; mp-85-87 %; re-crystallized with methanol; Rf-0.78; mobile phase, benzene:methanol (3:1); IR cm-1-2850.79, 2927.9 CH3 symmetric and asymmetric stretching vibrations; 1620 C=C stretching vibration of aromatic ring; 3001.15, 671.86 C-H stretching and bending vibrations of aromatic ring; 1632.64 C=N stretching vibration of aromatic ring; 3215, 1535.23 N-H stretching and bending vibrations of aromatic ring; 672.49 C-S stretching vibration; 2757.91, 1465 C-H stretching and bending vibrations; 1698.33 C-O stretching vibration of carbonyl; 1416.5 C-H stretching vibration of cyclic CH2; 1249.05 C-N stretching vibration; 3077.22 N-H stretching vibration; 300 MHz 1HNMRδ values (DMSO-d6): 2.7 (s, 3H, 20CH3); 2.5-2.6 (m, 4H, 2CH2-NH-CH2); 3.35-3.45 (m, 4H, 3CH2-N-5CH2); 3.8 (s, 2H, SCH2CO); 7.30-7.60 (m, 3H-17CH, 18CH, 19CH, Ar); 4.4 (s, 1H, 1NH), and 8.1(s, NH).
2-[(2-Oxo-2-piperazin-1-ylethyl)thio]-5-methoxy- 1H-benzimidazole (A03), yield-47 %; mp-88-89 %; re-crystallized with methanol-water (70:30); Rf-0.80; mobile phase, benzene: methanol (3:1); IR cm-1-1145.71 C-O stretching vibration of methoxy group; 2842.82, 1126 CH3 stretching and bending vibrations; 1614 C=C stretching vibration of aromatic ring; 3017.72, 682.19 C-H stretching and bending vibrations of aromatic ring; 1634 C=N stretching vibration of aromatic ring; 3225.98 N-H stretching vibration of aromatic ring; 665.08 C-S stretching vibration; 2657 C-H stretching vibration; 1689.12 C-O stretching vibration of carbonyl; 1402 C-H stretching vibration of cyclic CH2; 3057.46 N-H stretching vibration; 300 MHz 1HNMRδ values (DMSO-d6): 3.2 (s, 3H, 20 OCH3); 2.5-2.7 (m, 4H, 2CH2-NH-CH2); 3.3-3.4 (m, 4H, 3CH2-N-5CH2); 3.75 (s, 2H, SCH2CO); 7.25-7.7 (m, 3H-17CH, 18CH, 19CH, Ar); 4.45 (s, 1H, 1NH); and 8.15(s, NH).
2-[(2-Oxo-2-piperazin-1-ylethyl)thio]-5-chloro-1Hbenzimidazole (A04), yield-41 %; mp-89-91 %; re-crystallized with ethanol; Rf-0.72; mobile phase, benzene:methanol (3:1); IR cm-1-740.67 C-Cl stretching vibration; 1620 C=C stretching vibration of aromatic ring; 2983.88, 891.11 C-H stretching and bending vibrations of aromatic ring; 1620.21 C=N stretching vibration of aromatic ring; 3159, 1537.27 N-H stretching and bending vibrations of aromatic ring; 619.15 C-S stretching vibration; 2600.90 C-H stretching vibration; 1683.50 C-O stretching vibration of carbonyl; 1359.82 C-H stretching vibration of cyclic CH2; 3078 N-H stretching vibration; 300 MHz 1HNMR δ values (DMSO-d6): 2.4-2.65 (m, 4H, 2CH2-NHCH 2); 3.35-3.42 (m, 4H, 3CH2-N-5CH2); 3.63 (s, 2H, SCH2CO); 7.05-7.45 (m, 3H-17CH, 18CH, 19CH, Ar); 4.26 (s, 1H, 1NH); and 7.83 (s, NH).
2-[(2-Oxo-2-piperazin-1-ylethyl)thio]-5-bromo-1Hbenzimidazole (A05), yield-77 %; mp-96-98 %; re-crystallized with ethanol; Rf-0.77; mobile phase, benzene: methanol (3:1); IR cm-1-599.86 C-Br stretching vibration; 1564.27 C=C stretching vibration of aromatic ring; 2823.79C-H stretching vibration of aromatic ring; 1334.74 C=N stretching vibration of aromatic ring; 3446.79 N-H stretching vibration of aromatic ring; 690.52 C-S stretching vibration; 2640.55 C-H stretching vibration; 1681.93 C-O stretching vibration of carbonyl; 1398.12 C-H stretching vibration of cyclic CH2; 3080.32 N-H stretching vibration; 300 MHz 1HNMR δ values (CDCl3): 2.2 (m, 4H, 2CH2- NH-CH2); 3.1-3.3 (m, 4H, 3CH2-N-5CH2); 3.7 (s, 2H, SCH2CO); 7-7.2 (m, 3H-17CH, 18CH, 19CH, Ar); 4.3 (s, 1H, 1NH); and 7.3 (s, NH). 2-{[2-Oxo-2-(4-benzhydrylpiperazin-1-yl)ethyl]thio}- 1H-benzimidazole (A06), yield-47 %; mp-151-153 %; re-crystallized with methanol; Rf-0.62; mobile phase, benzene:methanol (3:1); IR cm-1-585.49 C=C stretching vibration of aromatic ring; 2868.15, 740 C-H stretching and bending vibrations of aromatic ring; 1512.19 C=N stretching vibration of aromatic ring; 3433.29, 1537.27 N-H stretching and bending vibration of aromatic ring; 663.51 C-S stretching vibration; 2715.77 C-H stretching vibration; 1359.82 C-H bending vibration; 1726.29 C-O stretching vibration of carbonyl; 300 MHz 1HNMR δ values (DMSO-d6): 2.2-2.4 (m, 4H, 2CH2-N-CH2); 3.3-3.5 (m, 4H, 3CH2-N-5CH2); 4.25 (s, 2H, SCH2CO); 7-7.50 (m, 14H-Ar); 3.75 (s, 1H, 7CH); and 8.1 (s, NH). 2-{[2-Oxo-2-(4-benzhydrylpiperazin-1-yl)ethyl] thio}- 5-methyl-1H-benzimidazole (A07), yield-59 %; mp-180-183 %; re-crystallized with methanol; Rf- 0.60; mobile phase, benzene:methanol (3:1); IR cm- 1-2868, 2951.09 CH3 Asymmetric and symmetric stretching vibrations;1524.49 C=C stretching vibration of aromatic ring; 3024.38, 802.39 C-H stretching and bending vibrations of aromatic ring; 1585.49 C=N stretching vibration of aromatic ring; 3321.42, 1527.62 N-H stretching and bending vibration of aromatic ring; 619.16 C-S stretching vibration; 2657.91 C-H stretching vibration; 1330.88 C-H bending vibration;1716.97 C-O stretching vibration of carbonyl; 300 MHz1 HNMR δ values (DMSO-d6): 2.5-2.6 (m, 4H, 2CH2- N-CH2); 3.35-3.4 (m, 4H, 3CH2-N-5CH2); 4.15 (s, 2H, SCH2CO); 7.1-7.60 (m, 13H-Ar); 3.7 (s, 1H, 7CH); 2.44 (s, 3H, CH3) and 8 (s, NH).
2-{[2-Oxo-2-(4-benzhydrylpiperazin-1-yl)ethyl]thio}- 5-methoxy-1H-benzimidazole (A08), yield- 66 %; mp-147-149 %; re-crystallized with ethanol; Rf- 0.65; mobile phase, benzene: methanol (3:1); IR cm-1- 1278.81 C-O stretching vibration of methoxy group; 2850, 1408.04 CH3 stretching and bending vibrations; 1585 C=C stretching vibration of aromatic ring; 3059 C-H stretching vibration of aromatic ring; 1658 C=N stretching vibration of aromatic ring; 3396.64 N-H stretching vibration of aromatic ring; 640.37 C-S stretching vibration; 2831 C-H stretching vibration; 1726.29 C-O stretching vibration of carbonyl; 1392 C-H stretching vibration of cyclic CH2; 300 MHz 1HNMR δ values (DMSO-d6): 3.7 (s, 3H, OCH3); 2.2- 2.3 (m, 4H, 2CH2-N-CH2); 3.32-3.41 (m, 4H, 3CH2-N5CH2); 4.2 (s, 2H, SCH2CO); 2.7 (s, 1H, 7CH); 7.1-7.45 (m, 13H, Ar); and 8.15 (s, 1H, 1NH).
2-{[2-Oxo-2-(4-benzhydrylpiperazin-1-yl)ethyl]thio}- 5-chloro-1H-benzimidazole (A09), yield- 67 %; mp-191-193 %; re-crystallized with methanol; Rf-0.68; mobile phase, benzene:methanol (3:1); IR cm-1-704.02 C-Cl stretching vibration; 1512.19, 1408 C=C stretching vibration of aromatic ring; 3101.54 C-H stretching vibration of aromatic ring; 1585.49 C=N stretching vibration of aromatic ring; 3433.29 N-H stretching vibration of aromatic ring; 663.51 C-S stretching vibration; 2868.15 C-H stretching vibration; 1722 C-O stretching vibration of carbonyl; 1359.82 C-H stretching vibration of cyclic CH2; 300 MHz 1HNMR δ values (DMSO-d6): 2.3 (m, 4H, 2CH2-N-CH2); 3.3- 3.45 (m, 4H, 3CH2-N-5CH2); 3.7 (s, 2H, SCH2CO); 2.5 (s, 1H, 7CH); 7.1-7.49 (m, 13H, Ar); and 7.9 (s, 1H, 1NH).
2-{[2-Oxo-2-(4-benzhydrylpiperazin-1-yl)ethyl]thio}- 5-bromo-1H-benzimidazole (A10), yield-73 %; mp-163- 165 %; re-crystallized with methanol; Rf-0.61; mobile phase, benzene: methanol (3:1); IR cm-1-615.29C-Br stretching vibration; 1505.05 C=C stretching vibration of aromatic ring; 3051.39 C-H stretching vibration of aromatic ring; 1558.55 C=N stretching vibration of aromatic ring; 3657.04 N-H stretching vibration of aromatic ring; 709.80 C-S stretching vibration; 2859.84 C-H stretching vibration; 1687.71 C-O stretching vibration of carbonyl; 1327.03 C-H stretching vibration of cyclic CH2; 300 MHz 1HNMR δ values (DMSO-d6): 2.4-2.55 (m, 4H, 2CH2-N-CH2); 3.13-3.25 (m, 4H, 3CH2-N-5CH2); 4.4 (s, 2H, SCH2CO); 3.65 (s, 1H, 7CH); 7.2-7.6 (m, 13H, Ar); and 8.13(s, 1H, 1NH).
Result and Discussion
From the literature it was observed that, the benzimidazole substituted at 5th position with different halogen atoms such as chloro, bromo, fluoro, and with alkyl and or alkoxy groups such as methyl, methoxy contributed to antiinflammatory activity. Piperazine attached to benzimidazole and indoles were found to have potent antiinflammatory activity[19]. Thus, it was decided to incorporate various substitutions at 5 th and 2nd position of benzimidazole-2-thiol with halogen, alkyl, alkoxy groups and benzyl, piperazine moiety, respectively.
The substituted benzimidazolin-2-thione was prepared from 4-substituted-1,2-diaminobenzene by heating with carbon disulfide, potassium hydroxide in ethanolmethod described by Wang et al.[20]. The 5th position was substituted by –CH3, -OCH3, -Cl and –Br groups to enhance the antiinflammatory activity of benzimidazolin-2-thione analogues.
Synthesis of 5-substituted-1H-benzimidazol-2- ylthioacetylpiperazine derivatives is shown in figure 1. The synthesis of first intermediate (i.e. 5(6)-(un)substituted- (1H-benzimidazol-2-ylthio)acetic acids) involved the reaction of thione derivatives with chloroacetic acid in the presence of sodium hydroxide (via William’s reaction) followed by second intermediate (i.e.1,3- thiazolo[3,2-a]-benzimidazol-3(2H)ones), which involved heating of first intermediate with acetic anhydride in pyridine medium on steam bath. The final product was obtained under reflux condition for 5 h, which resulted into hydrolysis of second intermediate followed by condensation with piperazine derivatives in ethanol[21]. In a basic medium, the condensed thiazole cycle underwent a cleavage. Because of these properties of the above-mentioned compounds, we chose the reaction between [1,3]-thiazolo[3,2-a]benzimidazol- 3(2H)-one and amines among the different methods for synthesis of (benzimidazol-2-ylthio)acetamides. This reaction offered the possibility for preparing large number of compounds by means of a simple procedure through introduction of various substituents both in the 5(6)-position of thiazolobenzimidazole ring and in the second place of thiazole cycle and this was preferred because of the simple work-up procedure and the higher purity of the synthesized substances. The yields were in an average range of 40-80 %. The thin layer chromatography (TLC) was recorded using mobile phase benzene:methanol (3:1). The yield, melting point and Rf value of each compound are shown in Table 1.
Table 1: Details of 5-Substituted-1H-Benzimidazol-2-YL-Thioacetylpiperazine and Benzhydrylpiperazine Derivatives
| Compound code | Compound | % Yield | MP ( ? ) | Rf |
|---|---|---|---|---|
| A01 | 1H-benzimidazol-2-ylthioacetylpiperazine (R= H) | 58 | 95-98 | 0.86 |
| A02 | 5-Methyl-1H-benzimidazol-2-ylthioacetylpiperazine (R= CH3) | 60 | 85-87 | 0.78 |
| A03 | 5-Methoxy-1H-benzimidazol-2-ylthioacetylpiperazine(R= OCH3) | 47 | 88-89 | 0.80 |
| A04 | 5-Chloro-1H-benzimidazol-2-ylthioacetylpiperazine (R= Cl) | 45 | 89-91 | 0.72 |
| A05 | 5-Bromo-1H-benzimidazol-2-ylthioacetylpiperazine (R= Br) | 77 | 96-98 | 0.77 |
| A06 | 1H-benzimidazol-2-ylthioacetylbenzhydrylpiperazine (R= H) | 47 | 151-153 | 0.62 |
| A07 | 5-Methyl-1H-benzimidazol-2-ylthioacetylbenzhydrylpiperazine (R= CH3) | 59 | 180-183 | 0.60 |
| A08 | 5-Methoxy-1H-benzimidazol-2-ylthioacetylbenzhydrylpiperazine (R= OCH3) | 66 | 147-149 | 0.65 |
| A09 | 5-Chloro-1H-benzimidazol-2-ylthioacetylbenzhydrylpiperazine (R= Cl) | 67 | 191-193 | 0.68 |
| A10 | 5-Bromo-1H-benzimidazol-2-ylthioacetylbenzhydrylpiperazine (R= Br) | 73 | 163-165 | 0.61 |
MP is melting point and Rf is retention factor
All the starting materials used for each reaction were assessed by recording physical constant of compounds and compared with those in literature. The starting materials were also subjected to TLC using precoated silica gel GF254 plates to ensure their purity. The reactions were also monitored using TLC. The title compounds were purified by recrystallization. Purity of intermediates and final products was assessed by single spot of TLC plate using suitable solvent systems. The melting points of the synthesized compounds were determined by open capillary method. All intermediates were characterized by recording their physical constants and IR spectroscopy. The IR spectra of the synthesized compounds exhibited expected absorption bands for functional groups. The IR data of 2-mercaptobenzimidazole stretching vibration at 2560- 2575 cm-1 due to S-H, stretching vibration at 3050-3250 cm-1 due to N-H was observed. For final compounds, characteristic absorption bands at 1640- 1660 were observed and absence of S-H stretching vibration at 2550-2650 indicated the substitution at thio group. N-H stretching vibration observed at 3050- 3200 cm-1, C-N vibration observed at 1250-1380 cm-1, C-C stretching vibration observed at 1450-1600 cm-1 and C-H stretching observed at 2800-2950 cm-1. 1HNMR spectra of the title compounds depicted expected delta values for the protons. In NMR spectra of the synthesized compounds, the signal due to S-CH2 methylene protons, present in all title compounds appeared at 4.2-4.6 ppm, as singlet indicated the substitution at the thio group; N-H proton was observed at 8.0-10.00 ppm as broad singlet.
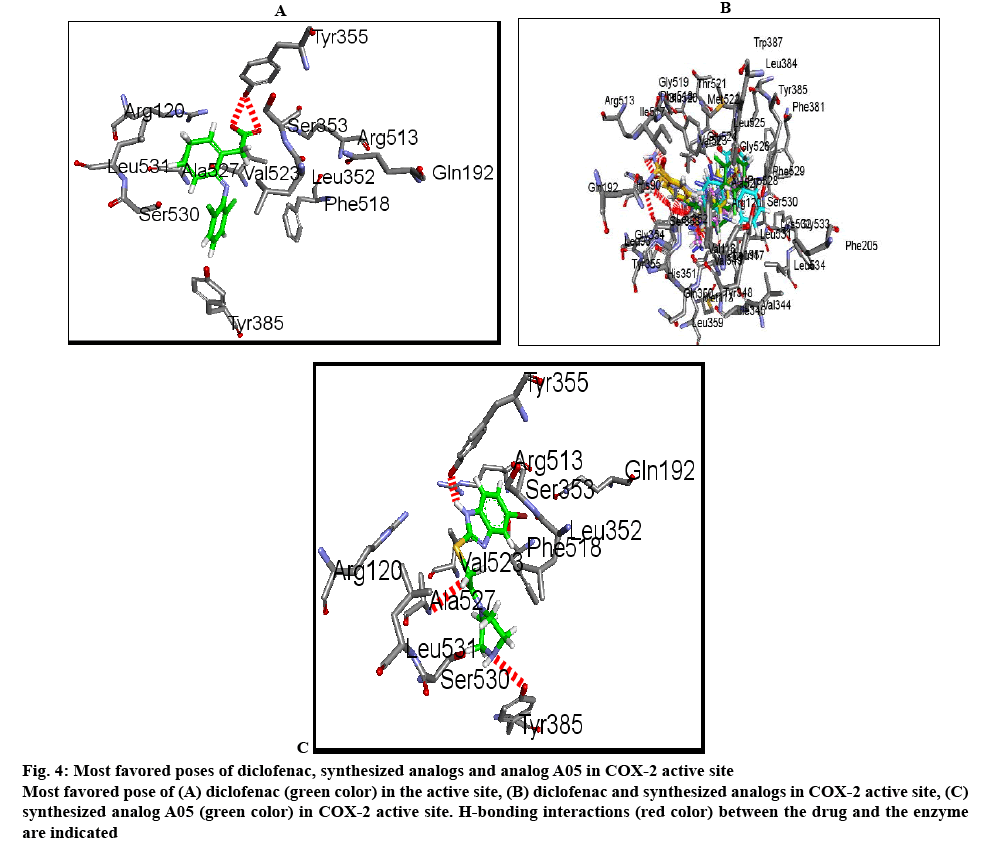
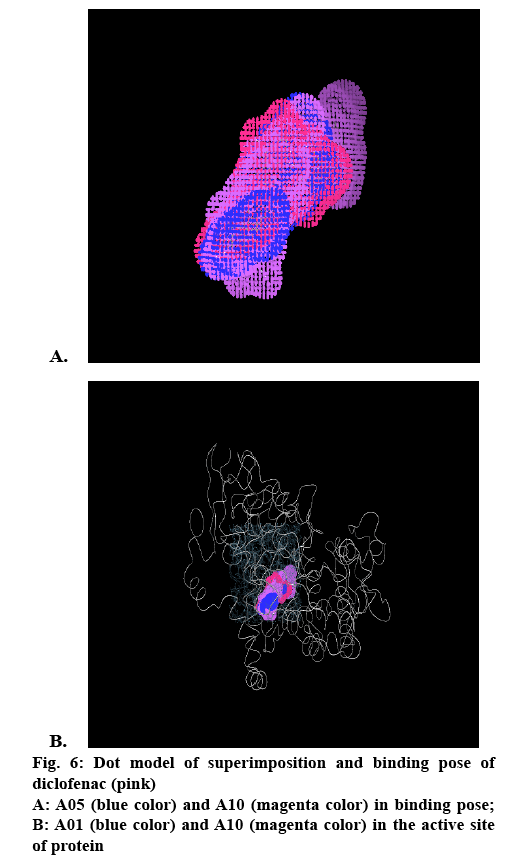
The cox-2 active site pocket (binding region) consists of three differential regions[22] viz, hydrophobic region consisting of Tyr385, Trp387, Phe518, Ala261, Tyr248 and Leu352; mouth of the active site with three hydrophilic residues flanking its entrance, Arg120, Glu524, Tyr355 and arranged to form an H-bond network; side pocket, defined by several conserved residues including His90, Arg513 and Val523. The important H-bonding interactions reported were with Arg120 and Ser530, hydrophobic interaction between aromatic ring of ligand with aromatic pocket formed by the side chains of Tyr385, Trp387, Phe518, Ala201, Tyr348 and Leu352. Arg120 and Glu524 act as a gate for ligand entrance to the active site[23]. Molecular studies[24] suggested that this gate does not open easily, and that Glu524 side chain fluctuates to interact with either Arg120 or Arg513[22]. Salt bridge is formed due to Arg120 and Glu524 in enzyme which prevents the entry of the inhibitors into the active site of cox-2, the formation of bond between inhibitor with Arg120 prevents this salt bridge formation and allow inhibitors to enter into the active site of the enzyme. It was observed that for cox-2 selective inhibition, interaction with Arg513 and Val523 is required while non-selective inhibitors interact with Arg120, Tyr384, Ser530 and Tyr355[22]. In the present study, the approach of molecular docking of naproxen (NPS5) to the enzyme cox-2 was used to explore the active site and to provide clues to favourable binding interactions that can be used to modify the existing drugs. To investigate further about selectivity (cox-1 or cox-2 inhibition) of our ligands, we docked selective cox-2 inhibitors celecoxib and rofecoxib along with our ligands. It was found that celecoxib’s and rofecoxib’s selective inhibition towards cox-2 is due to the interaction with the Leu352, Ser353, Phe518, Arg513 and Val523[25]. These types of interactions were not observed in case of synthesized analogues. For NPS5, the interactions were between the carboxylate group of NPS5 and with the residues Arg120 and Tyr355, the C=O portion of the carboxyl group interacted with Arg120, Tyr355 and the O portion interacted with Tyr355. In diclofenac, the (C=O and O portions) of carboxyl group interacted with same Tyr355 and Ser530, where in case of A02 along with the Tyr355, additional interactions with Tyr385, Ala527 were observed but there was no interaction with Arg120. These results indicated that the additional interactions in case of A02 might be attributed to the piperazine and C=O moieties which probably might have contributed to its greater biological activity. A05 also displayed H-bond interactions similar to A02. A01-05 showed interactions similar to that of standard, with additional interactions with Tyr- 385, Ala-527 and Ser-530. This was found to be in agreement with their biological activity where A05 showed the maximum percent inhibition followed by A02. In case of A06-10, interactions were different to that of standard, as they showed interactions with Tyr-385 and Ser-530. Docking studies of acetylpiperazine and acetylbenzhydrylpiperazine derivatives showed a binding pattern very similar to inhibitor NPS5 and diclofenac along with some additional interactions and was found to be in agreement with the biological data where acetylpiperazine and acetylbenzhydrylpiperazine derivatives showed the maximum activity as compared to other derivatives. Figures 4A, B and C shows the most favored pose of diclofenac, synthesized analogs and A05 in the binding site, respectively and figure 5 shows the surface model of protein with standard diclofenac, figure 6A shows Dot model of superimposition of diclofenac along with A05 and A10 in binding pose, and fig. 6B shows the binding pose of diclofenac, A01 and A10 in the active site of protein.
Figure 4: Most favored poses of diclofenac, synthesized analogs and analog A05 in COX-2 active site
Most favored pose of (A) diclofenac (green color) in the active site, (B) diclofenac and synthesized analogs in COX-2 active site, (C) synthesized analog A05 (green color) in COX-2 active site. H-bonding interactions (red color) between the drug and the enzyme are indicated
The screening of synthesized compounds for the in vivo antiinflammatory activity was carried out by the carrageenan-induced rat paw oedema in rats; carrageenan treatment caused increase in the paw volume. However, the effect was more significant in the later phase and maximum inhibition was observed after 2nd h of carrageenan injection. The results of pharmacological testing indicated that the 5-substituted benzimidazol-2-thiol derivatives possessed significant antiinflammatory activity as shown in Table 2. All the synthesized compounds showed maximum % inhibition of rat paw edema at 2 h, which is shown Table 2. When compared to that of standard diclofenac, the synthesized compounds had shown two fold greater inhibition. By observing the results, it indicated that the substitution at position 5(6) with halogen like –Br, Cl, and with –CH3 group showed maximum inhibition and substitution of piperazine and benzhydrylpiperazine at 2nd position showed an additive effect for inhibition of paw volume. Compared to the standard, A05 and A02 showed highest inhibition (36.72, 71.99 and 66.59 %, respectively, followed by the remaining synthesized compounds.
Table 2: Percent Inhibition of Paw Volumes of 5(6)-(UN)Substituted-1H-Benzimidazol-2-YL-Thioacetylpiperazine Derivatives
| Compound/antiinflammatory activitya | Paw volume mean±SEMb | % Inhibition of paw volume |
|---|---|---|
| Standard | 1.158±0.0094 | 36.72 |
| A01 | 1.13±0.0002 | 39.41 |
| A02 | 1.15±0.0036 | 66.59 |
| A03 | 1.155±0.0003 | 38.36 |
| A04 | 1.155±0.0010 | 52.07 |
| A05 | 0.99±0.0036 | 71.99 |
| A06 | 1.115±0.0003 | 38.58 |
| A07 | 1.04±0.0036 | 40.10 |
| A08 | 1.155±0.0004 | 37.55 |
| A09 | 1.07±0.0036 | 41.95 |
| A10 | 1.09±0.0036 | 44.54 |
aPercent inhibition of paw volumes of compounds at 2 h. bSEM is standard error of mean (p<0.01). One-way ANOVA followed by Dunnett’s test is applied for statistical analysis. Drug treated groups were compared with control group
The descriptor values were verified with the standard values reported in the literature or recommended values of descriptors Table 3. It was found that all the analogues of 5(6)-substituted-benzimidazol-2-thione showed significant values of the properties studied. All the compounds obeyed Lipinski Rule of 5 violations, Jorgensen’s rule of three violations, good oral absorption and lipophilicity.
Table 3: Estimated ADME Value of Synthesized Compounds
| Molecular weight | Donor HB | Acceptor HB | QPlog Po/w | QPlogS | QPPCaco | QPlog BB | QPPMDCK | % Human oral Absorption |
|---|---|---|---|---|---|---|---|---|
| 276.356 | 2 | 6 | 0.675 | -1.184 | 102.503 | -0.26 | 122.105 | 66.883 |
| 290.382 | 2 | 6 | 0.949 | -1.684 | 102.718 | -0.292 | 122.387 | 68.506 |
| 306.382 | 2 | 6.75 | 0.769 | -1.392 | 102.769 | -0.345 | 122.46 | 67.458 |
| 310.801 | 2 | 6 | 1.127 | -1.848 | 102.609 | -0.114 | 292.581 | 69.54 |
| 355.252 | 2 | 6 | 1.233 | -2.003 | 102.854 | -0.109 | 315.008 | 70.18 |
| 442.578 | 1 | 6.5 | 4.197 | -4.486 | 204.754 | -0.257 | 261.717 | 92.889 |
| 456.604 | 1 | 6.5 | 4.496 | -5.041 | 205.191 | -0.279 | 262.317 | 94.653 |
| 472.604 | 1 | 7.25 | 4.278 | -4.664 | 205.287 | -0.336 | 262.473 | 93.383 |
| 477.023 | 1 | 6.5 | 4.668 | -5.191 | 204.967 | -0.104 | 627.123 | 95.65 |
| 521.474 | 1 | 6.5 | 4.776 | -5.35 | 205.411 | -0.095 | 675.235 | 83.345 |
HB = hydrogen bond
Molecular modelling studies indicated that the hetero atom substituted derivatives entered in to the active site of cox-2 and interacted with the active amino acid residues, which are involved in the catalysis. The hetero substituted ring occupied the hydrophobic pocket by interacting with Tyr385 and Ser530 where the interaction of C=O group with guanidine group of Arg120 prevented the formation of salt bridge, which helps in the opening of the entrance of the active site. Interaction with the hydrophilic site containing Tyr355 helped in proper binding. The presnet studies on acetylpiperazine and acetylbenzhydrylpiperazine derivatives were consistent with the above reported observations along with an additional interaction of its C=O group with amino group of Ala527. Molecular docking studies of NPS5, diclofenac and other ligands, indicated that the substitution of these compounds with heteroaryl moiety at the 2nd-position might lead to better interaction with the amino acid residues in the active site which might increase their biological activity. The binding energy (BE) obtained after docking is also in agreement with activity, where A05 shown highest BE 59.81 compared to other derivatives. (BE of all derivatives is shown in Table 4). Adding to this, the important amino acid residues which are responsible for selective cox-2 inhibition reported in the literature are Leu352, Ser353, Phe518, Arg513 and Val523 but interactions of synthesized derivatives with these amino acids were not observed. Furthermore, adding to above, modifications of synthesized derivatives with substitution of oxygen in heterocyclic ring and sulfonyl group attached to the aromatic ring, or to the amino group might add to the selective inhibition of cox-2. As most of NSAIDs have interactions with Arg-120, Tyr- 385 and Ser-530 and most of the synthesized derivatives also showed similar interaction, we may classify them as non-selective cox inhibitors. The structure activity relationship further revealed that the substitution of halogens such as bromo, chloro and alkyl group such as methyl at 5th position and heterocyclic ring such as piperazine ring showed maximum activity which is in agreement with the molecular modelling where they showed maximum number of H-bond interactions with the active site amino acid residues. Adding to this, substitution of benzhydrylpiperazine ring contributed to the antiinflammatory activity but somewhat less when compared to that of piperazine ring. These encouraging results further permit the execution of detailed studies of this class of derivatives. Different substitution at 5th and 2nd-position of benzimidazole-2-thiol could be studied to see how these impact antiinflammatory activity and could be further modified to obtain selective activity. There is a good scope for detailed pharmacological studies of these derivatives.
Table 4: Binding Energy Obtained from Molecular Docking
| Compound code | Binding Energy (BE) |
|---|---|
| Diclofenac | 51.12 |
| A01 | 53.74 |
| A02 | 57.44 |
| A03 | 49.76 |
| A04 | 58.89 |
| A05 | 59.81 |
| A06 | 52.29 |
| A07 | 55.04 |
| A08 | 48.60 |
| A09 | 56.70 |
| A10 | 57.42 |
References
- Preston PN. Benzimidazoles. In: Brown DJ, editor. Cumulative Index of Heterocyclic Systems Chemistry of Heterocyclic Compounds. Hoboken, New Jersey, United States: John Wiley & Sons, Inc.; 2008. p. 40.
- Mohamed BG, Abdel-Alim AA, Hussein MA. Synthesis of 1-acyl-2-alkylthio-1, 2, 4-triazolobenzimidazoles with antifungal, anti-inflammatory and analgesic effects. Acta Pharm 2006;56:31-48.
- Mertens A, Muller-Beckmann B, Kampe W, Holck JP, Von der Saal W. 2-Pyridyl-6, 7-dihydro-3H, 5H-pyrrolo [2, 3-f] benzimidazol-6-ones, a novel class of cardiotonic agents. J Med Chem 1987;30:1279-89.
- Scherrer RA. Anti-inflammatory Drugs: Chemistry and Pharmacology. Cambridge, Massachusetts, United States: Academic Press; 1974. p. 119-22.
- Nicholson RM, Murphy JR, Dearden JR. Hypothetical receptor model for anti-inflammatory agents. J Pharm Pharmacol 1982;34(Suppl):106.
- Gun P, Jensen NP. Nonsteroidal anti-inflammatory and anti-arthritic drugs. In: Blankley J, editor. Quantitative structure activity relationships of drugs. Cambridge, Massachusetts, United States: Academic Press; 1983. p. 285-326.
- Tsukamoto G, Yoshino K, Kohno T, Ohtaka H, Kagaya H, Ito K. 2-Substituted azole derivatives. Synthesis and anti-inflammatory activity of some 2-(substituted-pyridinyl)benzimidazoles. J Med Chem 1980;23:734-8.
- Ito K, Kagaya H, Satoh I, Tsukamoto G, Nose T. The studies of the mechanism of anti-inflammatory action of 2-(5-ethylpyridin-2-yl)benzimidazole(KB-1043). Arzneimittelforschun Arzneimittelforschungg 1982;32(2):117-22.
- Taniguchi K, Shigenaga S, Ogahara T, Fujitsu T, Matsuo M. Synthesis and Anti-inflammatory and Analgesic Properties of 2-Amino-1H-benzimidazole and 1, 2-Dihydro-2-iminocycloheptimidazole Derivatives. Chem Pharm Bull 1993;41(2):301-9.
- Gilman SC, Carlson RP, Chang J, Lewis AJ. The anti-inflammatory activity of the immunomodulator Wy-18, 251 (3-(p-chlorophenyl)thiazolo[3,2-a]benzimidazole-2-acetic acid). Agents Actions 1985;17:53-9.
- Gilman SC, Carlson RP, Lewis AJ. Immunomodulatory activity of Wy-18, 251 (3-(p-chlorophenyl)thiazolo[3,2-a]benzimidazole-2-acetic acid). J Immunopharmacol 1985;7:79-98.
- Lazer ES, Matteo MR, Possanza GJ. Benzimidazole derivatives with atypical anti-inflammatory activity. J Med Chem 1987;30:726-9.
- Chan FK. Primer: managing NSAID-induced ulcer complications-balancing gastrointestinal and cardiovascular risks. Nat Clin Pract Gastroenterol Hepatol 2006;3(10):563-73.
- Whittle BJ. Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur J Pharmacol 2004;500(1-3):427-39.
- Cryer B. The role of cyclooxygenase selective inhibitors in the gastrointestinal tract. Curr Gastroenterol Rep 2003;5(6):453-8.
- Bjorkman DJ. Nonsteroidal anti-inflammatory drug-induced gastrointestinal injury. Am J Med 1996;101(1A):25S-32S.
- Kimmey MB. NSAID, ulcers, and prostaglandins. J Rheumatol Suppl 1992;36:68-73.
- Richy F, Bruyere O, Ethgen O, Rabenda V, Bouvenot G, Audran M, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis 2004;63:759-66.
- Terzioglu N, van Rijn RM, Bakker RA, De Esch IJ, Leurs R. Synthesis and structure-activity relationships of indole and benzimidazolepiperazines as histamine H(4) receptor antagonists. Bioorg Med Chem Lett 2004;14:5251-6.
- Wang MW, Yanjun L. Synthesis of an active quaternary phosphonium salt and its application to the Wittig reaction: Kinetic study. J Chin Institute Chem Eng 2007;38:451-9.
- Mavrova ATs, Anichina KK, Vuchev DI, Tsenov JA, Denkova PS, Kondeva MS, et al. Antihelminthic activity of some newly synthesized 5(6)-(un)substituted-1H-benzimidazol-2-ylthioacetylpiperazine derivatives. Eur J Med Chem 2006;41:1412-20.
- Llorens O, Perez JJ, Palomer A, Mauleon D. Differential binding mode of diverse cyclooxygenase inhibitors. J Mol Graph Model 2002;20:359-71.
- Kanyalkar M, Coutinho E. Selectivity of Rofecoxib to COX-2 as revealed by Docking Studies. Asian Chem Lett 2004;8:241-8.
- Raghavendra NM, Jyothsna A, Venkateswara Rao A, Subrahmanyam CV. Synthesis, Pharmacological evaluation and docking studies of N-(benzo[d]thiazol-2-yl)-2-piperazin-1-yl)acetamide analogs as COX-2 inhibitors. Bioorg Med Chem Lett 2012;22:820-3.
- Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996;384:644-8.