- *Corresponding Author:
- Fatima S. Dasankoppa
Department of Pharmaceutics, KLE University College of Pharmacy, Vidyanagar, Hubballi-580 031
E-mail: fsdsankop@gmail.com
| Date of Submission | 02 January 2016 |
| Date of Revision | 20 October 2016 |
| Date of Acceptance | 01 December 2016 |
| Indian J Pharm Sci 2016;78(6):818-826 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Ion exchange resins are water-insoluble, cross-linked polymers containing salt forming groups in repeating positions on the polymer chain. Bitter cationic drugs get adsorbed on to weak cationic exchange resins of carboxylic acid functionally like Indion 204, Indion 234 and Tulsion 335 to forms the non-bitter complexes. The present investigation aims at taste masking of bitter clarithromycin using ion exchange resins, which forms complexes, inhibiting its release in saliva. The drug resin complex loading process was optimized for the content of resin, activation, swelling time, stirring time, influence of pH and temperature for maximum drug loading and were subjected to differential scanning calorimetry to confirm the complex formation. These complexes were used to prepare chewable tablets and statistically the taste was evaluated. Acid activated resins comprising of Indion 204, Indion 234 and Tulsion 335 with drug: resin ratio of 1:2, stirred in solution of pH 7-8 at 70º for 6 h had a maximum drug loading and masked the bitter taste of the drug. Differential scanning calorimetry of drug resin complex revealed that there was interaction leading to complex formation. Drug resin complex were formulated into chewable tablet formulations (F1-F9) and evaluated. The various pre-compression and post parameters were found to be within permissible limits. Formulations F3, F6 and F9 containing 1:2 ratios of drug resin complex of Indion 204, Indion 234 and Tulsion 335 revealed maximum taste masking. This was further confirmed by treatment of taste evaluation scores of the volunteers by ANOVA, Dunnet multiple comparison test and Tukey’s multiple comparison test. All the three optimized formulations had a significant difference of P<0.001 when compared to control F10. F6 formulation was widely accepted. Ion exchange complexation could efficiently mask the bitter taste of clarithromycin and achieve palatable taste suitable for pediatric use.
Keywords
Ion exchange resins, drug-resin complex, taste masking, ionic interaction, Indion 204, 234, Tulsion 335, Dunnet’s test, Tukey’s test
Oral dosage forms are mainly preferred due to the ease of their administration. The major problem faced by pharmaceutical industry is the bitterness of active pharmaceutical ingredient (API) leading to poor patient compliance. Most of the bitter drugs have amine functional groups, which is the cause of their obnoxious taste [1]. Masking of bitter and obnoxious taste of drugs in paediatric and geriatric formulations is a major challenge to the pharmacist to ensure patient compliance and product value [2]. Ion exchange resins are inexpensive and can be used to develop a simple, rapid and cost-effective method of taste masking [3]. Ion exchange resins are water-insoluble, cross-linked polymers containing salt forming groups in repeating positions on the polymer chain, have an affinity for oppositely charged counter ions, thus adsorbing the ions into the polymer matrix. The binding is generally an equilibrium process, resulting in continuous desorption or elution of drug from the resin as drug is getting absorbed into the body [4,5]. Ion exchange resins are used for taste masking, sustained release, targeted drug delivery and drug stabilization [6,7]. Bitter cationic drugs can get adsorbed on to weak cationic exchange resin with carboxylic acid functional groupings to form the complex, which is non-bitter [8].
Clarithromycin is a macrolide antibiotic, used in treatment of common paediatric infections of middle ear and upper respiratory tract as well as certain forms of pneumonia, which affect the elderly [9]. The drug is very bitter making oral administration difficult [10]. It is practically insoluble in water, exhibits polymorphism and poses stability problem by getting hydrolysed when in liquid formulation for a long time [11]. Hence, the objective of the present research was to formulate pharmaceutically acceptable chewable tablets of clarithromycin that become a useful and economic choice of a taste-masked composition for patients.
Materials and Methods
Clarithromycin was obtained as a gift sample from Ind-Swift Laboratories Limited, Punjab. Indion 204 and Indion 234 were obtained as gift samples from Ion Exchange India Ltd., Mumbai. Tulsion-335 was obtained as a gift sample from Thermax Ltd., Pune. Polyvinyl pyrrolidine was obtained from Himedia, Mumbai. Mannitol, talc and flavour were purchased from S. D. Fine-Chem. Ltd., Mumbai. Aspartame was procured from Nutra Sweet Company. Aerosil® was provided by Elegant Pharma, Hubballi and all the chemicals used were of either pharma or analytical grade. Taste evaluation of formulation was performed by volunteers in the age group of 21 to 25 years. The study protocol was explained and written consent was obtained from the volunteers.
Standard calibration curve of clarithromycin
Samples in the concentration range of 20-120 μg/ml were prepared in chloroform. To 10 ml of each dilution in a separating funnel, 5 ml of concentrated HCl was added followed by 10 ml of acetone and the reaction mixture was shaken gently for 5 min for forming a stable orange coloured complex [12]. The upper orangered coloured layer was separated and its absorbance was measured at 486 nm against the reagent blank.
Complexation with ion exchange resin
Drug loading on to the resin was optimized for various process parameters including activation conditions, swelling time, stirring time, pH, temperature and resindrug ratio [13].
Effect of activation conditions on resin drug loading capacity
Indion 204, Indion 234 and Tulsion 335 were washed with distilled water and subsequently with 1 N HCl and 1 N NaOH in separate processes for activation. The resin was repeatedly washed with water until neutral pH was reached. Drug-resin complexes (DRC) were prepared by adding 100 mg of activated resin that was swollen for 45 min in a beaker containing 50 ml distilled water, 100 mg of drug was added separately into each of the beaker containing the activated resin, to prepare slurry with the aid of magnetic stirrer for 6 h at 60°. The residue was washed with 100 ml of chloroform and filtered. From this solution, 0.5 ml was taken and diluted to 10 ml with chloroform. The unbound drug in the filtrate was estimated spectrophotometrically at λmax of 486 nm [12].
Optimization of resin concentration for maximum drug loading
Different quantities of acid activated resins to obtain resin:drug ratios of 1:1, 1:1.5 and 1:2 were placed in different beakers containing adequate quantities of deionized water and allowed to swell for 45 min. Hundred mg of drug was added and stirred using a magnetic stirrer for 6 h at 60°. The mixtures were filtered and residues were washed with adequate quantities of chloroform. The drug-loading efficiency of the resin was estimated [12]. The ratio of resin:drug revealing maximum loading of drug was the optimized ratio (Table 1).
| Resins | Percent drug bound | |||||
|---|---|---|---|---|---|---|
| Activation conditions | Resin ratio | |||||
| Inactivated | Acid | Base | 1:1 | 1:1.5 | 1:2 | |
| Indion 204 | 45.26±0.68 | 66.92±0.83 | 52.16±0.34 | 67.15±0.28 | 75.01±0.40 | 86.67±0.26 |
| Indion 234 | 44.59±0.77 | 66.30±0.72 | 50.54±0.64 | 67.87±0.56 | 75.01±0.29 | 86.81±0.36 |
| Tulsion 335 | 44.30±0.48 | 67.06±0.39 | 50.73±0.65 | 70.01±0.63 | 77.39±0.58 | 87.14±0.45 |
Table 1: Effect of activation conditions and resin concentrations on drug loading capacity
Optimization of swelling time of resin for maximum drug loading
Separate batches of acid-activated resins were soaked in adequate quantity of deionized water for 20, 40, 60 and 70 min at 60°. To each of the beaker drug was added at various ratios of resin:drug and mixtures were stirred for 6 h at 60°. Resin drug-loading efficiency was estimated [12]. The swelling time required for maximum drug loading was optimized (Table 2).
| Percent drug bound to various resins | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Swelling time (min) | Stirring time (min) | |||||||||
| Resins | 20 | 40 | 60 | 70 | 60 | 120 | 180 | 240 | 300 | 360 |
| Indion 204 |
79.29 ±0.94 |
83.67 ±0.70 |
86.91 ±0.46 |
86.91 ±0.59 |
65.96 ±0.36 |
71.20 ±0.55 |
76.91 ±0.88 |
80.72 ±0.69 |
84.76 ±0.46 |
86.67 ±0.78 |
| Indion 234 |
75.86 ±0.75 |
83.05 ±0.37 |
85.29 ±0.62 |
85.29 ±0.69 |
65.49 ±0.98 |
70.82 ±0.79 |
74.86 ±0.97 |
79.62 ±0.78 |
83.43 ±0.69 |
85.81 ±0.87 |
| Tulsion 335 |
70.96 ±0.64 |
83.14 ±0.47 |
87.86 ±0.63 |
88.10 ±0.78 |
66.20 ±0.35 |
70.96 ±0.48 |
75.01 ±0.68 |
79.29 ±0.46 |
82.62 ±0.73 |
86.19 ±0.61 |
Table 2: Optimization of swelling time and stirring time for maximum drug loading
Optimization of stirring time for maximum drug loading
Separate batches of acid-activated resins were soaked in adequate quantity of deionized water and drug was added and stirred for 60, 120, 180, 240, 300 and 360 min with the aid of a magnetic bead at 60°. Resin drug loading efficiency was estimated [12]. The time required for maximum drug loading was thus optimized (Table 2).
Optimization of processing temperature for maximum drug loading
Separate batches of acid-activated resins were soaked in adequate quantity of deionized water, drug was added at different temperatures namely; 30°, 40°, 50°, 60° and 70° using temperature-controlled magnetic stirrer for 6 h. The amount of bound drug was estimated [12]. From this optimum temperature for maximum drug loading was arrived at Table 3.
| Percent drug bound to various resins | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Resins | Temperature (°) | pH | |||||||
| 30 | 40 | 50 | 60 | 70 | 1.2 | 6 | 7 | 8 | |
| Indion 204 | 76.87 ±0.47 |
79.73 ±0.53 |
82.34 ±0.98 |
86.39 ±0.53 |
89.48 ±0.27 |
49.26 ±0.86 |
76.63 ±0.65 |
86.15 ±0.69 |
88.53 ±0.58 |
| Indion 234 | 74.9 ±0.46 |
78.06 ±0.73 |
82.87 ±0.59 |
85.77 ±0.28 |
87.72 ±0.85 |
48.07 ±0.38 |
75.20 ±0.87 |
84.01 ±0.59 |
86.39 ±0.66 |
| Tulsion 335 | 74.97 ±0.58 |
80.44 ±0.33 |
83.06 ±0.98 |
86.39 ±0.46 |
88.77 ±0.92 |
54.74 ±0.57 |
79.25 ±0.46 |
85.44 ±0.68 |
87.58 ±0.35 |
Results are mean±standard deviation (n=3)
Table 3: Optimization of temperature and ph for maximum drug loading
Optimization of pH for maximum drug loading
Separate batches of DRC in varying ratios were added to adequate quantities of solutions having pH 1.2, 6, 7 and 8 prepared from standard solutions of hydrochloric acid and sodium hydroxide in 100 ml beakers and stirred using a magnetic stirrer for 6 h at 60°. The drug loading efficiency was estimated [12] and the optimum pH value was arrived at Table 3.
Preparation of DRCs
Depending on the results of resin optimization study; batches of drug-resin mixtures at 1:1, 1:1.5 and 1:2 ratios were prepared as previously discussed at optimized conditions for maximum loading capacity and subjected to further evaluation [13].
Evaluation of the prepared DRCs, drug content
The drug content was determined by eluting the 250 mg of DRC with continuous stirring in 100 ml of 1 N HCl for 1 h to ensure complete elution [13]. The solution was filtered. After suitable dilution, the drug content was determined spectrophotometrically at λmax of 486 nm and the readings were obtained in triplicate.
Drug release
The drug release studies were performed by employing USP Type XXIII tablet dissolution apparatus. DRC equivalent to a 250 mg of clarithromycin was taken in 900 ml of pH 1.2 buffer. The temperature and rotation speed of the apparatus were maintained at 37±0.5° and 75 rpm, respectively. Aliquots were withdrawn after every 2 min for 30 min, were filtered using Whatman filter paper no. 1. Forty one and were analysed spectrophotometrically at λmax of 486 nm. The readings were taken in triplicate [13,14].
Experimental characterization of the formed ion exchange complex
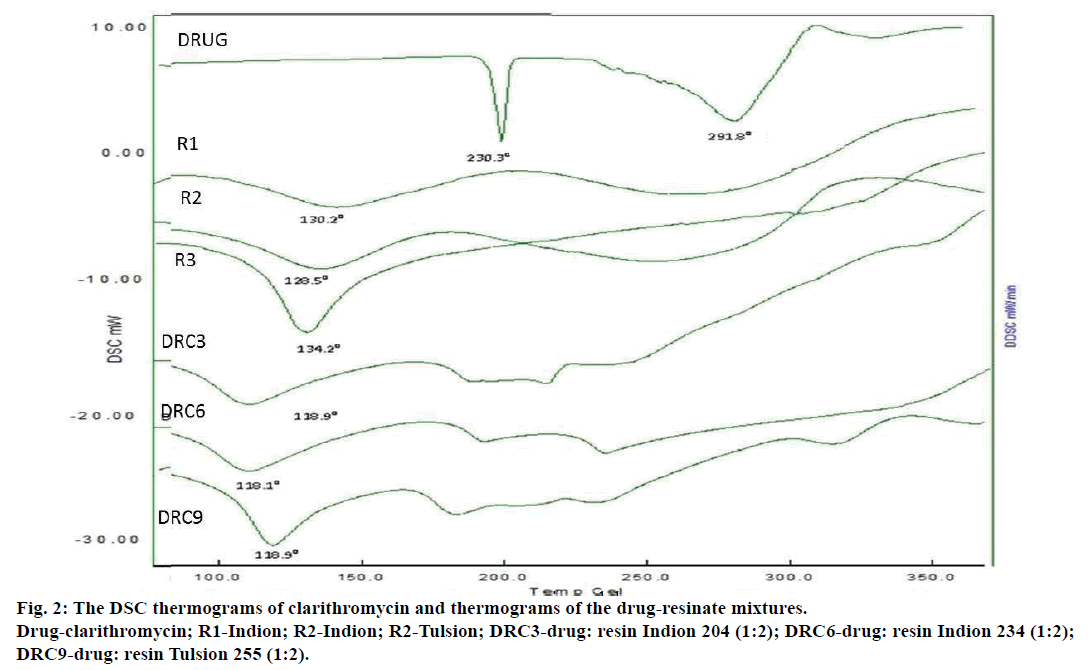
Differential scanning calorimetry (DSC) was carried out for the characterization of the complex formed. All the DRC were subjected to DSC and the thermograms were studied for drug polymer complex formation [13].
Micromeritics
The DRCs were evaluated for bulk density, tapped density, compressibility, angle of repose and Hausner’s ratio [15].
Formulation of the prepared DRC into chewable tablets
Accurately weighed amounts of the prepared complexes of clarithromycin resinates (Indion 204, Indion 234 and Tulsion 335) at 1:1, 1:1.5 and 1:2 drug:resin ratios equivalent to 250 mg of clarithromycin were mixed with other excipients and granulated using 2.5% w/v povidone solution in isopropyl alcohol as the granulating solution [13]. The dried granules were subjected to compression on a rotary tablet compression machine after lubrication with talc and aerosol. The formulations F1 to F9 represented with DRC and F10 represented without DRC (Table 4).
| Ingredients (mg) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Drug* | - | - | - | - | - | - | - | - | - | 250 |
| DRC Indion 204 |
641.02 (1:1) |
762.19 (1:1.5) |
815.21 (1:2) |
- | - | - | - | |||
| DRC Indion 234 |
- | - | - | 694.44 (1:1) |
791.13 (1:1.5) |
842.69 (1:2) |
- | - | - | - |
| DRC Tulsion 335 |
- | - | - | - | - | - | 666.66 (1:1) |
781.25 (1:1.5) |
842.69 (1:2) |
- |
| Polyvinyl pyrrolidine | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Aspartame | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| Aerosil | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Talc | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Flavor | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml |
| Isopropyl alcohol | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml |
| Mannitol | 306.48 | 185.31 | 132.29 | 253.06 | 156.37 | 114.81 | 280.84 | 166.25 | 114.81 | 647.5 |
| Total weight | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
Table 4: Formulation details for drug resinate complex (drc) chewable tablets
Evaluation parameters
Angle of repose, Carr’s index as well as the Hausner's ratio for drug powder and granule formulations of chewable tablets mixtures was determined and compared [13].
Evaluation of the prepared chewable tablets
Batches of 20 tablets of clarithromycin of different chewable formulae were evaluated for uniformity of tablet thickness, weight and diameter, weight variation, friability, hardness, time of disintegration and in vitro dissolution [13].
Assessment of the bitter taste of drug (bitterness threshold)
The bitter taste threshold value of clarithromycin was determined based on the bitter taste recognized by six volunteers. Before measurement, informed written consent was taken from each volunteer. Various concentrations (10-100 μg/ml) of drug were prepared in phosphate buffer pH 6.7. Mouth was rinsed with buffer solution and then, 10 ml of the most diluted solution was tasted by swirling it in the mouth mainly near the base of the tongue for 30 s. If the bitter sensation was no longer felt in the mouth after 30 s, the solution was spat out and waited for 1 min to ascertain whether this is due to delayed sensitivity [16]. Then the mouth was rinsed with safe drinking water. The recording was ‘-’, means did not detect any difference in taste; ‘+’ means detected some difference but was not able to be specify about the taste and ‘++’ means detected a bitter taste.
The next highest concentration was tasted after a time gap of 10 min. The threshold of bitterness of the drug is defined as the concentration at which more than half of the volunteer’s detected bitterness when holding the drug or formulation in their mouth. The threshold value was correspondingly selected from the different drug concentrations as the lowest concentration that had a bitter taste [13].
In vivo evaluation of bitter taste of formulations
Gustatory sensation tests of prepared chewable tablets were carried out. The pure drug formulation was kept as control during the study. The study protocol was explained and written consent was obtained from volunteers (n=6; 3 males and 3 females). Prepared formulae were kept in the mouth by each volunteer and the bitterness level was recorded against standard using numerical scale. The standard bitter quinine hydrochloride was used in concentrations of 0.01, 0.03, 0.10, 0.30 and 1.00 mM and the corresponding bitterness scores were defined as 0, 1, 2, 3 and 4, respectively. Before testing, volunteers were asked to keep the above standard quinine solutions in their mouth and were told the concentrations and bitterness scores of each solution [16]. After tasting a test formulation for 15 s, they were asked to give the sample a bitterness score. After testing the sample, volunteers rinsed their mouths well and waited for at least 20 min before tasting the next sample. The average scores for bitterness, mouth feel and overall acceptability for each formulation were calculated and the standard deviation was arrived.
Statistical analysis
The scores were then subjected to one way ANOVA [17] using GraphPad prism version 5.0 software. Dunnets multiple comparison test was used for comparing all the formulations F1 to F9 with the control formulation F10. Statistical significance was then interpreted. Tukey’s multiple comparison tests was used for comparing the scores within the groups with different ratios of the same resins and the formulations having highest significance levels from each group were compared.
Results and Discussion
Optimization of DRC and resin concentration for maximum drug loading was presented in Table 1. Optimization of swelling time and stirring time of resin for maximum drug loading has been shown in Table 2. Optimization of processing temperature and pH for maximum drug loading has been presented in Table 3. The loading capacity of the resin for clarithromycin was higher when activated in acidic rather than alkaline conditions. This could be explained by the stoichiometric nature of the exchange reaction between drug and resin in solution. All resins displayed increase in drug loading capacity with increase in swelling time. It was found that with increase in time of stirring of the solution, the drug loading gets increased and maximum drug loading was achieved within 6 h.
The effect of changing temperature on the exchange reactions is significant, since higher temperatures increased the ion diffusion. The percent drug loaded increased with raise in pH, which was due to the nature of the used resins and drug where the ionization was favoured at pH 8.
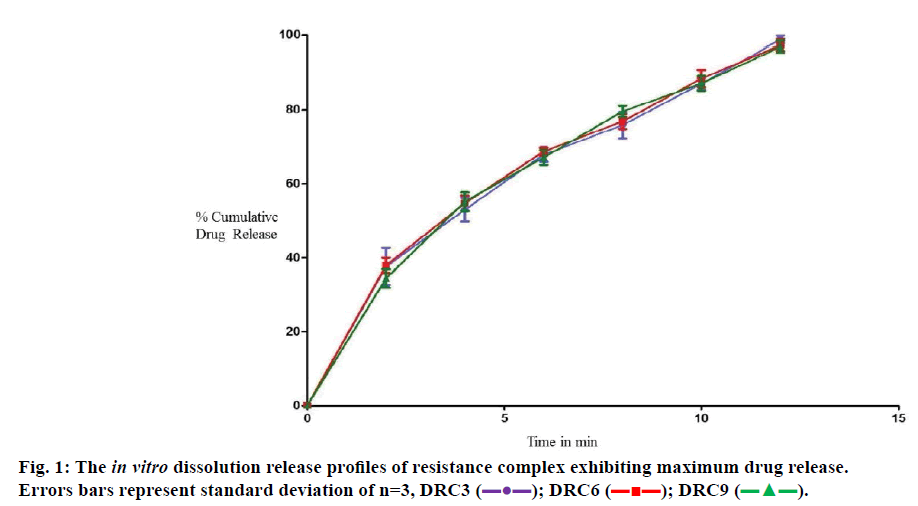
DRC displayed good micromeritic properties with angle of repose ranging from 26° 12±0.57 to 29° 38±0.47, bulk density values of 0.419±0.01 to 0.472±0.01 g/ cm3, tapped density of 0.505±0.07 to 0.575±0.07 g/ cm3, percent compressibility index of 13.461±0.89 to 17.913±0.73 and Hausner’s ratio of 1.155±0.08 to 1.218±0.07. DRC comprising of Indion 204, Indion 234 and Tulsion 335 at maximum drug-resin ratio (1:2) exhibited drug content in a range of 92.10±0.59%, 89.01±0.55% and 89.01±0.46%, respectively and faster dissolution when compared to the pure drug (Figure 1).
Evaluation of chewable tablets by DRC was transformed into granules and the pre-compression parameters were evaluated. The granules displayed angle of repose from 20° 57±0.48 to 25° 31±0.45, bulk density of 0.442±0.01 to 0.487±0.02 g/cm3, tapped density of 0.515±0.09 to 0.553±0.06 g/cm3, percent compressibility index values from 10.805±0.87 to 16.760±0.79 and Hausner’s ratio ranging from 1.121±0.06 to 1.201±0.06. All the prepared chewable tablet formulations (F1-F10) were subjected for evaluation of post-compression parameters. Diameter was found to be in a range of 13.36±0.05 to 15.15±0.05 mm, uniformity of thickness was found to be 3.08±0.012 to 3.25±0.022 mm, hardness was found to be in a range of 4.16±0.23 to 6.06±0.27 kg/cm3, friability ranged from 0.3±0.04 to 0.39±0.03% and the mean weight was found to be 998.04±0.60 to 1002.10±0.94 mg, disintegration time was in the range of 2.08±0.55 to 7.03±0.54 min and percentage drug content was from 97.44±0.89 to 98.99±0.68%.
All the formulations comprising of DRC displayed rapid drug release when compared to F10 formulations. F3, F6 and F9 formulations exhibited a release 98.64±0.332%, 99.05±0.112% and 98.85±0.221% in 14 min, respectively other formulations exhibited release within 20 to 22 min. F10 formulations displayed a release of 97.410±0.553% after 30 min. Thus, from the release studies it was evident that the ion-exchange resins can also act as dissolution enhancers apart from taste masking.
Threshold for bitterness of clarithromycin was detected by varying its concentration and threshold value was obtained from volunteers (n=6) (Table 5). The reference points for standard bitterness and palatability are shown in Table 6.
| Concentration (µg/ml) | Volunteers | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 10 | - | - | - | - | - | - |
| 20 | - | - | - | - | - | - |
| 30 | - | - | - | - | - | - |
| 40 | - | - | - | - | - | - |
| 50 | - | - | - | - | + | + |
| 60 | + | + | - | - | + | + |
| 70 | + | + | - | + | + | + |
| 80 | + | + | + | + | + | + |
| 90 | + | ++ | + | + | ++ | + |
| 100 | ++ | ++ | ++ | ++ | ++ | ++ |
-non bitter, +bitter, ++threshold bitterness
Table 5: Determination of threshold bitterness of clarithromycin
| Score | Mouth feel | Flavor | Overall acceptability |
|---|---|---|---|
| 0 | No | No | No |
| 1 | Gritty | Poor | Poor |
| 2 | Smooth | Acceptable | Acceptable |
| 3 | Creamy | Good | Good |
| 4 | Very creamy | Very good | Very good |
Table 6: Preference points for standard bitterness and palatability
Dunnet’s test was performed to evaluate bitterness and overall acceptability of F1 to F9 in comparison to F10 formulation without DRC (Table 7). Tukey’s test was performed to evaluate bitterness and overall acceptability by selecting one formulation which was significantly different from each of the three groups, i.e., F3 (from group of F1, F2 and F3), F6 (from group of F4, F5 and F6), F9 (from group of F7, F8 and F9) as shown in Table 8.
| Formulation code |
Mean score of F10±SD | Mean scores F1-F9±SD |
Summary |
|---|---|---|---|
| Bitterness scores | |||
| F10 vs. F1 | 3.83 ±0.4082 |
2.33±0.516 | *** |
| F10 vs. F2 | 1.16±0.408 | *** | |
| F10 vs. F3 | 0.16±0.408 | *** | |
| F10 vs. F4 | 2.16±0.408 | *** | |
| F10 vs. F5 | 1.33±0.516 | *** | |
| F10 vs. F6 | 0.00±0.000 | *** | |
| F10 vs. F7 | 2.00±0. 000 | *** | |
| F10 vs. F8 | 1.33±0.516 | *** | |
| F10 vs. F9 | 0.16±0.408 | *** | |
| Mouth feel scores | |||
| F10 vs. F1 | 2.00 ±0.6325 |
2.00±0.632 | ns |
| F10 vs. F2 | 2.167±0.98 | ns | |
| F10 vs. F3 | 2.00±0.894 | ns | |
| F10 vs. F4 | 1.83±0.752 | ns | |
| F10 vs. F5 | 2.00±0.894 | ns | |
| F10 vs. F6 | 2.00±0.894 | ns | |
| F10 vs. F7 | 2.00±0.632 | ns | |
| F10 vs. F8 | 1.83±0.752 | ns | |
| F10 vs. F9 | 2.00±0.632 | ns | |
| Acceptability scores | |||
| F10 vs. F1 | 0.333 ±0.5164 |
1.16±0.408 | * |
| F10 vs. F2 | 1.66±0.516 | *** | |
| F10 vs. F3 | 3.50±0.547 | *** | |
| F10 vs. F4 | 1.50±0.547 | ** | |
| F10 vs. F5 | 2.16±0.408 | *** | |
| F10 vs. F6 | 4.00±0.000 | *** | |
| F10 vs. F7 | 1.33±0.516 | * | |
| F10 vs. F8 | 1.66±0.816 | *** | |
| F10 vs. F9 | 3.33±0.516 | *** | |
***P<0.001, results are mean±standard deviation (n=3)
Table 7: Dunnet’s test for bitterness, mouth feel and overall acceptability evaluation for all the formulations
| Formulations code | Mean scores | Summary |
|---|---|---|
| Bitterness scores | ||
| F1vs. F2 | 2.33±0.516 vs. 1.16±0.408 | ** |
| F1 vs. F3 | 2.33±0.516 vs. 0.16±0.408 | *** |
| F2 vs. F3 | 1.16±0.408 vs. 0.160±0.408 | ** |
| F4 vs. F5 | 2.16±0.408 vs. 1.33±0.516 | ** |
| F4 vs. F6 | 2.16±0.408 vs. 0.00±0.000 | *** |
| F5 vs. F6 | 1.33±0.516 vs. 0.00±0.000 | *** |
| F7 vs. F8 | 2.00±0.000 vs. 1.33±0.516 | * |
| F7 vs. F9 | 2.00±0.000 vs. 0.16±0.408 | *** |
| F8 vs. F9 | 1.33±0.516 vs. 0.16±0.408 | *** |
| F3 vs. F6 | 0.16±0.408 vs. 0.00±0.000 | ns |
| F3 vs. F9 | 0.16±0.408 vs. 0.16±0.408 | ns |
| F6 vs. F9 | 0.00±0.000 vs. 0.16±0.408 | ns |
| Mouth feel scores | ||
| F1 vs. F2 | 2.00±0.632 vs. 2.167±0.983 | ns |
| F1 vs. F3 | 2.00±0.632 vs. 2.00±0.894 | ns |
| F2 vs. F3 | 2.16±0.983 vs. 2.00±0.894 | ns |
| F4 vs. F5 | 1.83±0.752 vs. 2.00±0.894 | ns |
| F4 vs. F6 | 1.83±0.752 vs. 2.00±0.894 | ns |
| F5 vs. F6 | 2.00±0.894 vs. 2.00±0.894 | ns |
| F7 vs. F8 | 2.00±0.632 vs. 1.83±0.752 | ns |
| F7 vs. F9 | 2.00±0.632 vs. 2.00±0.632 | ns |
| F8 vs. F9 | 1.83±0.752 vs. 2.00±0.632 | ns |
| F3 vs. F6 | 2.00±0.894 vs. 2.00±0.894 | ns |
| F3 vs. F9 | 2.00±0.894 vs. 2.00±0.632 | ns |
| F6 vs. F9 | 2.00±0.894 vs. 2.00±0.632 | ns |
| Acceptability scores | ||
| F1 vs. F2 | 1.16±0.408 vs. 1.66±0.516 | ns |
| F1 vs. F3 | 1.16±0.408 vs. 3.50±0.547 | *** |
| F2 vs. F3 | 1.66±0.516 vs. 3.50±0.547 | *** |
| F4 vs. F5 | 1.50±0.547 vs. 2.16±0.408 | * |
| F4 vs. F6 | 1.50±0.547 vs. 4.00±0.000 | *** |
| F5 vs. F6 | 2.16±0.408 vs. 4.00±0.000 | *** |
| F7 vs. F8 | 1.33±0.516 vs. 1.66±0.816 | ns |
| F7 vs. F9 | 1.33±0.516 vs. 3.33±0.516 | *** |
| F8 vs. F9 | 1.66±0.816 vs. 3.33±0.516 | ** |
| F3 vs. F6 | 3.50±0.547 vs. 4.00±0.000 | ns |
| F3 vs. F9 | 3.5±0.547 vs. 3.33±0.5164 | ns |
| F6 vs. F9 | 4.00±0.000 vs. 3.33±0.516 | * |
Table 8: Tukey’s test for bitterness and overall acceptability evaluation
The loading capacity of the resin for clarithromycin was higher when activated in acidic rather than alkaline conditions and this occurs in accordance with cationic nature of the used resin (Indion 204) since the COO- group of the Indion is loaded by H+ of the acid but not OH- of the base. The amount of drug loaded onto all the resins was higher with increasing polymer concentration. This can be explained by the stoichiometric nature of the exchange reaction between drug and resin in solution.
All the resins displayed increase in drug loading capacity with increase in swelling time; therefore time of swelling has to be established. Swelling and hydration increases the rate and extent of ion exchange process. In unswollen resin matrix, the exchangeable groups are latent and remain coiled towards their backbone. Swelling increases the surface area and these groups are oriented towards outside. It was found that with increase in time of stirring the solution, the drug loading gets increased and maximum drug loading was achieved within 6 h.
The temperature effect on the exchange reactions is significant since higher temperatures increase the ion diffusion rate through the exhaustive exchange zone which is markedly shrunk by temperature rise. The percentage drug loaded increased with increased pH, this is due to the nature of the used resin and drugs where the ionization was favoured at pH 8. The decreased complexation at higher pH is because at higher pH the solution becomes basic and cationic ion of resin gets saturated with basic solution, thereby hindering the drug attachment. The decreased complexation at lower pH is because in acidic environment the resin existed as a free acid in a non-ionic state and all the drug is released in the filtrate.
The evaluated parameters were within acceptable range. The in vitro dissolution studies were carried out for all the 9 DRC formulations and found to be excellent. The percentage drug released from 1:2 ratios of all the DRC was higher. The percentage of drug released from 1:2 ratio DRC6 of Indion 234 (DRC6) was found to be higher. This is due to the presence of more exchangeable H+ ions in stomach causing the rapid exchange of similar ions in DRC thereby releasing the drug. DSC thermograms of clarithromycin showed sharp characteristic endothermic peaks which completely disappeared in the thermograms of the drugresinate mixtures (DRC3, DRC6 and DRC9) thereby indicating interaction and complex formation. DSC thermograms of Drug, Resin and DRC are depicted in Figure 2. All micromeritic properties of the DRC were within the acceptable range. The formulations did display rapid drug release, but enhanced drug dissolution was observed in F6, F9 and F3 formulation due to higher ratio of drug to resin (1:2).
Taste evaluation test showed that the DRC-based formulae prepared at maximum resin concentration (1:2) of the formulations showed that there was no release in saliva to attain threshold bitterness concentrations thereby masking the bitter taste satisfactorily.
In case of Dunnet’s test for bitterness and overall acceptability evaluation, F1-F9 when compared to F10 gave P<0.001. Hence, all the formulations are significant when compared to F10. In case of Tukey’s test for bitterness and overall acceptability evaluation was carried out by selecting one formulations which was highly statistically significant from each of the three groups i.e., F3 (from group of F1, F2 and F3), F6 (from group of F4, F5 and F6) and F9 (from group of F7, F8 and F9). Hence, all the three formulations possess have good taste masking of the drug and good overall acceptability. F6 was widely accepted by the volunteers. In case of Dunnet’s test and Tukey’s test for mouth feel evaluation, F1-F9 when compared to F10 showed no significant difference. Hence, all the formulations have significantly same mouth feel when compared to F10. This concludes that addition of resins to the formulation did not alter the mouth feel of the chewable tablets. From the above data, it can be concluded that ion-exchange complexation of clarithromycin with Indion 204, Indion 234 and Tulsion 335 could efficiently mask the bitter taste and achieve palatable taste suitable for paediatric use.
Conflict of interest
There is no conflict of interest.
Financial support and sponsorship
Nil.
References
- Sharma S, Lewis S. Taste masking technologies: A Review. Int J Pharm Pharm Sci 2010;2:6-13.
- Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev Ind Pharm 2004;30:429-48.
- Roy G. Modifying bitterness: Mechanisms, ingredients and applications. Trends Food Sci Technol 1997;285-320.
- Borodkin S, Sundberg DP. Polycarboxylic acid ion-exchange resin adsorbates for taste coverage in chewable tablets. J Pharm Sci 1971;60:1523-7.
- Borodkin S, Yunker MH. Interaction of amine drugs with a polycarboxylic acid ion-exchange resin. J Pharm Sci 1970;59:481-6.
- Devi V, Krishna M. Advances in controlled and novel drug delivery. In: Jain NK, editor. 1st ed. New Delhi: CBS Publishers and Distributors; 2001. p. 290-306.
- Anand V, Kandarapu R, Garg S. Ion-exchange resins: Carrying drug delivery forward. Drug Discov Today 2001;6:905-14.
- Borodkin S. Ion exchange resins delivery systems. In: Tarcha PJ, editor. Polymers for drug delivery. 1st ed. Boca Raton: CRC Press, 1991. p. 215-30.
- Venkateswaramurthy N, Sambathkumar R, Vijayabaskaran M, Perumal P. Clarithromycin mucoadhesive microspheres for antihelicobacter pylori therapy: Formulation and in vitro evaluation. Int J Curr Pharmaceut Res 2010;2:24-27.
- Mou-ying Fu Lu, Borodkin S, Woodward L, Li P, Diesner C, Hernandez L, et al. A polymer carrier system for taste masking of macrolide antibiotics. Pharma Res 1991;8:706-12.
- Akre HS, Mundhada DR, Bhaskaran S, Asghar S, Gandhi GS. Dry suspension formulation of taste masked antibiotic drug for pediatric use. J Appl Pharm Sci 2012;02:166-71.
- Goyal A, Singhvi I. Visible spectrophotometric methods for estimation of clarithromycin from tablet formulation. Indian J Pharm Sci 2006:656-7.
- Helmy A, El Kady S, Khames A, Abd-elbary A. Preparation, characterization and in vitro/in vivo evaluation of indion-based chewable tablets of paracetamol and ibuprofen for pediatric use. J American Sci 2011;7:831-44.
- Patra S, Sahoo R, Panda RK, Himasankar K, Barik BB. In vitro evaluation of domperidone mouth dissolving tablets. Indian J Pharm Sci 2010;72:822-5.
- Bhise K, Shaikh S, Divyakumar Bora. Taste Mask, design and evaluation of an oral formulation using ion exchange resin as drug carrier. AAPS Pharm SciTech 2008;9:557-62.
- Uchida T,Miyanaga Y, Tanaka H, Wada K, Kurosaki S, Ohki T, et al. Quantitative evaluation of the bitterness of commercial medicines using a taste sensor. Chem Pharm Bull 2000;48:1843-5.
- Tavakoli N, Ghodrati M, Ghassemi-Dehkordi N, Sadeghi-Aliabadi H. Formulation and evaluation of a new herbal tablet from strawberry and grape leaves. Jundishapur J Nat Pharm Products 2008;3:19-25.