- *Corresponding Author:
- K. Pundarikakshudu
L. J. Institute of Pharmacy, Sanand Cross Road, Near Nagdev Kalyan Mandir, Sarkhej Gandhinagar Highway, Ahmedabad - 382 011, India
E-mail: p_kilambi@yahoo.com
| Date of Submission | 12 January 2006 |
| Date of Revision | 23 February 2007 |
| Date of Acceptance | 23 May 2007 |
| Indian J Pharm Sci, 2007, 69 (3): 394-401 |
Abstract
The purpose of the present investigation was to achieve colon specific delivery of sennosides using the polysaccharide pectin as a compression-coating agent. In this study, pectin along with hydroxypropylmethylcellulose was used for compression coating of the core tablets of calcium sennoside. Drug dissolution and erosion studies were carried out in pH 1.2 and phosphate buffer pH 7.4 using a pectinolytic enzyme. The system was designed based on the gastrointestinal transit time concept, assuming colon arrival time to be 6 h. It was found that pectin alone was not sufficient to protect the core tablets during entire gastrointestinal transit time. Addition of hydroxypropylmethylcellulose was required to control the erosion of tablets. In this investigation a 3 2 factorial design was constructed to investigate the influence of two variables; the amount of hydroxypropylmethylcellulose (X1) and coat weight of the tablets (X2) on the time taken for 50% erosion of tablet in presence of pectinase enzyme (TE 50 ) and average percent weight difference between tablets with and without enzyme at the 10 th hour (% WD). The results revealed that for protecting the calcium sennosides core tablets in upper gastrointestinal tract, the core tablets should be coated with lower amount of hydroxypropylmethylcellulose and higher amount of coat weight. The main effects were found to be statistically significant in nature. The amount of hydroxypropylmethylcellulose exhibited predominant action as compared to coat weight. In vivo performance was assessed by X-ray roentegenography study. The pectin-hydroxypropylmethylcellulose coating was found to be a promising colon delivery system for those drugs like sennosides.
Keywords
Colonic delivery, calcium sennoside, compression coating, pectinase enzyme, Roentegenography
During the last decade there has been an increased interest in the development of site-specific formulations for targeting drug delivery to the colon. The colon is a site for local and systemic drug delivery. A large number of polysaccharides are degraded by colonic bacteria and form the basis for a suitable carrier for colon targeted drug delivery system [1]. Pectin is one such heterogenous polysaccharide present in the cell wall of the plants. It consists mainly of Dgalacturonic acid and its methyl ester linked via ∝ (1-4) glycosidic bonds. Various reports suggest that pectin and its salt may be of value in designing drug delivery formulation to the colon [2-6]. It is non-toxic and almost totally degraded by colonic bacteria and is not digested by gastric or intestinal enzymes. A constraint of the polymer, pectin is its high solubility, but this is easily overcome either through choice of high methoxylated pectin and presence of additives.
Senna known as Tinnevelly Senna, family Leguminoseae, is a well-known herbal laxative drug and is included in many pharmacopoeias. The active constituents sennoside A and B, mainly act upon the large intestine and are therefore, especially suitable in habitual constipation. The glycosides are absorbed from the intestinal tract and the active anthraquinone are excreted into the colon, where they stimulate and increase the peristaltic movements of the colon by local action. This results in decreased absorption of water and thereby a bulky and softer fecal mass is produced. This suggests their action in lower bowl and no effect in stomach and small intestine [7-9]. By targeting we can ensure that entire quantity of sennoside is made available to colon thereby obviating any possible loss during passage through systemic circulation. Hence, one can expect optimal result, if the drug is targeted directly to the colon. We have reported on colon targeted drug delivery of sennosides [10-11].
The present study deals with the development of colonic drug delivery of sennosides using pectin. A 32 full factorial design was employed in this study to systematically design and develop colon specific calcium sennoside tablets.
Materials and Methods
Calcium sennoside (20%w/w) was gifted from Dishman Pharmaceuticals, Ahmedabad, India. Pectin USP, Hydroxypropylmethylcellulose (HPMC K4 M), cross linked polyvinyl pyrrolidone and polyvinyl pyrrolidone (PVP K 30) were gifted from Zydus Cadila Healthcare Ltd., Ahmedabad, India. Pectinase (Pectinex®) was obtained from Biocon India Limited, Bangalore, India. All other chemicals were of reagent grade.
Preparation of calcium sennoside core tablets
Calcium sennoside, 100 mg (20%w/w sennosides calculated as sennoside B) was dry mixed with cross-linked PVP (2%w/w) and PVP K30 (5% w/w). Isopropyl alcohol was added in sufficient quantity to granulate the powder blend. Granules were sieved through 1 mm aperture size sieve and dried at 45°. Granules were lubricated with talc (2%) and magnesium stearate (1%) and compressed using a rotary tablet press (Cadmach Machinery, India) having 12 mm size die. Weight variation, crushing strength; friability, thickness, disintegration time and dissolution in phosphate buffer pH 7.4 were performed for the core tablets. The average tablet weight was 105±0.45 mg. Tablet had a crushing strength and friability of 4.5 kgf and 0.21%, respectively.
Gelling and swelling study
Pectin and its physical blend with HPMC in varying proportions were subjected to gelling and swelling study. Different dissolution medium, 0.1 N HCl, pH 6.8 buffer and pH 7.4 buffer were used for the study. Physical blend (100 mg) of pectin and HPMC (100:0, 80:20, 60:40) was dispersed in 100 ml of each medium in graduated cylinder. Gelling and swelling were observed after standing for 24 h.
Experimental design
A 32 full factorial design was utilized in the present investigation [12]. The proportion of HPMC in coat (X1) and coat weight (X2) were used as independent variables. The experimental design is presented in Table 1. The chosen dependent variables were TE50 (time taken for 50% erosion of tablet in presence of pectinase enzyme) and% WD (average percent weight difference between tablets with and without enzyme at the 10th h).
| Number | Variable level | TE50 | %WD | T80 | |||
|---|---|---|---|---|---|---|---|
| X1 | X2 | With outP* | With P* | With out P* | With P* | ||
| 1 | -1 | -1 | 4.6 | 4.2 | 19.13 | 5.4 | 5.2 |
| 2 | -1 | 0 | 5.9 | 5.8 | 15.53 | 5.9 | 5.6 |
| 3 | -1 | 1 | 6.1 | 6 | 13.58 | 6.8 | 6.2 |
| 4 | 0 | -1 | 7.6 | 7 | 12.5 | 7.8 | 7.5 |
| 5 | 0 | 0 | 7.8 | 7.1 | 11.32 | 8.2 | 7.9 |
| 6 | 0 | 1 | 8.6 | 7.2 | 10.98 | 8.9 | 8.2 |
| 7 | 1 | -1 | 9.5 | 7.3 | 10.52 | 9.9 | 9.2 |
| 8 | 1 | 0 | 9.7 | 7.5 | 10.27 | 10.8 | 9.9 |
| 9 | 1 | 1 | 9.9 | 7.7 | 9.1 | 12.4 | 11 |
P* is Pectinase enzyme, TE50 is time taken for 50% erosion of tablet in presence of pectinase enzyme, %WD is average percent weight difference between tablets with and without enzyme at the 10th h. Translation of coded levels in actual units is as follows: coded levels for X1: HPMC proportion (mg) are, -1 is 0, 0 is 20 and +1 is 40, while for X2: weight of coat (mg) are, -1 is 250, 0 is 350 and +1 is 450, respectively. T80 is time taken for 80% sennosides released from formulation.
Table 1: Full Factorial Experimental Design Layout
Compression coating of calcium sennoside core tablets
Polymer blend of pectin and HPMC (100:0, 80:20, 60:40) was prepared by simple mixing. Calcium sennoside core tablets with satisfactory physical parameters were compression coated using different proportions of polymer blend at different levels according to the full factorial design layout (Table 1). Average 45% of polymer blend was added in 15 mm size die cavity of a rotary tablet press (Cadmach Machinery, India). Calcium sennosides core tablets were then placed in the center. Rest of the amount of polymer blend was added and tablets were compressed (250, 350 and 450 mg according to the design). The hardness of the tablet was adjusted to 5.5 kgf.
Erosion study for compression-coated tablets
The compression-coated tablets were subjected to erosion studies. The USP dissolution apparatus 2 was used at 50 rpm at 37±0.5°. First, medium used was 900 ml of 0.1 N HCl. The test was continued for 2 h, after 2 h the dissolution medium was discarded and refilled with phosphate buffer pH 7.4 and the test was continued for additional 4 h. After 6 h, depending on the design 3% pectinase was added to the dissolution medium and the test was continued for another 4 h. After each sampling time (2 h, 6 h, 8 h and 10 h) one tablet was removed and dried overnight at 45° in an oven and the remaining tablet mass was determined gravimetrically as described by Turkoglu et al [13]. Control study (without pectinase enzyme) was also carried out by continuing the dissolution study in phosphate buffer pH 7.4 for a predetermined time. The results of TE50 and %WD with and without enzyme are shown in Table 1.
In vitro dissolution studies of compression-coated tablets
In vitro dissolution studies were conducted using USP dissolution apparatus 2 at 50 rpm at 37±0.5°. During dissolution studies physiological condition of stomach to colon was mimicked using different dissolution media. First 900 ml of 0.1 N HCl solution was used as a dissolution medium and the study was continued up to 2 h. The medium was then discarded and refilled with phosphate buffer pH 7.4 and the study was continued for additional 4 h. After 6 h, depending on the design 3% pectinase was added to the dissolution medium and the test was continued for a predetermined time. Control study (without pectinase enzyme) was also carried out by continuing the dissolution study in phosphate buffer pH 7.4 for a predetermined time. Ten millilitres sample was withdrawn after each sampling hour (2 h) and replaced by fresh dissolution media. Calcium sennoside concentration was determined spectrophotometrically as per British Pharmacopoeia (BP) using Hitachi U 2000 spectrophotometer at 505 nm [14]. T80 (time taken for 80% sennosides to be released from formulation) of each batch are reported in Table 1.
In vivo roentgenography studies for compressioncoated tablets
Each core tablet (105 mg) for in vivo studies consisted barium sulphate 100 mg, Co-crystallized lactose-MCC and cross linked PVP (5% w/w) were dry mixed PVP K30 (5% w/w). Isopropyl alcohol was added in sufficient quantity to granulate the powder blend. The crushing strength, friability and diameter of the tablet were kept same as those obtained with sennosides tablets. The core tablet was compression coated with optimized formulation derived after factorial design study.
Healthy volunteers of 18-29 y of age participated in the study. They were non-alcoholics, non-smoking and were not on any drugs. The purpose of the study was fully explained and each volunteer had given his written consent. The study design has been approved by Institutional Ethical Committee on Clinical Studies. After overnight fasting, each volunteer orally ingested compression coated barium sulphate core tablet with 200 ml of water. X-ray images of GIT were obtained with standard KUB AP view on 12×15 films using a 300 ma X-ray unit with 30 MAS and 55 KU techniques. All X-rays were taken at different time interval 3 h, 4 h and 7 h to follow the movement, location and the integrity of the tablet in the digestive tract.
Results and Discussion
Calcium sennoside core tablets (105±0.45 mg) were prepared by direct compression technique. Tablets had satisfactory hardness (4.5 kgf) and friability (0.21%). Disintegration time for the tablets was found to be less than 6 min. Core tablets were tested for dissolution rate in phosphate buffer pH 7.4. The tablets released 100% of calcium sennoside within 0.5 h.
Physical blend of pectin and HPMC in varying proportions were subjected to gelling and swelling study. It was observed that loose gel was formed when pectin was used alone suggesting that tablets coated with only pectin would be eroded very fast. While the physical blend of pectin and HPMC gave stiff gelling properties. Swelling of only pectin as well as its combination with HPMC was satisfactory. For a system where erosion of the outer coat plays vital role, the coating should have good swelling as well as stiff gel formation. Thus, combination of pectin and HPMC would be essential to achieve good swelling and gelling of formulation.
The responses are measured and polynomial equation is derived by carrying out multiple regression analysis and F- statistics to identify statistically significant. Y= b0+b1X1+b2X2+b12X1X2+b11X12+ b22X22(Eqn.1), where Y is dependent variable, b0 is the arithmetic mean response of the nine runs; bi is the estimated coefficient for the factor Xi. The main effects (X1 and X2) represent the average results of changing one factor at a time from its low to high value. The interaction (X1X2) shows how the response changes when two factors are changed simultaneously. The polynomial terms (X12 and X22) are included to investigate non-linearity.
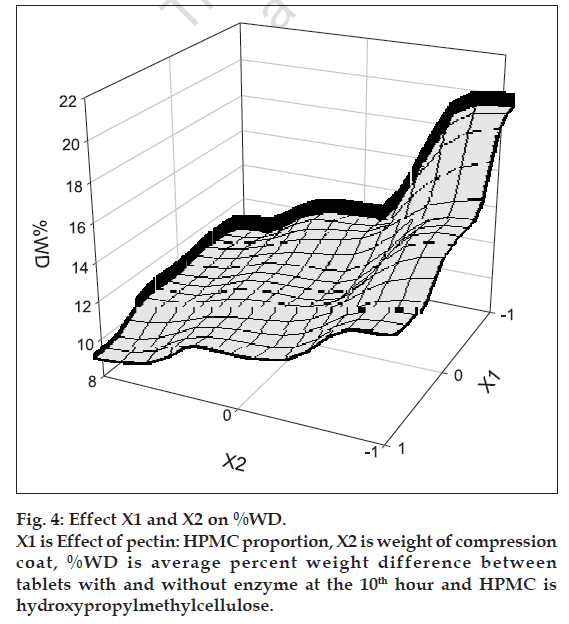
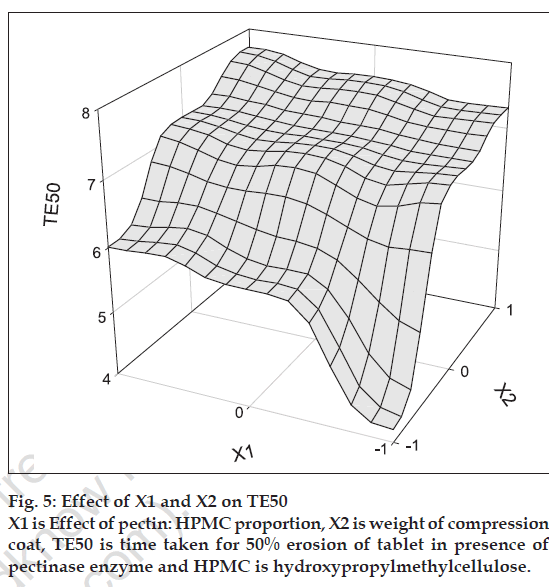
Thus, a systematic study was adopted by applying 32 full factorial design. The amount of HPMC in coat (X1) and coat weight (X2) were used as independent variables. Dependent variables chosen were TE50 and %WD. The following polynomial equation was evolved for relating the time required for 50% erosion of tablets in presence of pectinase with independent variables. TE50= 8.06+2.08X1+0.48X2–0.28X1X2– 0.38X1 2–0.08X2 2..Eqn. 2., (R2= 0.991, F= 67.705, DF= 8). The value of TE50 for the nine batches showed a wide variation from a minimum of 4.6 h to a maximum of 9.9 h, indicating that the independent variables influence the selected dependent variables. The main effects (X1 and X2) are significant (P<0.05). The interaction and polynomial terms are insignificant. The coefficient value of X1 (2.08) is more than that of X2 (0.48) indicating that X1 is more effective in relation to TE50 than X2. The erosion of tablet largely depends on X1.%WD= 0.163-0.082X1-0.010 X2+0.013X1X2+0.035X1 2–0.010X2 2..Eqn.3, (R2= 0.97, F= 17.46, DF= 8). The value of %WD for the nine batches showed a wide variation from a minimum of 9.10 to a maximum of 19.73 h, indicating that the independent variables have some influence on the selected dependent variables. The main effect (X1) is significant (P<0.05). The interaction is significant. The coefficient value of X1 X2 (0.013) and X1 2 (0.035) indicate that X1 is more effective for %WD than X2.
The polynomial equation can be used to draw conclusions after considering the magnitude of the coefficient and the mathematical sign it carries, i.e. positive or negative. Surface response plots were generated based on the Eqns. 2 and 3.
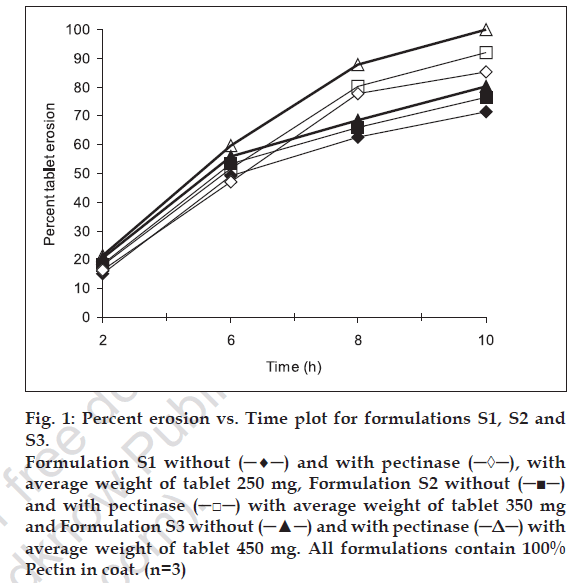
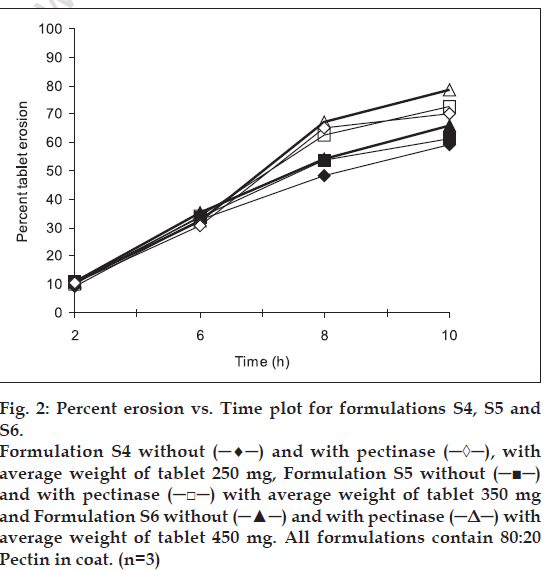
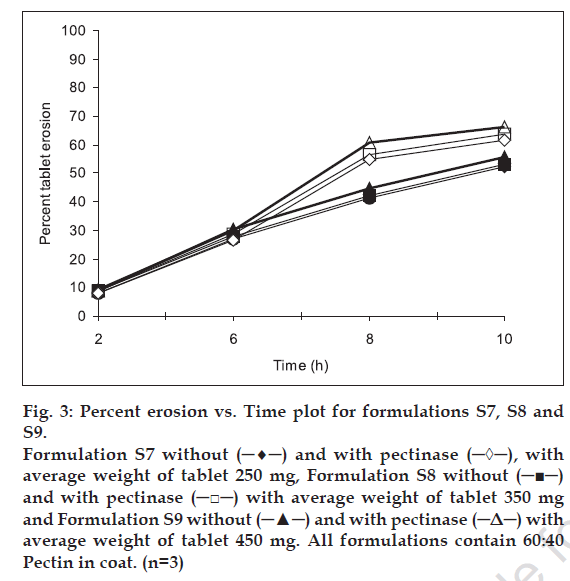
Erosion study of compression-coated tablets was carried out in different dissolution medium to mimic the physiological condition of gastrointestinal tract. Three levels of pectin: HPMC ratio i.e. 100:0, 80:20, 60:40 and three levels of coating thickness i.e. 250, 350 and 450 mg, were studied. figs. 1-3 show the percent erosion for batches S1-S3, S4-S6 and S7-S9. The values of TE50 and %WD of tablets in presence of enzyme and without enzyme are given in Table 1.
Fig 1: Percent erosin vs.Time plot for formulations S1, S2 and S3.
Formulation S1 without (─♦─) and with pectinase (─◊─), with
average weight of tablet 250 mg, Formulation S2 without (─■─)
and with pectinase (─□─) with average weight of tablet 350 mg
and Formulation S3 without (─▲─) and with pectinase (─Δ─) with
average weight of tablet 450 mg. All formulations contain 100%
Pectin in coat. (n=3)
Fig 2: Percent erosion vs. Time plot for formulations S4, S5 and S6. Formulation S4 without (─♦─) and with pectinase (─◊─), with average weight of tablet 250 mg, Formulation S5 without (─■─) and with pectinase (─□─) with average weight of tablet 350 mg and Formulation S6 without (─▲─) and with pectinase (─Δ─) with average weight of tablet 450 mg. All formulations contain 80:20 Pectin in coat. (n=3)
Fig 3: Percent erosion vs. Time plot for formulations S7, S8 and S9. Formulation S7 without (─♦─) and with pectinase (─◊─), with average weight of tablet 250 mg, Formulation S8 without (─■─) and with pectinase (─□─) with average weight of tablet 350 mg and Formulation S9 without (─▲─) and with pectinase (─Δ─) with average weight of tablet 450 mg. All formulations contain 60:40 Pectin in coat. (n=3)
The fastest erosion was seen in batches with 100% pectin and no amount of HPMC. Among all the batches, the slowest erosion pattern was observed with batches (S7, S8 and S9) containing 60: 40 pectin: HPMC ratio (fig. 3). Thus we can conclude that HPMC assist in slow erosion pattern of the tablets. At the 6 h point where pectinase was added to the dissolution medium, the tablets of batches S1, S2 and S3 had already lost more than 50% of their weight while for batches S7-S9 resulted in the high difference in percent erosion of tablets i.e. 7.3-7.7. Thus, 60:40 pectin:HPMC combination was too slow for the GI transit time concept. Results of erosion study with respect to coat weight were also found significant. The surface response plots (figs. 4 and 5) indicate that higher amount of X1 and higher amount of X2 favors higher %WD and lower TE50. Higher coat weight i.e. 450 mg, could help in protecting system up to 6 h. Hence, 80:20, Pectin: HPMC ratio at 450 mg coat weight (batch S6) was considered to be optimum for delivering sennosides in the colon with no release in stomach and upper intestine.
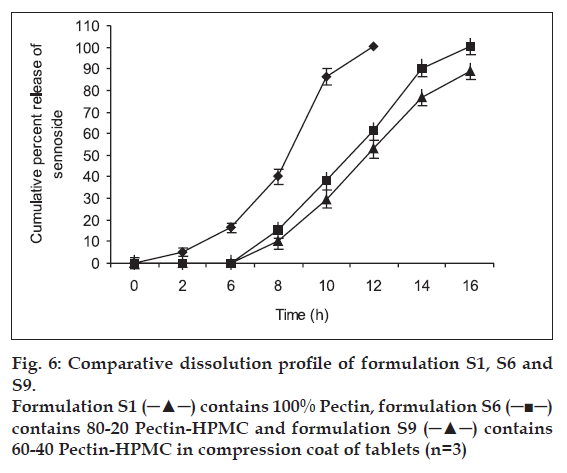
colon targeting, it is desirable that the system remains intact in the physiological environment of stomach and upper intestine and releases the drug in the colon. For the laxative drugs like, calcium sennosides acting locally on the colon they are required to be released at site of action without loss of drug in the upper GI tract. Hence, an attempt was made to formulate a dosage form, which triggers drug release in colon. The compression coat was designed to undergo bacterial degradation in the colon and rapidly disintegrate core in the colon. To determine the effect of pectinolytic enzyme, dissolution studies were carried out with and without the enzyme, pectinase. The enzyme was added at the 6 h to simulate the colon arrival time under normal conditions. At the 2 h sampling intervals sennoside was detected for all the batches. T80 (80% drug released from tablet) of all batches are shown in Table 1. Dissolution was continued up to 16 h depending on the tablet erosion pattern. Comparative dissolution profile of batches S3, S6 and S9 shown in fig. 6 indicates that as the amount of HPMC increases drug release in the initial hours can be retarded. T80 values for the nine batches show a wide variation i.e. the response ranges from a minimum of 5.2 to 11.0 h. The data clearly indicates that the T80 value is dependent on the factors selected. At lower level of HPMC erosion of tablet was fast and hence, premature drug release was observed. Batches S1 to S3 showed T80 of 5.2, 5.5, 6.2 h and 5.4, 5.9, 6.8 with and without pectinase enzyme, respectively indicates the effect of coat weight on time taken for 80% drug release. As the coat weight increased value of T80 also increased. Higher HPMC levels (batches S7 to S9) show slower drug release. T80 values were found to be 9.2, 9.9, 11.0 h and 9.9, 10.8, 12.4 h with and without enzyme, respectively. Hence, the final selection was done from batches S4, S5 and S6 showing T80 values 7.5, 7.9 and 8.2 in presence of pectinase. Batch S6 was selected as optimized formulation as the enzyme concentration taken for the dissolution study may be less than that actually present in the healthy human colon. From the overall dissolution data it can be concluded that HPMC plays a major role in optimization of the formulation, coat thickness helps in protecting core tablet up to 6 h and addition of pectinase in dissolution media is essential to mimic the colon environment.
The in vitro drug release studies in the presence of rat caecal content showed that coat formulation S6 containing Pectin: HPMC (80:20) was optimal for targeted delivery of drug to colon. However, the evaluation of the dosage forms in humans renders support to in vitro studies. Hence, Roentegenography studies were carried out in three healthy human volunteers to access the in vivo performance of the optimized batch. The studies were carried out using barium sulphate as X-ray opaque material. As per the advice of a well-known radiologist of the city, the time and amount of radiation exposed to volunteers would be more if γ-scintigraphy technique is adopted. Also, γ-scintigraphy technique utilizes the radiotracers, which may harm the volunteers. The same technique has been utilized successfully in our previous work [10,15]. Further, X-ray visulatization of tablet disintegration performance has been studied by researcher16. Hence, it was proposed to use Roentegenography technique, which is comparatively safer technique.
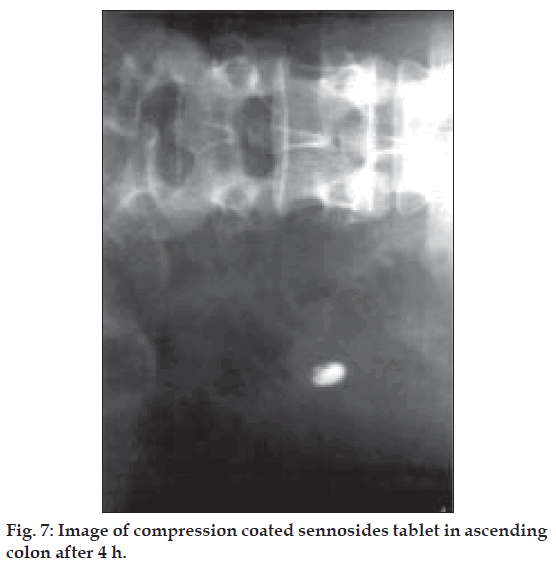
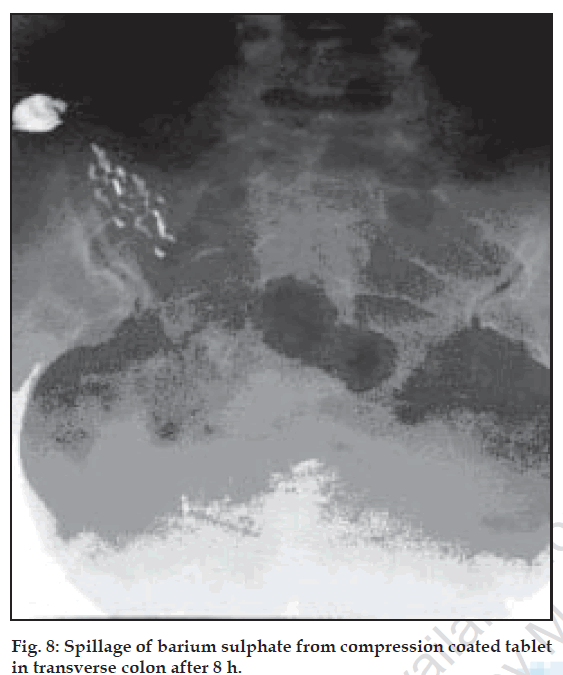
The compression-coated tablet of barium sulphate (455 mg) was ingested by the healthy volunteers and images were taken at different time intervals i.e. 4 h, 8 h. The colonic arrival time of the tablet was accessed and found to be 2-4 h in all volunteers. It was observed that tablet was swollen but remained intact till 4 h (fig. 7). Barium sulphate was not released either in stomach or in small intestine of volunteers. At 8th h, in the colon the tablets were found broken. X-ray showing ‘spillage’ of barium sulphate in ascending colon proves that on entering the colon, the coating of the tablets began to disintegrate because of bacterial enzymatic action (fig. 8). The process of degradation of pectin and solubalisation of HPMC appeared to have initiated at around 4th h after ingestion of tablet.
The above results indicate the efficiency of the formulation S6 to protect the core up till it reach to colon. Sennosides will only be made available in the colon, which will further give the pharmacological action. Thus, pectin and HPMC in the form of coat is capable to protect the drug from being released completely in the physiological environment of stomach and small intestine.
Calcium sennoside tablets were successfully prepared using pectin and HPMC to deliver the sennosides in the colon. The systems made from a mixture of these polymers swell and form a hydrogel layer when placed in an aqueous medium. For optimization of the formulation 32 full factorial design was adopted. The amount of HPMC in coat (X1) and coat weight (X2) were used as independent variables. Dependent variables chosen were TE50 and % WD. The study showed both independent variables showed significant effect on the chosen dependent variables. The pure pectin coat was found insufficient to protect calcium sennoside core till the 6 h where pectinase was added based on the time delivery concept. The polymer blend of pectin: HPMC in 80:20 ratio provided an intermediate erosion pattern for the colonic delivery of sennoside tablets. Hence, it can be concluded that the drugs like, sennosides can be delivered to colon without releasing in stomach and upper intestine using optimized combination of pectin and HPMC. Roentegenography, a comparatively safer technique, to view the in vivo behavior of formulation was adopted. Results of Roentegenography studies of batch S6 (Pectin: HPMC (80:20) and 450 mg coat weight) showed successful drug delivery to the colon in healthy human volunteers.
Acknowledgements
The authors gratefully acknowledge the support from Prof. B. M. Peerzada and Prof. Manish Shah for providing facilities. The authors also gratefully acknowledge Mr. A. C. Shah, Vice president, Troikka Pharmaceuticals, Ahmedabad, India for the gift sample of Pectinax and Dr. Avani F. Amin for her help in statistical design.
References
- Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci 2003;6:33-66.
- Rubinstein A, Radai R. In vitro and in vivo analysis of colon specificity of calcium pectinate formulations. Eur J Pharm Biopharm 1995;41:291-5.
- Wakerly Z, Fell J, Attwood D, Perkins D. Studies on drug release from pectin/ethylcellulose film coated tablets: A potential colonic delivery system. Int J Pharm 1997;153:219-24.
- Sriamornsak P. Investigation of pectin as a carrier for oral delivery of proteins using calcium pectinate gel beads. Int J Pharm 1998;169:213-20.
- Macleod G, Collett J, Fell J. The potential use of mixed films of pectin, Chitosan and HPMC for bimodal drug release. J Control Release 1999;58:303-10.
- Turkoglu M, Takka SD, Baran H, Sakr A. Pectin hydroxymethyl cellulose drug delivery system for colon targeting. Pharm Ind 1999;61:662-5.
- Fairbairn JW. The active constituents of the vegetable purgatives containing anthracene derivatives. J Pharm Pharmacol 1949;1:683- 6.
- Franz G. The Senna drug and its chemistry, Pharmacology 1993;47:2-6.
- Reynolds JE. editor. In: Martindale: The extra pharmacopoeia, 32nd ed. London; Royal Pharmaceutical Society: 1996. p. 1212.
- Munira M, Pundarikakshudu K. Studies on the development and optimization of oral colon targeted drug delivery system for sennoside in the treatment of constipation. Drug Delivery Technol 2004;6:71-7.
- Munira M, Pundarikakshudu K. Studies on formulation development of colon targeted drug delivery system for sennosides. J Pharm Pharma Sci. Available from: http://www.ualberta.ca/~csps/JPPS7/Issue7(3).htm. [Last updated on 2004 Dec].
- Bolton S. In: Pharmaceutical statistics, 3rd ed. New York; Marcel Dekker Inc.: 1997. p. 326.
- Turkoglu M, Timucin U. In vitro evaluation of pectin-HPMC compression coated 5-aminosalicylic acid tablets for colonic delivery. Eur J Pharm Biopharm 2002;53:65-73.
- British pharmacopoeia, 15th ed. British pharmacopoeia commission. Health ministry. London; 1993. p. 592.
- Munira M, Pundarikakshudu K. Optimization and pharmacotechnical evaluation of compression coated colon specific drug delivery system of Triphala using factorial design, Drug Dev Res 2005;65:34-42.
- Wallace H., Steinberg GH, Josheph N, Hasting H. Methods for determining in vivo tablet disintegration. J Pharm Sci 1965;54:747-52.