- *Corresponding Author:
- A. Singh
College of Pharmaceutical Sciences, Rajkumar Goel Institute of Technology, 5 km stone (opp. Jain Tubes Co. Ltd.) Ghaziabad-201 003
E-mail: cuteanu15@gmail.com
| Date of Submission | March 12, 2012 |
| Date of Revision | March 04, 2012 |
| Date of Acceptance | March 17, 2011 |
| Indian J Pharm Sci, 2012, 74 (2): 101-106 |
Abstract
In majority of individuals blood pressure rises in the early morning hours, which lead to serious cardiovascular complications. Formulation of pulsatile system makes it possible to deliver drug at definite period of time when symptoms of the disease condition are most critical. The purpose of the present work was to develop pulsatile release tablet of losartan potassium for chronotherapy in hypertension. The prepared system consisted of a core tablet coated with versatile and safe hydrophilic cellulosic ethers such as, hydroxypropyl methylcellulose, hydroxypropyl cellulose and sodium carboxy methylcellulose to produce burst release after predetermined lag time. Various formulation factors were studied through series of test and in vitro dissolution study. It was found that core tablets containing superdisintegrant failed to produce burst drug release pattern while effervescent agent was able to do so. Results also reveal that coating composition and coating level affects lag time. Formulation containing effervescent agent in core and coated with 200 mg hydroxypropyl cellulose provide lag time of 4.5 h with 73% drug release in 6 h that followed a sigmoidal release pattern. These values were close to the desired objective of producing lag time of 5â??6 h followed by fast drug release. This approach can thus provide a useful means for timed release of losartan and is helpful for patients with morning surge.
Keywords
Chronotherapy, hypertension, lag time, losartan potassium, pulsatile, sigmoidal
Over the last 30 years, numerous technical advancements has occurred in formulations, biodegradable polymers, and the understanding of pharmacokinetics has resulted in new techniques of drug delivery. Apart from the controlled, sustained, prolonged and targeted delivery systems, a new delivery system known as pulsatile delivery system has drawn attention of scientists, which is based on the concept of chronotherapeutics [1]. A pulsatile drug delivery system is one that delivers drug molecule in rapid and transient manner within a short time period immediately after a predetermined off release period [2]. The rationale for use of proposed system is to deliver drug at a time when disease condition is in the most morbid and mortal state during 24 h. Particular rhythm in onset and extent of symptoms were observed in diseases such as, bronchial asthma, rheumatic disease, ulcer, cancer, diabetes, attention deficit syndrome, hypercholesterolemia, neurological disorder, myocardial infarction, angina pectoris and hypertension [3]. Several pulsed release formulations have been developed recently, wherein tablets or capsules are the basis of pulsatile formulation that addresses emerging chronotherapeutic requirements [4].
In cardiovascular diseases the focus is to optimally deliver the antihypertensive or antianginal drug in higher amounts in early morning and lower amount at night. Holter monitoring of the electrical properties of the heart has revealed 24 h variation in the occurrence of ventricular premature beats with the peak in events, in diurnally active persons, between 6 a.m. and noon. The main sources of such a time?dependency in cardiovascular physiology and pathophysiology are the external interfering stimuli, primarily physical and mental activity or stress, sleep–wake cycle, and the endogenous rhythmicity [5]. Drugs that are capable of reducing the morning increase in norepinephrine and angiotensin II, have a more cardio protective effect and a better blood pressure (BP) lowering effect [6]. Day?night patterns have been documented for BP, arrhythmias, angina, myocardial infarction, and stroke, among other cardiovascular maladies. Risk of ischemic events is highest during the first few hours of the daily activity span and lowest during sleep [7].
The renin angiotensin–aldosterone system is activated in the morning. Hence blood pressure rises rapidly in the morning, the so?called morning surge, and that this rise has been associated with increased risk of cardiovascular events such as stroke and myocardial infarction [8].
Losartan potassium is an angiotensin antagonist, which has been reported to lower the blood pressure without disrupting the diurnal variation in hypertensive patients. However, no effort has been made to develop a system for angiotensin antagonist that deliver drug at a specified time and show maximum effect at time when needed most. Hence, an attempt was made to formulate pulsatile drug delivery system of losartan potassium which can deliver the drug after lag time of 5?6 h. The system consisted of fast dissolving core tablet coated with various hydrophilic cellulosic polymer. The effect of various parameters like type and weight of coating polymer, and excipients used in core tablet affects desired results and this was investigated.
Materials and Methods
Losartan potassium was obtained as a gift sample from Unichem Laboratories Ltd., Mumbai, India. Hydroxypropyl methylcellulose K4M (HPMC K4M) and hydroxypropyl methylcellulose E50 (HPMC E50) was obtained as a gift from Colorcon Asia Private Limited, Goa, India. Hydroxypropyl cellulose (HPC, M.W. 100) was obtained from Across organics. All other materials were obtained from Central Drug House, New Delhi.
Preparation of core tablets
Core tablets of 120 mg weight were prepared by direct compression method using 7 mm concave punch with single station tablet compression machine (Anant electrical, India). Two types of core tablets were prepared, one containing superdisintegrant crospovidone (C) and other containing effervescent agent (E) for producing burst release from final coated tablet. Optimised formulation containing effervescent agent contained 50 mg losartan potassium, effervescent agent (NaHCO3:citric acid, 1:0.76), sodium chloride and microcrystalline cellulose (15.66% w/w each based on total core weight) as filler, talc and magnesium stearate (1% w/w each based on total core weight) as lubricant and glidant. Optimized batch containing crospovidone contained 50 mg losartan potassium, 12% crosspovidone, 27.66% lactose, 16.66% starch and 1% each talc and magnesium stearate in 120 mg tablet.
Preparation of coating granules
Three types of granules were prepared by using three different polymers i.e. sodium carboxymethyl cellulose (NaCMC), HPMC K4M, and HPMC E50. Granules were formed by simple mixing of polymer with 5% w/v alcoholic solution of polyvinyl pyrollidone K30 to form dough. The formed dough was passed through sieve, dried and resieved through sieve no. 20. Finally mixed with talc and magnesium stearate. In addition to above polymers hydroxypropyl cellulose was directly used (without granulation) for compression coating because of its directly compressible character.
Compression coating of core tablet
Two hundred milligrams of prepared granules was used for shell formation in each tablet. Press coating of tablet was performed. Half the amount of powder from 200 mg was filled into the die to form a powder bed. In centre core, tablet formulation is placed. Over this remaining half of the granules was filled into die and contents were compressed using concave punches of 10 mm diameter. Hardness of tablet was maintained between 6?8 kg. Various Batches of coated tablet is given in Table 1. Also the thickness of coating polymer was varied to determine the effect of coating thickness on lag time.
| Formulation | Coating polymer | Core tablet |
|---|---|---|
| HC1 | HPMC E50 | C |
| HC2 | HPMC K4M | C |
| HC3 | HPC | C |
| HC4 | NaCMC | C |
| HE1 | HPMC E50 | E |
| HE2 | HPC | E |
| HE3 | NaCMC | E |
| HE4 | HPMC K4M | E |
Table 1: Pulsatile release tablet formulations enclosing optimized core tablets
Film coating of compression coated tablet
Film coating of pulsatile release tablet was done to enhance its physical appearance. Coating solution contained titanium dioxide 3% w/v, alcohol 200 proof 50% v/v, propylene glycol 2.11% w/v, hydroxypropyl methylcellulose (15 cps) 2?4% w/v and methylene chloride q.s. [9]. Coating solution was applied by dip coating technique using pipette (10 ml) attached to vaccum pump. Vaccum pump produced suction force that allowed tablet to adhere to pipette mouth. This adhered tablet was then partially dipped in coating solution to allow coat formation at three sides of tablet. The forth side was coated when other three sides dried.
Physicochemical characterization of tablets
The thickness of tablet (n=5) was determined using digital micrometer (Digimatic micrometer, Mitutoyo Products). The hardness of tablet (n=3) was determined using Pfizer tablet hardness tester. Weight variation test of tablet (n=20) was carried out as per official method [10]. Disintegration time of core tablet was determined using tablet disintegration test machine. For determining drug content of core tablet, 3 tablets (n=3) were crushed individually, pulverized and dissolved in 100 ml of methanol. The drug solution was filtered. Aliquots of filtrate was diluted suitably with methanol and analyzed spectrophotometrically at 250 nm.
Lag time and drug release
The lag time was determined by visual observation of the pulsatile tablet in USP II paddle apparatus (medium: 0.1 N HCl for 2 h followed by phosphate buffer, pH 6.8, at 37°, rotation speed 50 rpm) and was determined as time point, when the outer coating ruptured (n=3).
in vitro dissolution studies
The dissolution study of marketed formulation of losartan was carried out using paddle type dissolution apparatus (Hicon dissolution rate test apparatus, Grover enterprises) at 37±0.5° in 900 ml 0.1 N HCl at paddle speed of 50 rpm.
The in vitro dissolution study of various pulsatile tablets was carried out in 900 ml of 0.1 N HCl for first 2 h, followed by 900 ml of phosphate buffer (pH 6.8). The dissolution medium was maintained at temperature 37±0.5°. The paddle speed was set at 50 rpm. At different time intervals 5 ml of sample was withdrawn and analysed by UV/ Vis spectrophotometer at 250 nm. At each time of withdrawal, 5 ml of fresh corresponding medium was replaced into dissolution vessel. The time when 10% or more of losartan potassium dissolved in medium was defined as end of lag time in this experiment. The test was performed in triplicate.
Stability studies
After determining drug content, the optimized formulation (HE2) was subjected to accelerated stability studies according to ICH guidelines (40±2º/75±5% RH) for a period of 3 months in stability chamber (Hicon, Delhi, India). Tablets were packed in amber colored wide mouth glass bottle hermetically sealed. The samples were taken out at 15, 30, 60 and 90 days and evaluated for the drug content and in vitro release study.
Results and Discussion
Different tablet formulations of losartan potassium were made by direct compression. The tablets of batch C and E showed uniform thickness (2.50±0.25 mm and 2.58±0.14 mm, respectively). Tablets were studied for hardness, disintegration and weight variation. The hardness of core tablets was found to be 1±0.2 kg/cm2. The disintegration time taken by both batches varied. Fast disintegrating core tablets were prepared to achieve pulsatile release by incorporating superdisintegrants and effervescent agents in core tablet separately. Three superdisintegrants, sodium starch glycolate, crosscarmelose sodium and crosspovidone, were used to produce core tablets. The core tablet containing crosspovidone produced fastest disintegration of tablets. Batch C showed fasted disintegration with 4±0.41 min in all batches containing crosspovidone. Core tablets containing effervescent agents were also formulated. Batch E produced fastest disintegration within 0.95±0.40 min in formulations with effervescent agent.
Various batches of pulsatile release tablets were formulated by compression coating of polymeric granules over core tablet (Table 1). These pulsatile tablets were film coated to enhance appearance. Coating solution containing 2% HPMC 15 cps produced tablets with glossy appearance and concealed surface imperfection.
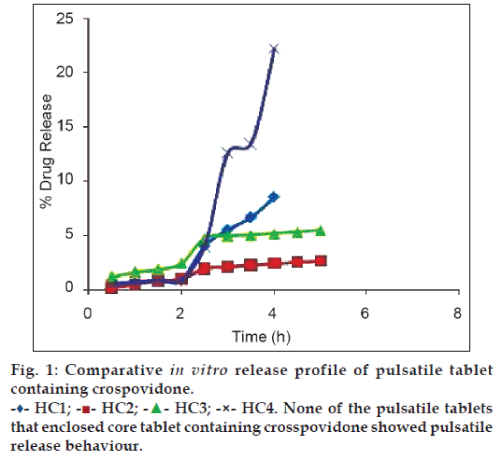
in vitro releases of the final formulations were carried out to determine whether the drug release profile after lag time followed a sigmoidal pattern. The results demonstrate that not all formulations showed pulsatile release with a distinct lag time. Batch HC1 to HC4 failed to produce burst release with a defined lag time (fig. 1). The reason for this is that crospovidone contained within core tablet did not produce requisite extent of internal stress required to exceed the mechanical strength of outer polymeric coat and rupture the coat. Moreover batches showed different percent of drug release depending on physicochemical properties of the polymer used for coating.
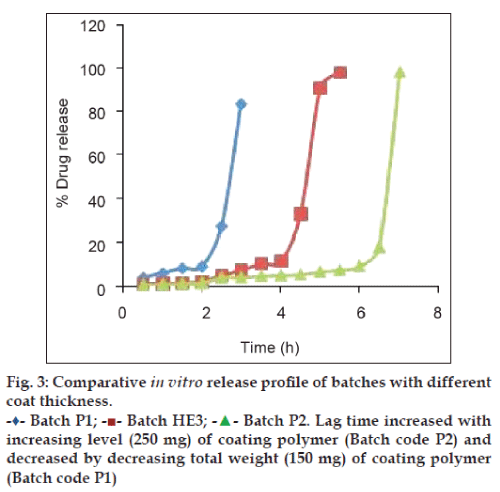
Among formulations enclosing core tablet with effervescent agent, all except HE4 produced desired drug release profile (fig. 2). The reason for pulsatile release behaviour by batch HE1, HE2, HE3 is that the internal pressure that is created by the effervescent core is more than the mechanical strength of outer polymeric coat. Similar to formulation HC1 to HC4, formulation HE1 to HE4 produced varying percent of drug release based on physicochemical property of coating polymers. They also produced varying lag time. Formulation HE4 failed to produce pulsatile release behaviour because its outer coat was made of HPMC K4M, which is swellable high viscosity grade. This provided the coat sufficient strength that could not be overcome by internal pressure generated by the effervescent agent. The results demonstrate that among the two HPMC grades used for coating, Tablet coated with grade HPMC E50 (Batch HE1) produced defined lag time while that coated with HPMC K4M (HE4) failed. This is due to difference in viscosity of both the grades of HPMC. HPMC E50 used is an erodible grade with low viscosity of 52 mPaS while HPMC K4M is swellable grade with high viscosity of 4697 mPaS [11]. In contrast to erodible polymers, swellable polymers have higher degree of cross?linked macromolecular networks (hydrogels) that fails to dissolve even after extensive water uptake and posses greater mechanical strength and hence HPMC K4M coated tablet did not produce burst release [12]. Similarly, in formulation HE2 which is coated with hydroxypropyl cellulose, duration of lag phase depended on hydration, swelling and dissolution of coat. This showed release profile very similar to tablet coated with HPMC E50 with slight increase in lag time of 4.5 h.
Batch HE3 coated with carboxymethyl cellulose sodium showed least lag time because of its high permeation and high water absorbing character [11], which leads to quick hydration of core tablet that evolves gas generating internal pressure. Also carboxymethyl cellulose sodium has lower extent of cross linking and gelling character than other three polymers that provided lesser extent of mechanical strength to its coat.
The aim of the proposed work was to develop pulsatile release tablet with lag time of 5?6 h and fast drug release thereafter. From the release profile depicted in fig. 2, both batch HE1 and HE2 can be selected as optimised batch. This is because batch HE2 produced lag time of 4.5 h with 73% drug release in 6 h and batch HE1 produced lag time of 4 h with 98% drug release in 5.5 h. However, batch HE2 was selected as the optimised batch because it produced lag time that is more close to the desired lag time and also its physical appearance after film coating is more elegant than that of HE1 batch.
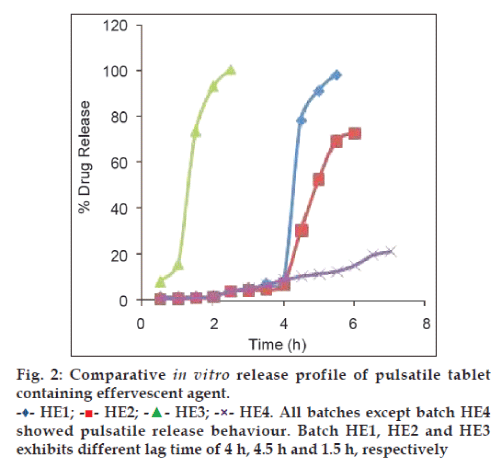
Fig. 3, demonstrates comparative release profile of batches with different coat thickness. It was observed that lag time increased with increasing level (250 mg) of coating polymer (Batch code P2) and decreased by decreasing total weight (150 mg) of coating polymer (Batch code P1). This is because as coating level increases mechanical strength of coat also increases and medium permeation rate at higher coating thickness reduces.
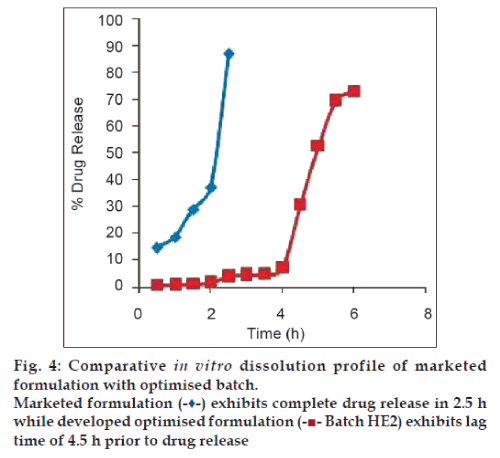
Fig. 4, demonstrates comparative in vitro release profile of marketed formulation with optimised batch HE2. 87% drug release in 2.5 h. was observed for marketed product. From the study on circadian behaviour of blood pressure and cardiac events, it is apparent that release of losartan potassium in pulsatile manner would be more effective than the conventional pattern achieved from marketed product. This suggests superiority of proposed formulation over marketed product.
Results of accelerated stability studies reveal that the tablet did not show any change in physical appearance during the study period. Drug content (n=3; mean±SD) was found to be 99.3±0.31%. This signifies that optimised batch has stability and potency at accelerated conditions within 3 months. The dissolution profile of these tablets also did not show any significant variation.
Over the past decade, increasing interest has been directed towards oral pulsatile delivery especially because of its potential suitability for meeting chronopharmaceutical needs related to widespread diseases with circadian symptom variation. The designated pulsatile system characterised in this study demonstrate that after oral administration, the coat rupture determined the lag time. In the given system the coat ruptured due to internal pressure generated by the gas released from effervescent agent on hydration, which is present in core tablet. Polymer HPMC E50, HPC and NaCMC produced pulsatile behaviour while HPMC K4M failed due to greater viscosity and gelling character that provided greater mechanical strength to coat. The swelling polymer i.e. superdisintegrants failed to produce the extent of internal stress needed to rupture outer coat.
Batch HE2 was selected as optimised formulation with lag time of 4.5 h. and complete drug release up to 6 h. It was also determined that lag time was directly proportional to viscosity of coating polymer and coating level. Thus this approach can provide a useful means for pulsatile release (with single pulse) of losartan potassium and may be helpful for patients with morning surge in blood pressure.
References
- Smolensky MH, Peppas NA. Chronobiology, drug delivery, and chronotherapeutics. Adv Drug Deliv Rev 2007;59:828?51.
- Geest BGD, Mehuys E, Laekeman G, Demeester J, Smedt SC. Pulsed drug delivery. Expert Opin Drug Deliv 2006;3:459?62.
- Youan BB. Chronopharmaceutics: gimmick or clinically relevant approach to drug delivery? J Control Release 2004;98:337?53.
- Shidhaye SS, Lotlikar VM, Ghule AM, Phutane PK, Kadam VJ. Pulsatile delivery systems: An approach for chronotherapeutic diseases. Sys Rev Pharm 2010;1:55?61.
- Portaluppi F, Hermida RC. Circadian rhythms in cardiac arrhythmias and opportunities for their chronotherapy. Adv Drug Deliv Rev 2007;59:940–51.
- Izzedine H, Launay?Vacher V, Deray G. Abnormal blood pressure circadian rhythm: A target organ damage? Int J Cardiol2006;107:343–9.
- Smolensky MH. Chronobiology and chronotherapeutics applications to cardiovascular medicine. Am J Hypertens 1996;9:11S?21.
- Pasic J, Shapiro D, Motivala S, Hui KK. Blood pressure morning surge and hostility. Am J Hypertens 1998;11:245–50.
- Niazi SK. Handbook of pharmaceutical manufacturing formulation: compressed solid products.Vol. 1. USA: CRC Press; 2004. p. 273.
- Indian Pharmacopoeia. Vol. 1. Ghaziabad: Indian Pharmacopoeia Commission, Government of India, Ministry of Health and Family Welfare; 2007. p. 182.
- Raymond RC, Sheskey JP, Weller PJ. Handbook of pharmaceutical Excipients. 4th ed. London: Pharmaceutical Press; 2003. p. 551, 916.
- Gazzaniga A, Palugan L, Foppoli A, Sangalli ME. Oral pulsatile delivery systems based on swellable hydrophilic polymers. Eur J Pharm Biopharm 2008;68:11?8.