- *Corresponding Author:
- Shaila Lewis
Department of Pharmaceutics, Manipal College of Pharmaceutical Sciences, MAHE, Manipal - 576 104, India.
E-mail: s.lewis@manipal.edu
| Date of Submission | 3 October 2005 |
| Date of Decision | 25 March 2006 |
| Date of Acceptance | 27 December 2006 |
| Indian J Pharm Sci, 2006, 68 (6): 829-831 |
Abstract
The availability of nicotine-replacement therapies is very low in India, as there are only a few importers. Currently, negligible forms of transdermal patches are available, they are expensive, and not easily accessible to the common man. In this study, an attempt was made to develop transdermal patches of nicotine, which are cost effective and conducive to the Indian market. Two types of patches, monolayered and bilayered, were prepared. The monolayered patch bore a rate- controlling membrane, whereas the bilayered, served as matrix type. The physical charecteristics of the patches were evaluated by standard techniques. The drug content was found to be uniform in the patches. In vitro release studies of transdermal patches showed a biphasic release pattern, with diffusion as the dominating mechanism of drug release for the matrix type, while the membrane-controlled released nicotine, gradually over the 24 h study.
Tobacco smoking related diseases constitute a major health problem all over the world. Chronic tobacco smoking affects all the systems of the body. Nicotine replacement therapy has therefore been developed as an aid to smoking cessation. Though a variety of nicotine replacement products are commercially available in the market, number of practical problems are associated with each nicotine replacement therapy product available [1]. Drug absorption via the mucosal epithelium of the oral cavity is an established route of systemic drug delivery, which is especially useful if absorption after oral administration is incomplete or ineffective. Furthermore, oral transmucosal drug delivery provides removal of dosage form in case of need. Buccal administration of drug is highly acceptable by patients and the buccal mucosa is relatively permeable with rich blood supply [2]. The aim of this work was to develop mucoadhesive tablets of nicotine for buccal administration and to carryout its release, in vivo and pharmacokinetic study.
Nicotine was obtained from Merck, Germany, HPMC, K4M from BDH laboratories, Sodium alginate from S.D. Fine Chemicals, Chitosan from Central Institute of Fisheries Tech., Cochin, India and Carbopol 934P was purchased from B. F. Goodrich and Co. Germany. Biaxially-oriented polypropylene (BOPP) film was supplied by Pidilite, India. The rest of the ingredients and reagents were of analytical grade.
Tablets were prepared by the direct compression of the drug with mucoadhesive polymers. Nicotine was adsorbed on aerosil (colloidal silicon dioxide). To this, the rest of the excipients were added in geometric progression and blended to obtain uniform mixing. The powder was compressed on a single station tablet machine using a flat November - punch of 9.5 mm diameter. Compositions of the buccal adhesive tablet formulations are shown in Table 1.
| Ingredients (mg) | A | B | C | D | E | F | G | H | I | |
|---|---|---|---|---|---|---|---|---|---|---|
| Nicotine | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Aerosil | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| HPMC K4m | 24 | 12 | 18 | 6 | 30 | 20 | 10 | - | | |
| Sodium alginate | 0 | 12 | 6 | 18 | - | - | - | 5 | 20 | |
| Carbopol | - | - | - | - | 10 | 10 | 20 | - | - | |
| Chitosan - - - - | - | - | - | 25 | 10 | |||||
| Magnesium stearate 1 | 1 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Starch DCq.sto 100 | 100 100 | 100 | 100 | 100 | 100 | 100 | 100 | |||
| q.s.: Quality sufficient | ||||||||||
Table 1: Composition of Various Mucoadhesive Tablets
BOPP film was used as backing membrane. The adhesive used in making the film was water based acrylic emulsion which is a pressure sensitive adhesive. This film was cut into the required circular shape and attached manually to the tablet. The tablets were evaluated for Thickness, Hardness and weight uniformity.
In vitro bioadhesion studies were carried out using a modified physical balance [3]. The dissolution behavior of buccal tablets was determined using the USP XXIII method (apparatus II-paddle) [4]. Preliminary in vivo evaluation of placebo tablets were conducted on five healthy volunteers after obtaining approval from the hospital ethical committee (approval No. KH/26-12/2002), KMC, Manipal. The objectives of the study were to determine the time of adhesion, to investigate the acceptibility of different polymers and to determine any irritation or side-effects produced by the formulations. Pharmacokinetic study of nicotine buccal tablets was conducted in six healthy south Indian adult male smokers. The study was unblinded and conducted in age group between 25-40 y. Blood samples were withdrawn from the cubital forearm vein before drug administration and then 0.5, 1, 2, 3, 4, 6, 8, 12 and 24 h after administration of the buccal tablet. Blood samples were collected in heparinised tubes, centrifuged and plasma was separated by centrifugation and frozen till further analysis. The nicotine concentration in plasma was quantitatively determined by HPLC [5]. Calculation of pharmacokinetic parameters was done using PK Solution 2.0 software supplied by Summit Research Services Montrose, USA, which is based on noncompartmental pharmacokinetic analysis.
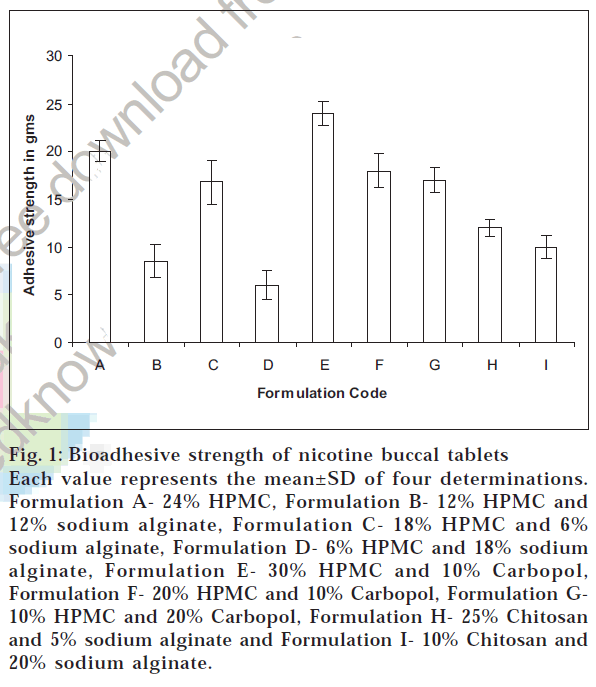
Physical parameters were found to be fairly good. The tablets showed thickness in the range of 1.20 to 1.50 mm. Hardness and friability were satisfactory. In vitro bioadhesion studies showed a linear dependence of the mucoadhesive capacity versus the polymer percentage as shown by adhesion force (fig. 1). From the results it can be seen that polymer having high molecular weight and high viscosity exhibited higher adhesion and increasing contact time had little effect on this. Results of in vitro release of nicotine from buccal adhesive tablets notably differed depending on quality and quantity of mucoadhesive components (fig. 2).
Fig. 1: Bioadhesive strength of nicotine buccal tablets
Each value represents the mean±SD of four determinations. Formulation A- 24% HPMC, Formulation B- 12% HPMC and 12% sodium alginate, Formulation C- 18% HPMC and 6% sodium alginate, Formulation D- 6% HPMC and 18% sodium alginate, Formulation E- 30% HPMC and 10% Carbopol, Formulation F- 20% HPMC and 10% Carbopol, Formulation G10% HPMC and 20% Carbopol, Formulation H- 25% Chitosan and 5% sodium alginate and Formulation I- 10% Chitosan and 20% sodium alginate.
The release rate of nicotine from mucoadhesive tablets decreased with increasing concentration of HPMC. Formulation A containing only HPMC exhibited less drug release compared to formulation B and C. The tablets of formulation A released the drug at a controlled rate, controlled by the swelling of the polymer, followed by drug diffusion through swollen polymer and then further followed by slow erosion of the polymer. The ratio of the bioadhesive polymers HPMC and sodium alginate also altered the release rate. Sodium alginate is more hydrophilic than HPMC. It can swell rapidly, therefore decrease of sodium alginate content delays drug release. On contact of the tablets with water, sodium alginate rapidly hydrates and swells to form a gel layer over the tablet surface. Decreasing concentration of sodium alginate from B to C decreased the release of nicotine. Tablets with low alginate content hydrate less readily resulting in a slower release rate.
Formulation E with 30%HPMC showed a retarded release, which may be due to the gelling and slow dissolution of the polymer [6]. Formulation G released the drug at a faster rate due to higher carbopol. As the carbopol content increased, there was an increase in the amount of drug released.
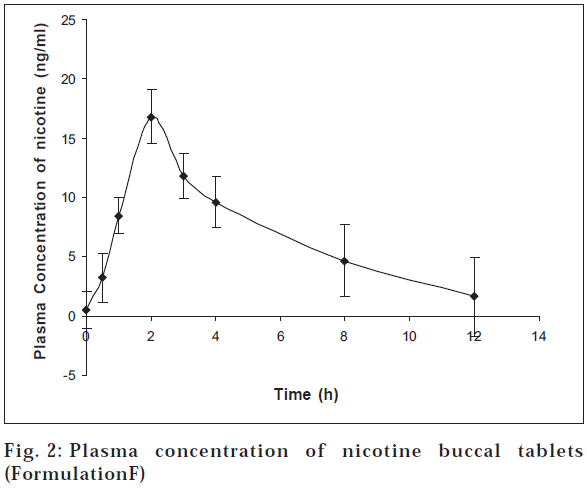
In case of chitosan-sodium alginate, when tablets came into contact with buffer, chitosan rapidly absorbed a large quantity of water swelled and then eroded gradually. The chitosan/alginate tablets of formulation I, having a higher alginate content displayed a faster release pattern compared to formulation H. In case of formulation H with lower alginate content, chitosan swelled due to water uptake. This swelling and lack of erosion gave a retarding effect on nicotine drug release. In vivo evaluation of mucoadhesive tablets confirmed that the mucoadhesive tablets did not cause any irritation or hindrance to the volunteers and their taste was acceptable. Pharmacokinetic study of nicotine buccal tablets indicated that trace amounts of nicotine (mean nicotine levels of 0.48 ng/ml) were detected in the plasma before application of the buccal tablet. The presence of nicotine before system application (t=0 h) may be due to either passive exposure of volunteers to cigarette smoke in the environment or insufficient time for nicotine to clear from the body after an overnight abstention from cigarette smoking. Following the administration of the dosage form, plasma nicotine concentrations were observed to rise rapidly in the first two hours. Peak plasma concentration (Cmax) of nicotine after administration of the buccal dosage form was achieved in two hours (tmax) and found to be 16.8 ng/ml. It indicates rapid absorption of nicotine from buccal formulation. The buccal mucosa may be a more favorable site for absorption of nicotine. Non keratinized and strongly supplied with blood, with a dense capillary network, it constitutes a relatively large drug absorption area. Drug can thus reach the systemic circulation directly through capillary vessels, bypassing the first pass metabolism in the intestines and liver or avoiding inactivation in the stomach [7]. This in turn contributes to higher bioavailability after administration of a smaller dose of drug. Area under the concentration-time curve AUC(0-24) was found to be 82.4±24.0 ng/ml for 5 mg of buccal tablet. The high Cmax value of buccal tablets indicates rapid absorption into systemic circulation (tmax =2 h), which can be readily used in smoking cessation for break through cravings.
Since the buccal tablet remains stationary at the buccal mucosa, it is in constant contact with the buccal mucosa. Hence it is able to provide good bioavailability. The mucoadhesive buccal tablets showed compliance in human volunteers. The impermeable backing layer facilitates unidirectional release of nicotine, which was supported by the absence of bitter taste. Since the bioadhesive tablets eventually eroded, the removal of the device was not needed and bioadhesive tablets can be inserted repeatedly if desired. The results of the pharmacokinetic study indicate that the developed buccal dosage form is able to continuously deliver nicotine within the mouth to the buccal membranes. Hence the developed mucoadhesive tablet for buccal administration has a potential clinical usefulness as nicotine replacement therapy for smoking cessation.
References
- Park, C.R. and Munday, D.L., Int. J. Pharm., 2002, 237, 215.
- Shojaei, A.H., J. Pharm. Sci., 1998, 1, 15.
- Gupta, A., Garg, S. and Khar, R.K., Indian Drugs., 1992, 30, 152.
- Yong, C.S., Jung, J.H., Rhee, J.D., Kim, C.K. and Choi, H.G., Drug Develop. Ind. Pharm., 2001, 27, 447.
- Lewis S., Subramanian G. and Udupa, N., Indian Drugs., 2004, 41,28.
- Mumtaz, A.M. and Ch’ng, H.S., Int. J. Pharm.,1995, 121, 129.
- Harris, D. and Robinson, J.R., J. Pharm. Sci., 1992, 81, 1.