- *Corresponding Author:
- H. N. Shivakumar
Department of Pharmaceutical Technology, K. L. E. S’s College of Pharmacy, Rajajinagar 2nd Block, Bangalore-560 010
E-mail: shivakumarhn@yahoo.co.in
| Date of Submission | 3-Mar-2005 |
| Date of Decision | 24-June-2005 |
| Date of Acceptance | 12-Feb-2006 |
| Indian J Pharm Sci, 2006, 68 (1):76-82 |
Abstract
An oral controlled onset extended release dosage form intended to approximate the chronobiology of rheumatoid arthritis is proposed for site-specific release to the colon. The multiparticulate system consisting of drug-loaded cellulose acetate cores encapsulated within Eudragit S-100 microcapsules was designed for chronotherapeutic delivery of ketoprofen. Drug-loaded cellulose acetate cores were prepared by emulsion solvent evaporation technique in an oily phase at different drug:polymer ratios (1:1, 2:1 and 4:1). These cores were successfully microencapsulated with Eudragit S-100 following the same technique at the core:coat ratio of 1:5. Scanning electron microscopy (SEM) revealed that the cellulose acetate cores were discrete, uniform and spherical with a porous and rough surface, whereas the Eudragit microcapsules were discrete and spherical with a smooth and dense surface. In vitro drug release studies of the Eudragit microcapsules were performed in different pH conditions following pH-progression method for a period of 16 h. The release studies indicated that the microcapsules posses both pH-sensitive and controlled-release properties, showing limited drug release below pH 7.0 (6.40 to 8.94%), following which the cellulose acetate cores effectively controlled the drug release for a period of 11 h in pH 7.5. The differential scanning calorimetric and powder X-ray diffraction studies demonstrated that ketoprofen was present in dissolved state in the cellulose acetate polymeric matrix, which could explain the controlled drug release from the cores. The release of ketoprofen from Eudragit microcapsules in pH 7.5 depended on the cellulose acetate levels and was characterized by Higuchi's diffusion model.
Introduction
Chronotherapeutics refers to a clinical practice of synchronizing drug delivery in a manner consistent with the body’s circadian rhythm, including disease states, to produce maximum health benefit and minimum harm [1]. The site-specific delivery of drugs to the colon has implications in a number of therapeutic areas, which include topical treatment of colonic disorders such as Crohn’s disease, ulcerative colitis, constipation, colorectal cancer, spastic colon and irritable bowel syndrome. A colonic delivery system would additionally be valuable when a delay in absorption is therapeutically desirable in treatment of diseases like rheumatoid arthritis, which are influenced by circadian rhythms [2]. The disease is known to have the peak symptoms when awaking from nighttime sleep.
Ketoprofen is a potent non-steroidal anti-inflammatory drug with a short biological which is prescribed for longterm longterm treatment of musculoskeletal and joint disorders such as rheumatoid arthritis, osteoarthritis, alkylosing spondilytis and acute gout [3]. The mechanism of action of ketoprofen is mainly associated to the inhibition of the body’s ability to synthesize prostaglandin. Adverse effects on the gastric mucosa have been observed when the drug is administered orally. Ketoprofen is rapidly absorbed from the gastrointestinal tract and reaches high bioavailability (>92%). The drug has been reported to be transported across the intestinal epithelial cells by trans-cellular passive diffusion [4].
Colon-specific delivery can be achieved with a suitable mechanism that triggers off the drug release upon reaching the colon. The physiological change in the pH of the gastrointestinal tract has been extensively exploited to convey the actives to the colon. Methods based on pH-sensitive delivery, such as delayed-onset dosage forms, could be a simple and practical means for colon targeting. Several polymers, particularly Eudragit S-100 [5] and Eudragit™S [6], have been investigated for colonic delivery. These polymers have been designed to be soluble at pH values higher than 7, keeping in mind the pH prevalent in the large intestine. As reported, the pH of the colon in normal subjects drops from 7.5±0.4 in the terminal ileum to 6.4±0.6 in the ascending colon [7]. However, the major disadvantage of these systems is the possibility of the drug being released in the terminal ileum rather than the colon. This problem was thought to be solved by utilizing two polymers: one having pHsensitive, and the other imparting a controlled-release property. Eudragit S was used to prevent the drug release till the formulation reaches the terminal ileum, whereas cellulose acetate avoids the complete release in the ileum and effectively conveys the drug to the colon.
A multiparticulate system presents several advantages in comparison to single unit forms in that they exhibit higher colonic residence time, more predictable gastric emptying, and cause less local irritation [8]. With all these considerations in mind, a multiparticulate system consisting of drug-loaded cellulose acetate cores encapsulated within Eudragit S-100 microcapsules was designed for chronotherapeutic delivery of ketoprofen. As a core forming polymer, cellulose acetate, whose application in the microencapsulation has been extensively investigated, was selected [9]. With this system, the aim was to minimize drug release in the upper part of the gastrointestinal tract and target the drug to the colon.
Materials and Methods
Ketoprofen was kindly donated by Rhone Poulenc (I) Ltd., Mumbai. Eudragit S-100 was generously donated by Rohm Pharma, Darmstadt, Germany. Cellulose acetate was supplied by Rolex Chemicals, Mumbai. The rest of the chemicals of analytical grade, supplied by S. D. Fine Chemicals, Mumbai, included light liquid paraffin, Span-80, acetone, n-hexane, and methanol.
Preparation of cellulose acetate cores containing ketoprofen
Cellulose acetate cores containing ketoprofen were prepared by emulsion-solvent evaporation technique in an oily phase [10]. Cellulose acetate was dissolved in acetone to get a homogenous polymer solution (1% w/v). Ketoprofen was dissolved in 10 ml of the polymer solution at drug:polymer ratio of 1:1, 2:1 and 4:1, and the resulting solution was added in thin streams to 70 ml of liquid paraffin containing 1% w/w of span-80. About 10 ml of acetone was added to the external phase to produce a stable o/o emulsion. The system was maintained under constant stirring (1000 rpm) using a variable speed propeller stirrer (RQ 125 D, Remi Udyog Ltd., Mumbai) for a period of 3 h to allow complete solvent evaporation. The cellulose acetate cores formed were separated, washed with n-hexane, and dried for 48 h in a vacuum desiccator.
Microencapsulation of drug-loaded cellulose acetate core
Cellulose acetate cores containing ketoprofen were encapsulated following the same technique with Eudragit S-100 [10]. The drug-loaded cellulose acetate cores (100 mg) were suspended in 5 ml of ethanolic solution of Eudragit S-100 (10% w/v) and emulsified into 70 ml of liquid paraffin containing 1% w/w of span-80. Emulsification was maintained using a variable-speed propeller stirrer at 1000 rpm to allow complete solvent evaporation. The microcapsules formed were separated, washed with nhexane, and dried for 48 h in a vacuum desiccator.
IR spectra of ketoprofen, cellulose acetate, Eudragit S and the microcapsules were recorded in a FTIR spectrophotometer (Jasco FTIR, 460 plus) to check the chemical integrity of the drug in the microcapsules [11].
Scanning electron microscopy (SEM)
Morphology and surface topography of the microparticles were examined by scanning electron microscopy12 (SEMJeol, JSM-840A, Japan). The samples were mounted on the SEM sample stab, using a double-sided sticking tape and coated with gold (200A°) under reduced pressure (0.001 torr) for 5 min to improve the conductivity using an Ion sputtering device (Jeol, JFC-1100 E, Japan). The coated samples were observed under the SEM and photomicrographs of suitable magnifications obtained.
Particle-size distribution
The particle-size distribution of the microparticles was determined using optical microscopy [12]. The projected diameter of a total of 200 microparticles from each batch was observed. The size distribution data got were attempted to fit into normal and log normal distribution, and the equivalent diameter based on surface number basis (dsn) was computed using Hatch-choate equation [13].
Estimation of drug content
An accurately weighed quantity of the microparticles was dissolved in acetone. The acetone was evaporated and the residue left behind was vortexed with 75% methanol for 30 min to extract the drug. The dispersion was filtered and the absorbance of the filtrate was measured at 258 nm after appropriate dilution in a UV-visible spectrophotometer (Jasco V-530, Japan). The drug content was estimated in triplicate using a calibration curve constructed in the same solvent. Polymers did not interfere with the assay at this wavelength.
Thermal analysis [11]
Samples of the ketoprofen, cellulose acetate, physical mixtures and the cellulose acetate cores were taken in a flat-bottomed aluminium pans and heated over a temperature range of 40-180° at a constant rate of 5°/min with purging of nitrogen (50ml/min) using alumina as a reference standard in a differential scanning calorimeter (Perkin Elmer DSC, Pyris-1).
X-ray powder diffraction studies [11]
The diffraction studies were carried out in a powder XRay diffractometer (Philips, PW 1050/37) with a vertical goniometer using Cu K α radiation with Ni filter at a voltage of 40 kV and a current of 20 mA. Powder XRD patterns for ketoprofen, cellulose acetate, physical mixture and cellulose acetate cores were obtained by scanning from 0 to 50° 2θ.
In vitro drug release studies
Dissolution studies of the Eudragit microcapsules were carried out in triplicate employing USP XIII dissolution rate test apparatus-1 (Electrolab, TDT-06T) following pH progression method simulating the gastrointestinal tract conditions [10]. Weighed quantities of the microcapsules were loaded into the basket of the dissolution apparatus, and the pH changes were performed, starting with 900 ml of 0.1 N hydrochloric acid for 2 h, mixed phosphate buffer of pH 5.5 for 1 h, phosphate buffer of pH 6.8 for 2 h, followed by mixed phosphate buffer of pH 7.5 till the end of the test. The temperature of the dissolution fluid was maintained at 37±0.5° with a stirring speed of 100 rpm. The samples were withdrawn every hour, filtered through a Millipore filter (0.22 µm), and assayed spectrophotometrically at 258 nm for the samples of pH 1.2, and at 260 nm for the rest of the samples. However, the dissolution studies of the cellulose acetate cores were also performed under the same set of experimental conditions using mixed phosphate buffer of pH 7.5 as the dissolution fluid mimicking the pH at the end of the small intestine [7].
Results and Discussion
The compositions of different batches of cellulose acetate cores are shown in Table 1. Cellulose acetate cores containing ketoprofen were prepared by emulsion solvent evaporation technique, where the organic solution containing the drug and cellulose acetate in acetone was emulsified into an external oil phase of liquid paraffin. A small amount of acetone added to the external oily phase was known to avoid rapid diffusion of the organic solvent into the oily phase. This would prevent immediate polymer precipitation before the organic solution could be dispersed into droplets in the oily phase leading to formation of a stable emulsion. Span 80 (1% w/w) was used as an emulsifier to stabilize the o/o emulsion produced.
| Formulation Code | Drug to cellulose acetate Ratio | dsn *(mm) | Drug loading* (% w / w) |
Entrapment efficiency* (% w / w) |
KH *(% h-1/2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 1:1 | 715.96 | ± 6.05 | 39.25 | ± 0.83 | 87.36 | ± 1.78 | 16.11 | ± 0.77 | ||
| C2 | 2:1 | 712.01 | ± 3.69 | 51.16 | ± 1.23 | 81.25 | ± 1.25 | 19.72 | ± 0.79 | ||
| C3 | 4:1 | 709 ± 11.75 | 59.25 | ± 1.33 | 77.11 | ± 0.58 | 23.28 | ± 1.22 | |||
Table 1: Composition And Physical Characteristics Of Ketoprofen Loaded Cellulose Acetate Cores
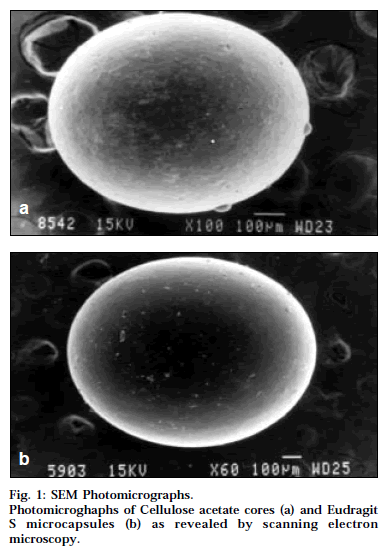
Photomicrographs of the cellulose acetate cores are shown in Fig. 1a. It is vivid from SEM photomicrographs that the cores were discrete, uniform and spherical with a porous and rough surface. The rough surface of the cores can be attributed to the rapid solvent diffusion and quick precipitation of cellulose acetate during the formation of o/o emulsion. It has been reported that microspheres with a porous and rough surface were produced by solvent evaporation technique with crystalline polymers [14].
The viscosity of the polymer solution used during microencapsulation is known to determine the size of the microspheres produced [10]. The viscosity of the organic solution depended on the polymer concentration in the solution, organic solvent used and the temperature. Since all the three batches of cellulose acetate cores were prepared using the organic solution having the same polymer concentration (1% w/v of cellulose acetate), there was no significant difference in the emulsion globule size. Accordingly, the cellulose acetate cores of the three batches did not vary significantly in their size. The size distribution data obtained from optical microscopy, when represented as log-probability plots, gave straight lines indicating a log-normal distribution in all the three batches of cellulose acetate cores produced. The surface number diameters (dsn) of the three batches of drug-loaded cores, as computed using Hatch-choate equation, are represented in Table 1.
The percent drug loading and entrapment efficiency of the three batches of drug loaded cores are depicted in Table 1. The values of entrapment efficiency were found to decrease with increase in the initial drug loading, which can be ascribed to better drug entrapment within the cores with increase in cellulose acetate levels. The drug loss during the microencapsulation process can possibly be related to the partitioning of the drug to the oil phase.
The in vitro release profiles of cellulose acetate cores are portrayed in Fig. 2. As the cellulose acetate cores remain protected by the Eudragit S-100 coat below pH 7, the dissolution tests of the cellulose acetate cores were conducted using phosphate buffer of pH 7.5 also mimicking the pH prevalent at the end of the small intestine [7]. The dissolution studies indicated that the cores were characterized by an initial burst effect during the first hour. The burst effect was reduced with increasing cellulose acetate levels in the cores, which may be attributed to better drug entrapment within the cores with increase in cellulose acetate levels. The slow release phase was followed by a controlled release phase, during which the drug release depended on the cellulose acetate levels in the cores. The mechanism of drug release was found to be characterized by Higuchi’s diffusion model [15] as plots of amount of drug released versus square root of time were found to be linear. The values of Higuchi rate constant (KH) were found to range between 16.1 to 23.3 %h-1/2 with a distinct increasing trend as the cellulose acetate levels in the cores decreased. The effect of varying cellulose acetate levels in matrix diffusional systems on drug release has been documented [9].
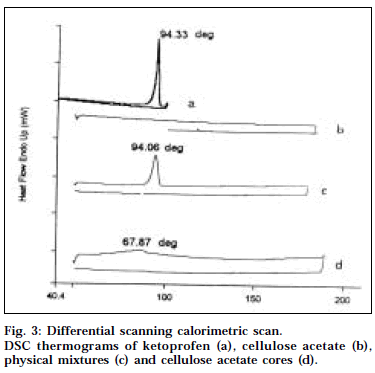
DSC has been one of the most widely used calorimetric techniques to characterize the solubility and physical state of drug in the polymeric matrix. Fig. 3 depicts the DSC thermograms of ketoprofen, cellulose acetate, physical mixtures and cellulose acetate cores. The DSC thermogram of ketoprofen exhibited a single sharp endothermic peak at 94.34° corresponding to its melting transition temperature [16]. This peak was also observed in the thermogram of the physical mixture, even though slightly broadened but shifted to lower temperature (94.06°). This may be possibly due to fact that presence of cellulose acetate in the physical mixture depresses the melting point of ketoprofen and broadens its melting point endotherm. The thermograms of the drug-loaded cellulose acetate cores showed no such characteristic peak, indicating that the drug was present in the dissolved state in cellulose acetate polymer matrix.
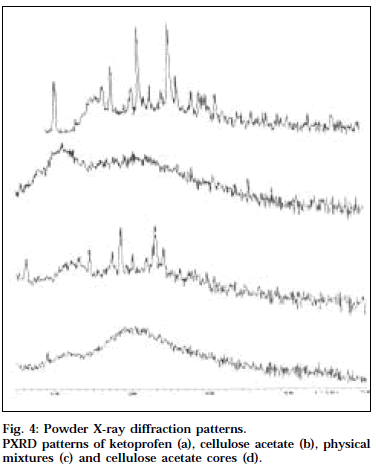
Powder XRD technique has been extensively utilized along with DSC to study the physical state of drug in the polymer matrix. Powder XRD patterns for ketoprofen, cellulose acetate, physical mixture and cellulose acetate cores are shown in Fig. 4. The crystalline nature of ketoprofen was clearly demonstrated by its characteristic PXRD pattern containing well-defined peaks. The PXRD diffractogram of the physical mixture of the drug and cellulose acetate also exhibited the characteristic diffraction pattern of the crystalline drug, indicating that the drug was dispersed in cellulose acetate in the physical mixture, The PXRD spectra of the cores did not reveal any such characteristic PXRD pattern corresponding to the crystalline drug, confirming the fact that the drug existed in the dissolved state in the cellulose acetate polymer matrix. These results could explain the controlled drug release from the cellulose acetate cores.
The second part of the research work was focused on microencapsulation of the cellulose acetate cores with a pH-sensitive acrylic polymer. The drug-loaded cores were microencapsulated following the emulsion solvent evaporation technique with Eudragit S-100 that dissolves at pH of above 7. Eudragit S was selected to protect the cellulose acetate cores in the upper part of the gastrointestinal tract, avoiding any significant drug release before reaching the colon. Once the acrylic coat dissolves, it was expected that the cellulose acetate cores would effectively control the drug release at the target site.
As a part of the research work, a preliminary screening study was undertaken to select a suitable organic solvent that would dissolve Eudragit S-100 and, at the same time, maintain the integrity of the cellulose acetate cores. Ethanol was chosen as solvent as it met the above said criteria; moreover, ethanol diffuses quickly into the external oily phase, resulting in encapsulation of the drugloaded cellulose acetate cores. Table 2 depicts the compositions of different batches of Eudragit microcapsules.
| FormulationCode | Core | Coat to core ratio | d sn *(mm) | Drug loading* (% w / w) |
EncapsulationEfficiency* (% w / w) | K H *(% h-1/2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | C1 | 5:1 | 1026.15 | ± 14.69 | 6.40 | ± 0.13 | 85.77 | ± 1.13 | 15.79 | ± 0.86 | |||
| E2 | C2 | 5:1 | 1031.06 | ± 10.21 | 8.27 | ± 0.25 | 77.55 | ± 1.61 | 18.68 | ± 0.83 | |||
| E3 | C3 | 5:1 | 1018.63 ± 5.02 | 9.67 | ± 0.28 | 70.90 | ± 0.98 | 22.31 ± 1.09 | |||||
Table 2: Composition And Physical Characteristics Of Eudragit S-100 Microcapsules Containing Drug Loaded Cellulose Acetate Cores
SEM revealed that the Eudragit microcapsules were discrete, uniform and spherical, with a smooth and dense surface. The photomicrographs of the Eudragit microcapsules are portrayed in Fig. 1b. It has been already established that microspheres with a smooth and dense surface were produced by solvent evaporation technique with amorphous polymers [14].
As mentioned earlier, the particle size of the microcapsules produced depended on the viscosity of the polymer solution used. As all the three batches of microcapsules were produced using the polymer solution having the same Eudragit concentration (10% w/v), the microcapsules of the three batches did not differ significantly in their particle size. The particle size distribution data, as determined by optical microscopy when represented as log-probability plots, gave straight lines indicating a log-normal distribution in all the three batches of microcapsules produced. The surface number diameters (dsn) of the three batches of microcapsules, along with their percentage drug loading and encapsulation efficiency values, are represented in Table 2. The values of percentage drug loading and encapsulation efficiency portray that emulsion solvent evaporation technique allows favourable drug encapsulation using Eudragit S 100.
IR Spectrophotometry has been employed as a useful tool to identify the drug excipient interaction. The IR spectra of ketoprofen and the microcapsules were identical. The principal IR absorption peaks of ketoprofen at 1698 cm-1 (carboxylic acid carbonyl) and 1655 cm-1 (ketonic carbonyl) appeared in the spectra of ketoprofen as well as the microcapsules. These observations indicated no chemical interaction between the drug and other excipients used.
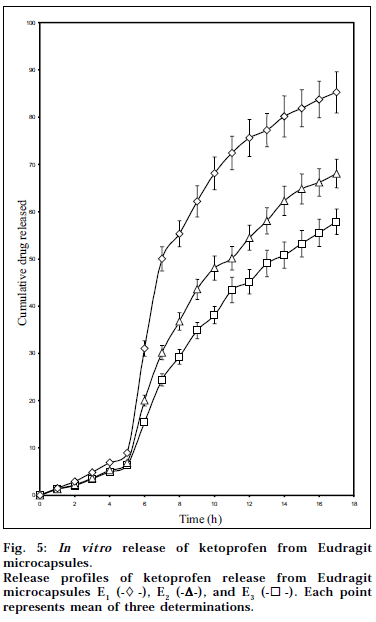
Fig. 5 portrays the in vitro drug release profiles of Eudragit microcapsules as determined by pH progression method. The studies showed that the microcapsules exhibited both pH-sensitive and controlled-release properties. The drug release depended on the pH of the dissolution media and the cellulose acetate levels in the microcapsules. A limited drug release was observed from the microcapsules below pH 7 during the first five hours of dissolution (6.39 to 8.94 %), which can be ascribed to the pH-sensitive nature of Eudragit S-100 coating. Eudragit S-100 is a pH-sensitive acrylic polymer having a threshold pH of 7 [5]. It was observed that once the acrylic coating dissolved at pH 7.5, the cellulose acetate cores effectively controlled the drug release for a period of 11 h. As revealed by the DSC and PXRD studies, the physical state of ketoprofen in the cellulose acetate polymeric matrix could explain the controlled release of the drug from the cellulose acetate cores. Diffusion of the drug through the cellulose acetate polymeric matrix was the rate-controlling step that could characterize the mechanism of drug release. The mechanism of drug release from the microcapsules in pH 7.5 was found to be diffusion controlled and was characterized by Higuchi’s diffusion model. The values of kinetic constant ranged between 15.79 to 22.31% h-1/2, showing a distinct increasing trend as the cellulose acetate levels in the Eudragit microcapsules decreased.
A multiparticulate system having both pH-sensitive and controlled-release property is described for chronotherapeutic delivery of ketoprofen. The results collectively prove that dual coated microcapsules with enteric and controlled release properties can be successfully developed using double microencapsulation procedure. Bedtime administration of such a device could improve the anti-inflammatory therapy in the management of rheumatoid arthritis.
Acknowledgements
The authors are grateful to Prof. B. G. Shivananda, Principal, Al-Ameen College of Pharmacy, for his constant support during the present research. They are also thankful to Rhone Poulenc (I) Ltd., Mumbai, for providing the gift sample of ketoprofen.
References
- Smolensky, M.H. and Labreque, G., Pharmaceutical News, 1997, 4, 10.
- Kinget, R., Willbrord, K., Leisbeth, V. and Guy, V., J. DrugTarget.,1998, 6, 129.
- Sweetman, S.C., In: Martindale, The Complete Drug Reference, 33rdEdn., Pharmaceutical Press, London, U.K., 2002, 47.
- Hilgendorf, C., SpahnLangguth, H., Regardh, C.G., Lipka, E., Amidon, G.L. and Langguth, P., J. Pharm. Sci., 2000, 89, 63.
- Ashford, M., Fell, J.T., Attwood, D., Woodhead, P.J., Int. J.Pharm., 1993, 91, 241.
- Karin, K., Jyrki, H., Johanna, V., Martii, M., Osmo, A. and Jouko, Y., Int. J. Pharm., 2000, 99, 187.
- Evans, D.F., Pye, G., Branley, R., Clarke, A.G., Dyson, T.J. and Hardcastle, J.D., Gut, 29, 1988, 1035.
- Follonier, N. and Doelker, E., S.T.P. Pharm. Sci., 1992, 2, 141.
- Chowdary, K.P.R., and Vijayaratna, J., Indian J. Pharm. Sci., 1990, 52, 279.
- Rodriguez, M., Jose, L., Vila-Jato, and Dolores, T., J. Control.Release. 1998, 55, 67.
- Chowdary, K.P.R. and GirijaSankar, G., Drug. Develop. Ind.Pharm., 1997, 23, 325.
- Singh, B., and Agarwal, R., Indian J. Pharm. Sci., 2002, 64, 378.
- Martin, A., Bustamante, P., Chun, A.H.C. In: Physical Pharmacy, 4thEdn., Waverly Pvt. Ltd., New Delhi 1995, 423.
- Mathiowitz, E., Kreitz, M.R., and Peppas, L.B., In: Mathiowitz E., Eds., Encyclopedia of Controlled Drug Release, Vol 2, John Wiley and Sons Inc, New York, 1999, 493.
- Higuchi, T., J. Pharm. Sci., 1963, 52, 1145.
- Liversidge, G.G., In Florey, K., Eds. Analytical Profiles of Drug Substances, Vol 10, Academic Press, New York, 443.
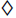 -), C2 (-Δ-), and C3 (-◼-). Each point represents mean of
three determinations.
-), C2 (-Δ-), and C3 (-◼-). Each point represents mean of
three determinations.