- *Corresponding Author:
- Min Li
Department of Traditional Chinese Medicine Center, Beijing Luhe Hospital Capital Medical University, Tongzhou, Beijing 101199, China
E-mail: lm2006002022@163.com
| Date of Received | 10 June 2021 |
| Date of Revision | 04 October 2022 |
| Date of Acceptance | 09 March 2023 |
| Indian J Pharm Sci 2023;85(2):412-418 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect and mechanism of Danggui Buxue decoction on high glucose-induced apoptotic and oxidative injury in human retinal vascular endothelial cells. Human retinal vascular endothelial cells were treated with high glucose to mimic the diabetic retinopathy condition in vitro. The 3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyl-2H-tetrazolium bromide assay and flow cytometry were used to investigate cell viability and apoptosis. Western blot was applied to detect the proteins of apoptosis-related markers and forkhead box O4. The oxidative injury was assessed using enzyme-linked immunosorbent assay analysis. Human retinal vascular endothelial cells in high glucose group showed the decrease of cell viability and superoxide dismutase activity, as well as the increase of cell apoptosis rate, reactive oxygen species level and malondialdehyde content, which were reversed by Danggui Buxue decoction treatment in a dose-dependent manner. Subsequently, we found that forkhead box O4 level was elevated in high glucose-induced human retinal vascular endothelial cells, while Danggui Buxue decoction dose-dependently down-regulated forkhead box O4 level in human retinal vascular endothelial cells. Deficiency of forkhead box O4 could reverse high glucose-induced apoptotic and oxidative injury in human retinal vascular endothelial cells. Moreover, the inhibitory effects of Danggui Buxue decoction on high glucose-induced human retinal vascular endothelial cells were attenuated by forkhead box O4 overexpression. Danggui Buxue decoction may inhibit the apoptosis and oxidative damage of human retinal vascular endothelial cells induced by high glucose by down-regulating forkhead box O4.
Keywords
Danggui Buxue decoction, retina, forkhead box O4, retinal vascular endothelial cell
Diabetes Retinopathy (DR) is a retinal vascular injury in diabetes patients, which has adverse effects on the vision of patients and can lead to blindness in serious cases[1]. Previous studies have shown that blood glucose in patients continues to rise during DR, which can promote apoptosis and oxidative damage of retinal micro vascular endothelium in the body in turn[2]. Due to the complex pathogenesis of DR, there is no effective treatment for the cure of DR patients and it is of great significance to find a safe and effective treatment for DR patients.
Danggui Buxue Decoction (DBD) is a conventional traditional Chinese medicine first described in “Neiwaishang Bianhuo Lun” written by Dong-yuan Li[3] is composed of the root of the traditional Chinese medicine Astragalus (Huangqi, HQ) and angelica (Danggui, DG)[4]. Recently, pharmacological study has proposed the anti-inflammatory, antioxidant, anti-apoptosis and anticancer effects of DBD[5,6]. For example, Li et al.[7] showed that DBD had protective effects on inflammatory bowel disease via repressing inflammation but promoting intestinal mucosal repair. Additionally, DBD improved the function of heart through increasing mitochondrial autophagy but impairing cardiomyocytes apoptosis[8].
However, there are few reports on the research of DBD on the progression of DR. Forkhead Box O4 (FOXO4) belongs to the FOXO family that has been proved to participate in modulating cellular homeostasis, metabolism, apoptosis and cell cycle through transcriptional activity[9]. FOXO4 exists in a variety of tissues, organs and its expression increases in pancreatic B cells, which is closely related to diabetes and the related complications[10,11]. Besides, it was also showed that Alpha-Melanocyte-Stimulating Hormone (α-MSH) decreased FOXO4 in diabetic retinas and High Glucose (HG)-challenged endothelial cells and FOXO4 up-regulation could reverse the suppressing functions of α-MSH in apoptosis and oxidative damage in Retinal Vascular Endothelial Cells (RVECs) under HG treatment[12]. However, whether FOXO4 was involved in the regulatory effects of DBD in DR remain unclear.
Here, this study used HG-induced RVECs to explore whether DBD had protective effects on HG-induced RVECs and its potential mechanisms.
Materials and Methods
Cell culture and treatment:
Human RVECs (HRVECs) were purchased from American Type Culture Collection (ATCC) (Rockville, Maryland, United States of America (USA)) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Solarbio, Beijing, China) containing 10 % Fetal Bovine Serum (FBS) (Solarbio), 2 mM L-glutamine (Solarbio) and 1 % penicillin-streptomycin (Solarbio) at 37° with 5 % Carbon dioxide (CO2).
In HG group, HRVECs were cultured in RPMI-1640 complete medium containing 30 mmol/l glucose. Cells cultured with 5.5 mmol/l glucose were used as control. HRVECs were incubated with DBD at concentrations of 100, 200 or 400 mg/l for 30 min, followed by HG stimulation.
Vector construction:
The specific small interfering Ribonucleic Acid (siRNA) targeting FOXO4 (si-FOXO4), FOXO4 plasmid cloning Deoxyribonucleic Acid (pcDNA) 3.1 overexpressing vector (FOXO4) and the matching control (si-NC or vector) were synthesized by Gene Pharma (Shanghai, China). Then they were transfected into HRVECs according to the instructions of Lipofectamine™ 3000 (Invitrogen). After transfection, cells were subjected to HG stimulation and then incubated in HG condition for subsequent analysis.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) assay:
HRVECs collected from different groups were seeded in 96-well plates and then 20 μl of MTT solution (Solarbio) was incubated with the cells for 4 h, followed by reacting with 150 μl dimethyl sulfoxide. The absorbance value at 490 nm was tested with a spectrophotometer.
Flow cytometry:
HRVECs collected from different groups were adjusted to 1.0×106/ml, then cells were incubated with 10 μl Annexin V-Fluorescein Isothiocyante (FITC) and 10 μl Propidium Iodide (PI) (BD Biosciences, San Diego, California, USA) in dark for 10 min. The apoptosis was detected by flow cytometry.
Western blot:
The protein was extracted using Radio- Immunoprecipitation Assay (RIPA) buffer (Beyotime, Shanghai, China) and then separated by 10 % Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel (the voltage was set at 90 V, the duration was 3 h), followed by shifting onto Polyvinylidene Difluoride (PVDF) membranes. Thereafter, the membranes were probed with primary antibodies against B-cell lymphoma 2 (Bcl-2) (ab692, 1:2000), Bcl-2 Associated X-Protein (BAX) (ab32503, 1:2000), Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (ab181602, 1:5000), FOXO4 (ab128908, 1:2000) at 4° for 12 h and appropriate secondary antibodies for 1 h at 37°. The antibodies were provided by Abcam (Cambridge, Massachusetts, USA). An Electrochemiluminescence (ECL) assay kit (Beyotime) was applied to assess the protein bands.
Enzyme-Linked Immunosorbent Assay (ELISA):
HRVECs collected from different groups were centrifuged at 2000 g for 10 min and cell supernatant was collected. Then the contents of Reactive Oxygen Species (ROS), Malondialdehyde (MDA) and Superoxide Dismutase (SOD) were detected using the ELISA kits (Abcam) based on the manufacturer’s instructions.
Statistical analysis:
Results were manifested as the mean±Standard Deviation (SD). Comparisons between groups were conducted using Student’s t-test (two group) or oneway analysis of variance (more than two group). p<0.05 indicated statistically significant difference.
Results and Discussion
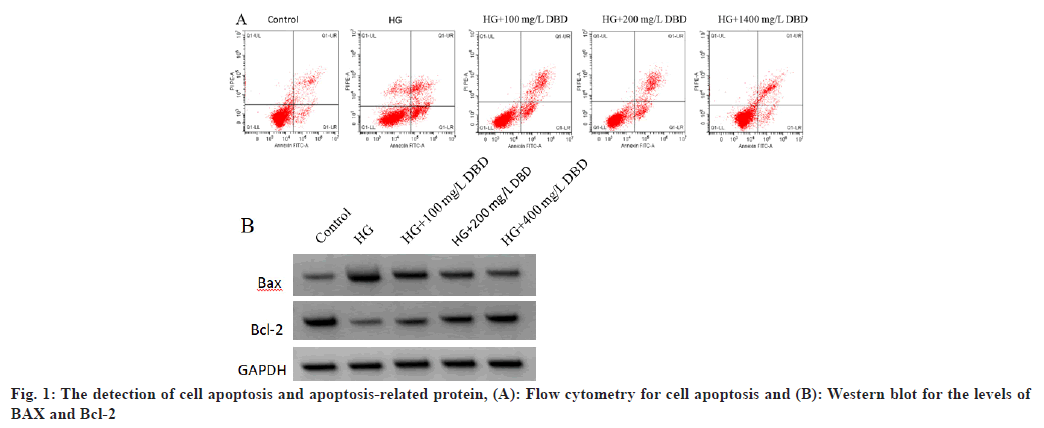
As shown in Table 1, fig. 1A and fig. 1B, HG stimulation lead to the inhibition of HRVEC viability and the promotion of cell apoptosis evidenced by increased apoptosis rate and pro-apoptotic BAX protein as well as decreased anti-apoptotic Bcl-2 protein; besides that, DBD treatment could reverse HG induced cell viability inhibition and cell apoptosis promotion in a dose-dependent manner.
| Group | Cell viability (%) | Apoptosis rate (%) | Bcl-2 | BAX |
|---|---|---|---|---|
| Control | 98.7±8.4 | 8.5±0.4 | 0.72±0.06 | 0.33±0.03 |
| HG | 41.5±3.9* | 27.2±2.3* | 0.29±0.02* | 0.76±0.07* |
| HG+100 mg/l DBD | 53.2±5.3# | 22.4±1.9# | 0.38±0.03# | 0.62±0.06# |
| HG+200 mg/l DBD | 65.6±5.4# | 18.5±1.4# | 0.45±0.04# | 0.54±0.05# |
| HG+400 mg/l DBD | 72.5±7.1# | 12.7±1.2# | 0.61±0.05# | 0.46±0.04# |
| F | 109.071 | 200.886 | 151.25 | 87.733 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with the control, *p<0.05 and compared with the HG, #p<0.05
Table 1: The Effects of DBD on the Viability and Apoptosis of HRVECs
HG stimulation markedly increased the ROS level and MDA content, but decreased the SOD activity in HRVECs (Table 2). Compared with HG group, DBD treatment dose-dependently decreased the contents of ROS and MDA, and increased SOD level in HRVECs as shown in Table 2.
| Group | ROS (μmol/g) | MDA (nmol/mg) | SOD (U/mg) |
|---|---|---|---|
| Control | 42.5±5.4 | 3.3±0.5 | 135.6±11.8 |
| HG | 98.7±8.8* | 10.2±1.2* | 72.3±6.7* |
| HG+100 mg/l DBD | 86.9±7.2# | 8.4±0.5# | 88.2±8.4# |
| HG+200 mg/l DBD | 71.3±7.1# | 6.2±0.7# | 95.3±8.7# |
| HG+400 mg/l DBD | 56.9±4.5# | 4.8±0.4# | 116.7±10.4# |
| F | 99.728 | 132.429 | 63.152 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared with the control, *p<0.05 and compared with the HG, #p<0.05
Table 2: The Effects of DBD on The Oxidative Stress of HRVECs
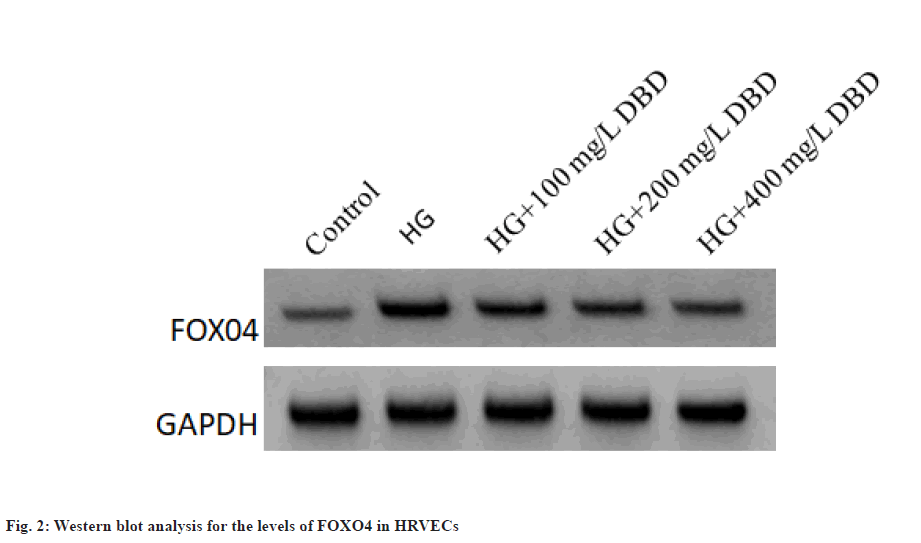
The expression of FOXO4 protein in the HG group was increased significantly related to the control group. In addition, DBD treatment dose-dependently decreased the levels of FOXO4 in HRVECs as shown in fig. 2 and Table 3.
| Group | FOXO4 protein |
|---|---|
| Control | 0.34±0.04 |
| HG | 0.75±0.06* |
| HG+100 mg/l DBD | 0.62±0.05# |
| HG+200 mg/l DBD | 0.53±0.05# |
| HG+400 mg/l DBD | 0.45±0.04# |
| F | 94.081 |
| p | 0 |
Note: Compared with the control, *p<0.05 and compared with the HG, #p<0.05
Table 3: The Effects of DBD on the Expression of FOXO4 in HRVECs
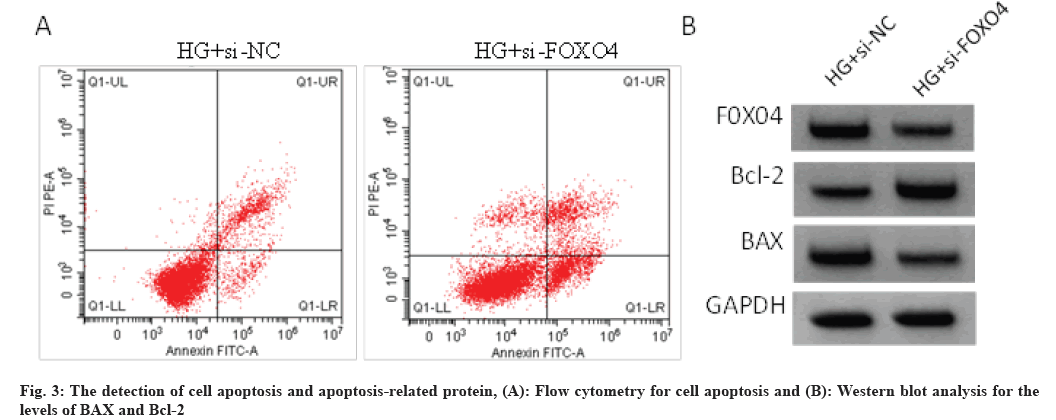
As shown in Table 4 and fig. 3, the transfection of si-FOXO4 significantly reduced the levels of FOXO4 compared with si-NC transfection. Besides that, knockdown of FOXO4 abolished HG-induced inhibition of cell viability, promotion of cell apoptosis, decline of Bcl-2 levels and elevation of BAX levels in HRVECs. Knockdown of FOXO4 markedly reduced the levels of ROS and MDA, while increased the content of SOD in HRVECs under HG stimulation as shown in Table 5.
| Group | FOXO4 | Cell viability (%) | Apoptosis rate (%) | Bcl-2 | BAX |
|---|---|---|---|---|---|
| HG+si-NC | 0.72±0.04 | 43.5±3.8 | 27.8±2.9 | 0.31±0.04 | 0.73±0.06 |
| HG+si-FOXO4 | 0.47±0.04* | 62.7±6.5* | 13.5±1.1* | 0.52±0.04* | 0.54±0.04* |
| t | 13.258 | 13.159 | 21.403 | 11.137 | 7.904 |
| p | 0 | 0 | 0 | 0 | 0 |
Note: Compared with the HG+si-NC group, *p<0.05
Table 4: The Effects of FOXO4 Down-Regulation on the Viability and Apoptosis of HRVECS
| Group | ROS (μmol/g) | MDA (nmol/mg) | SOD (U/mg) |
|---|---|---|---|
| HG+si-NC | 95.3±8.5 | 9.7±0.9 | 76.9±6.8 |
| HG+si-FOXO4 | 62.8±6.4* | 6.2±0.6* | 92.5±8.3* |
| t | 9.164 | 9.707 | 4.362 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared with the HG si-NC, *p<0.05
Table 5: The Effects of FOXO4 Down-Regulation on the Oxidative Stress of HRVECS
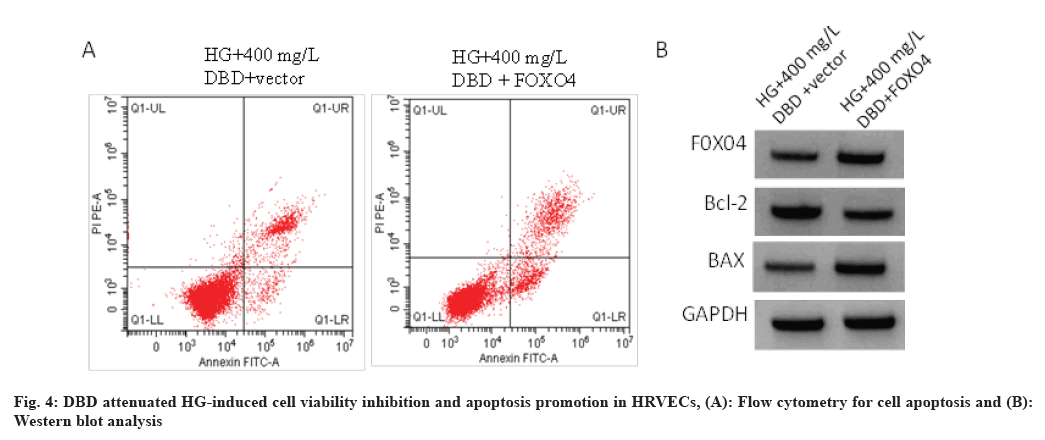
As shown in Table 6, DBD treatment led to the decrease of FOXO4 protein level in HG-induced HRVECs, which was rescued by FOXO4 transfection. Functionally, DBD attenuated HG-induced cell viability inhibition and apoptosis promotion in HRVECs, while these effects mediated by DBD treatment were abolished by FOXO4 overexpression as shown in fig. 4 and Table 6.
| Group | FOXO4 | Cell viability (%) | Cell apoptosis (%) | Bcl-2 | BAX | ROS (μmol/g) | MDA (nmol/mg) | SOD (U/mg) |
|---|---|---|---|---|---|---|---|---|
| HG+400 mg/l DBD+vector | 0.42±0.04 | 74.7±6.9 | 11.9±1.4 | 0.59±0.05 | 0.43±0.05 | 52.4±5.1 | 4.9±0.5 | 121.7±11.3 |
| HG+400 mg/l DBD+FOXO4 | 0.58±0.05* | 52.8±4.6* | 16.2±1.5* | 0.43±0.04* | 0.61±0.04* | 63.3±5.4* | 6.3±0.5* | 104.7±8.2* |
| t | 7.496 | 7.923 | 6.287 | 7.496 | 8.433 | 4.402 | 5.940 | 3.653 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 |
Note: Compared with the HG+400 mg/l DBD+FOXO4 group, *p<0.05
Table 6: DBD Treatment Regulates HG-Induced HRVEC Injury via FOXO4
Hyperglycemia remains the major cause of DR, which is able to induce the damage of endothelial cells via stimulating pathological metabolism and biochemical changes, thus initiating multifactorial pathology in DR process[13-16]. Therefore, protecting endothelial cells from hyperglycemia-induced damage may be beneficial for the development of novel therapeutic method for DR prevention. MDA is one of the products of lipid peroxidation and its expression can indirectly reflect the oxidative stress level of cells, SOD is an important antioxidant enzyme, which can remove oxygen free radicals[17-19]. Oxidative stress reflects an imbalance between pro/ anti-oxidant and is harmful to cells owing to the excessive generation and accumulation of ROS[20,21]. In the current work, we showed that the apoptosis rate, the levels of MDA and ROS in the HRVECs were significantly increased after HG stimulation, while SOD activity and the viability in the cells was decreased, suggesting that HG induced excessive oxidative and apoptotic injury in HRVECs.
DBD is a conventional traditional Chinese medicine that is widely used due to its hematopoietic effect and extraordinary immune regulation[22]. Besides, it has been proved that DBD plays a significant role in diabetes and diabetes-related complication. For example, DBD could ameliorate the water intake and body weight loss in diabetic atherosclerosis rat model by inhibiting inflammatory response[23]. DBD decreased HG-induced extracellular matrix accumulation and proliferation inhibition in glomerular meningeal cell, thereby involving in regulating diabetic nephropathy[24,25]. Herein, we found that DBD treatment could dose-dependently reverse HG-induced cell viability inhibition, cell apoptosis promotion and oxidative suppression in HRVECs. In all, DBD impaired HG-induced oxidative and apoptotic injury in HRVECs, implying the therapeutic value of DND in DR.
FOXO4 is widely expressed in the body and can mediate insulin-like growth factor to participate in cell growth, differentiation, apoptosis, oxidative stress and other key processes, and is closely related to diabetes and its complications[26,27]. For example, inhibition of FOXO4 could activate C1q/ TNF-Related Protein-3 (CTRP3), thereby inhibiting HG-stimulated oxidative injury in retinal pericytes during DR[28]. Moreover, Zhang et al.[29] showed that microRNA 29a/b inhibition was able to promote DR exasperation via destroying the function of Müller cells by increasing FOXO4. In this work, we confirmed that FOXO4 expression was elevated in HG-induced HRVECs, moreover, we also confirmed that knockdown of FOXO4 could protect HRVEC from HG-induced oxidative and apoptotic injury in HRVECs. Importantly, we also discovered that DBD treatment was able to decrease the levels of FOXO4 in a dose-dependently manner in HRVECs. Functionally, overexpression of FOXO4 abolished the protective effects of DBD on HG-stimulated HRVECs.
Collectively, DBD could inhibit HG-induced oxidative and apoptotic injury in HRVECs via decreasing the expression of FOXO4, implying that DBD may be developed into a novel and effective approach for DR intervention.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lechner J, O'Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vision Res 2017;139:7-14.
[Crossref] [Google Scholar] [PubMed]
- Devi TS, Hosoya KI, Terasaki T, Singh LP. Critical role of TXNIP in oxidative stress, DNA damage and retinal pericyte apoptosis under high glucose: Implications for diabetic retinopathy. Exp Cell Res 2013;319(7):1001-2.
[Crossref] [Google Scholar] [PubMed]
- Gong AG, Li N, Lau KM, Lee PS, Yan L, Xu ML, et al. Calycosin orchestrates the functions of Danggui Buxue Tang, a Chinese herbal decoction composing of astragali radix and Angelica sinensis radix: An evaluation by using calycosin-knock out herbal extract. J Ethnopharmacol 2015;168:150-7.
[Crossref] [Google Scholar] [PubMed]
- Hu G, Yang P, Zeng Y, Zhang S, Song J. Danggui Buxue decoction promotes angiogenesis by up-regulation of VEGFR1/2 expressions and down-regulation of sVEGFR1/2 expression in myocardial infarction rat. J Chin Med Assoc 2018;81(1):37-46.
[Crossref] [Google Scholar] [PubMed]
- Hu G, Yang P, Zeng Y, Zhang S, Song J. Danggui Buxue decoction promotes angiogenesis by up-regulation of VEGFR1/2 expressions and down-regulation of sVEGFR1/2 expression in myocardial infarction rat. J Chin Med Assoc 2018;81(1):37-46.
[Crossref] [Google Scholar] [PubMed]
- Shi XQ, Yue SJ, Tang YP, Chen YY, Zhou GS, Zhang J, et al. A network pharmacology approach to investigate the blood enriching mechanism of Danggui Buxue decoction. J Ethnopharmacol 2019;235:227-42.
[Crossref] [Google Scholar] [PubMed]
- Li C, Zhu F, Wang S, Wang J, Wu B. Danggui Buxue decoction ameliorates inflammatory bowel disease by improving inflammation and rebuilding intestinal mucosal barrier. Evid Based Complement Alternat Med 2021;2021.
[Crossref] [Google Scholar] [PubMed]
- Li TT, Guo YJ, Ren J, Liu H, Ji ES. Danggui Buxue decoction improves heart function of chronic intermittent hypoxia mice by promoting mitochondrial autophagy and inhibiting cardiomyocyte apoptosis. Zhongguo Zhong Yao Za Zhi 2022;47(11):3066-72.
[Crossref] [Google Scholar] [PubMed]
- Liu W, Li Y, Luo B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cell Mol Life Sci 2020;77(4):651-63.
[Crossref] [Google Scholar] [PubMed]
- Lee S, Dong HH. FOXO integration of insulin signaling with glucose and lipid metabolism. J Endocrinol 2017;233(2):R67-79.
[Crossref] [Google Scholar] [PubMed]
- Hong Q, Zhang L, Das B, Li Z, Liu B, Cai G, et al. Increased podocyte sirtuin-1 function attenuates diabetic kidney injury. Kidney Int 2018;93(6):1330-43.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Dong L, Liu X, Jiang Y, Zhang L, Zhang X, et al. α-Melanocyte-stimulating hormone protects retinal vascular endothelial cells from oxidative stress and apoptosis in a rat model of diabetes. PloS One 2014;9(4):e93433.
[Crossref] [Google Scholar] [PubMed]
- Siasos G, Gouliopoulos N, Moschos MM, Oikonomou E, Kollia C, Konsola T, et al. Role of endothelial dysfunction and arterial stiffness in the development of diabetic retinopathy. Diabetes Care 2015;38(1):e9-10.
[Crossref] [Google Scholar] [PubMed]
- Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: A sensor for metabolic danger? Sci 2010;327(5963):296-300.
[Crossref] [Google Scholar] [PubMed]
- Frank RN. Diabetic retinopathy. N Engl J Med 2004;350(1):48-58.
- Stewart MW. Treatment of diabetic retinopathy: Recent advances and unresolved challenges. World J Diab 2016;7(16):333.
[Crossref] [Google Scholar] [PubMed]
- You L, Peng H, Liu J, Cai M, Wu H, Zhang Z, et al. Catalpol protects arpe-19 cells against oxidative stress viaactivation of the keap1/Nrf2/ARE pathway. Cells 2021;10(10):2635.
- Yilmaz MI, Romano M, Basarali MK, Elzagallaai A, Karaman M, Demir Z, et al. The effect of corrected inflammation, oxidative stress and endothelial dysfunction on FMD levels in patients with selected chronic diseases: A quasi-experimental study. Sci Rep 2020;10(1):9018.
[Crossref] [Google Scholar] [PubMed]
- Islam MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 2017;39(1):73-82.
[Crossref] [Google Scholar] [PubMed]
- Daenen K, Andries A, Mekahli D, van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol 2019;34(6):975-91.
[Crossref] [Google Scholar] [PubMed]
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: Harms and benefits for human health. Oxid Med Cell Longev 2017;2017.
[Crossref] [Google Scholar] [PubMed]
- Gao QT, Choi RC, Cheung AW, Zhu JT, Li J, Chu GK, et al. Danggui Buxue Tang–A Chinese herbal decoction activates the phosphorylations of extracellular signal-regulated kinase and estrogen receptor α in cultured MCF-7 cells. FEBS Lett 2007;581(2):233-40.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Chen S, Deng X, Yang X, Huang X. Danggui Buxue Tang decoction has an anti-inflammatory effect in diabetic atherosclerosis rat model. Diabetes Res Clin Prac 2006;74(2):194-6.
[Crossref] [Google Scholar] [PubMed]
- Ke HL, Zhang YW, Zhou BF, Zhen RT. Effects of Danggui Buxue Tang, a traditional Chinese herbal decoction, on high glucose-induced proliferation and expression of extracellular matrix proteins in glomerular mesangial cells. Nat Prod Res 2012;26(11):1022-6.
[Crossref] [Google Scholar] [PubMed]
- Zhang R, Han X, Huang T, Wang X. Danggui Buxue tang inhibited mesangial cell proliferation and extracellular matrix accumulation through GAS5/NF-κB pathway. Biosci Rep 2019;39(10):BSR20181740.
[Crossref] [Google Scholar] [PubMed]
- O’Neill BT, Lee KY, Klaus K, Softic S, Krumpoch MT, Fentz J, et al. Insulin and IGF-1 receptors regulate FOXO-mediated signaling in muscle proteostasis. J Clin Invest 2016;126(9):3433-46.
- Ponugoti B, Dong G, Graves DT. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res 2012;2012:939751.
[Crossref] [Google Scholar] [PubMed]
- Zeng X, Peng Y, Wang Y, Kang K. C1q/tumor necrosis factor-related protein-3 (CTRP3) activated by forkhead box O4 (FOXO4) down-regulation protects retinal pericytes against high glucose-induced oxidative damage through nuclear factor erythroid 2-related factor 2 (Nrf2)/Nuclear factor-kappaB (NF-κB) signaling. Bioengineered 2022;13(3):6080-91.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Wu L, Chen J, Lin S, Cai D, Chen C, et al. Downregulation of MicroRNA 29a/b exacerbated diabetic retinopathy by impairing the function of Müller cells via forkhead box protein O4. Diab Vasc Dis Res 2018;15(3):214-22.
[Crossref] [Google Scholar] [PubMed]