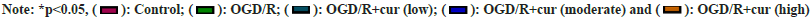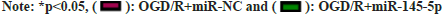- *Corresponding Author:
- Ruihua Sun
Laboratory Department, Wuwei Traditional Chinese Medicine Hospital, Wuwei 733000, China
E-mail: zldongtaiping@163.com
| Date of Received | 05 January 2022 |
| Date of Revision | 17 November 2022 |
| Date of Acceptance | 04 September 2023 |
| Indian J Pharm Sci 2023;85(5):1302-1308 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Curcumin can alleviate cerebral ischemia/reperfusion injury, but its mediated mechanism is still unclear. The study aimed to investigate whether curcumin mediates cerebral ischemia/reperfusion injury-impelled neuronal damage by microRNA-145-5p. An oxygen-glucose-deprivation/reperfusion model was conducted using rat hippocampal neuron HT22. Detection of catalase and superoxide dismutase activities and malondialdehyde content by a matching reagent kit was carried out to assess cellular oxidative stress. Interleukin-6, interleukin-10, and tumor necrosis factor-alpha were assessed for inflammation analysis. Relative microRNA-145-5p expression was detected by quantitative polymerase chain reaction. Oxygen-glucose-deprivation/reperfusion stimulation prompted the release of interleukin-10, interleukin-6, and tumor necrosis factor-alpha, lessened the activities of catalase and superoxide dismutase, as well as elevated the content of malondialdehyde in neurons HT22, but these alterations were undercut by curcumin in a concentration-dependent manner, suggesting that curcumin impaired oxygen-glucose-deprivation/reperfusion-prompted HT22 cell inflammation and oxidative stress. MicroRNA-145-5p silencing overtly reduced oxygen-glucose-deprivation/reperfusionprompted HT22 cell inflammation and oxidative stress. Curcumin treatment significantly decreased oxygenglucose- deprivation/reperfusion-mediated upregulation of microRNA-145-5p in neurons HT22. Moreover, in oxygen-glucose-deprivation/reperfusion-stimulated neurons HT22, the upregulation of microRNA-145-5p overexpression partly reversed cur-mediated changes regarding pro-inflammatory factors and indicators of oxidative stress. Curcumin could reduce microRNA-145-5p expression in oxygen-glucose-deprivation/ reperfusion-stimulated neurons, thus weakening neuronal inflammation and oxidative stress.
Keywords
Curcumin, microRNA-145-5p, ischemia/reperfusion, inflammatory response, oxidative stress, interleukin
Cerebrovascular disease induces damage to brain tissue owing to intracranial blood circulation disorders in the blood vessels of the brain[1]. This disease is more common in middle-aged and elderly individuals with acute onset, characterized by hemiplegia and speech disorders. Acute cerebrovascular diseases are generally divided into two categories; ischemic and hemorrhagic[2]. When ischemic patients undergo rapid reperfusion, neuronal damage continues to worsen, leading to neurological dysfunction (as Cerebral Ischemia/ Reperfusion (CI/R) injury)[3]. Evidence has confirmed that CI/R injury is associated with inflammation[4,5]. At present, there are two treatment options for ischemic cerebrovascular disease, namely thrombolysis and mechanical thrombectomy[6]. However, due to the impact of time windows and the risk of secondary cerebral hemorrhage, the benefits of recanalization are limited for most patients. Therefore, it is imperative to develop new treatment methods.
Curcumin (Cur) is a rare pigment with a diketone structure in the plant kingdom[7]. Cur is conducive to lowering blood lipids, anti-tumor, antiinflammatory, cholagogic, and antioxidant[8]. It has been proven that it can exert neuroprotective effects on CI/R models through multiple pathways such as antioxidant, anti-apoptotic, and antiinflammatory[ 9-11], but the specific mechanisms mediated by it need to be further explored.
MicroRNAs (miRs) are a large family of endogenous short, single-stranded posttranscriptional regulators that regulate gene expression[12]. miRs, powerful regulators of various cellular activities, can inherently alter the cellular environment by modulating target genes, including cell growth, differentiation, development and apoptosis[13]. The research on miRs mostly focuses on cancer research and attempts to use a certain miR as a specific marker for certain cancers. Increased evidence confirms that miRs regulate intracellular pathways of numerous inflammatory mediators. Recently, miRs have been shown to affect CI/R injury[14,15]. Animal studies and clinical investigations have shown abnormal alterations in the expression of miRs during this process[16,17]. For example, miR-146a could improve CI/R injury by inhibiting IPAK-1 in Oligodendrome Cells (OPCs), increasing myelin and reducing apoptosis of OPCs[18]. As another example, miR- 3473b lessened adaptive immunophysiological regulation in mice by targeting Suppressor of Cytokine Signaling-3 (SOCS3) in mouse BV2 cell lines[19]. The association of miR-145-5p has been demonstrated in various diseases[20], including CI/R injury[21]. However, whether cur can alleviate CI/R injury by regulating the expression of miR- 145-5p is unclear.
Here, we mainly studied whether cur can alleviate Oxygen-Glucose Deprivation/Re-Oxygenation (OGD/R)-mediated neuronal damage by down regulating miR-145-5p, providing some reference for CI/R injury prevention.
Materials and Methods
Cell culture and treatment:
The growth of rat hippocampal neurons HT22 (American Type Culture Collection, Manassas, Virginia, United States of America (USA)) was maintained in Dulbecco's Modified Eagle Medium (DMEM) (high glucose) (Santa Cruz, California, USA) containing Fetal Bovine Serum (FBS) 10 % (Cell Signaling Technology, Massachusetts, USA) and placed in a cell incubator with 5 % Carbon dioxide (CO2) at 37°. For cur treatment, cur (Nanjing Guangrun Biological Products Co., Ltd, Nanjing, China) was dissolved in dimethyl sulfoxide (Solarbio, Beijing, China) to prepare cur solutions with different concentrations (20 μg/g for low dose, 40 μg/g for medium dose and 60 μg/g for high dose).
Cell transfection:
In miR-145-5p, miR-145-5p mimic, and their negative controls in miR-NC and miR-NC were used for the silencing or overexpression of miR-145-5p. Transfection was executed using Lipofectamine 3000 (Thermo, Waltham, Massachusetts, USA) with opti-Minimum Essential Medium (MEM) (Thermo) according to the producer’s guidelines.
Construction of OGD/R-stimulated neurons HT22:
For the construction of OGD-stimulated neuronal models, neurons HT22 were cultured with glucosefree DMEM (Santa Cruz) and placed in a hypoxic incubator containing 1 % O2, 5 % CO2, and 94 % Nitrogen (N2) at 37° for 3 h to induce OGD injury. After 3 h of OGD treatment, it was replaced with normal medium and deoxygenated for 24 h.
Measurement of pro-inflammatory factors:
Measurement of Tumor Necrosis Factor-Alpha (TNF-α), Interleukin (IL)-6, and IL-10 levels in the supernatant was performed following the instructions attached to the matching kit (Enzymelinked Biotech, Shanghai, China).
Indicators related to oxidative stress:
The cultured neurons HT22 were collected from each group. Various oxidative stress indicators were detected according to the instructions of Malondialdehyde (MDA), Catalase (CAT), and Superoxide Dismutase (SOD) detection kits (Hangzhou Sijiqing Biotechnology Co., Ltd., China).
Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR):
Isolation of total Ribonucleic Acid (RNA) was performed using the PicoPure™ RNA Isolation Kit (Thermo). The synthesis of complementary Deoxyribonucleic Acid (DNA) from total RNA (400 ng) was performed using the miScript II RT Kit (QIAGEN, Gaithersburg, Maryland, USA). RT-qPCR was conducted (TaKaRa, Japan), with three replicates for each sample. The cycle conditions were a total of 40 cycles, including 95° for 5 min, 95° for 30 s, and 60° for 30 s; 72° for 30 s; the cycle conditions at 60° extended for 5 min. miR- 145-5p; forward 5’-ACACTCCAGCTGGGGTCCAGTTTTCCCAGGA- 3’, reverse 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG AGGGATTC-3’; U6; forward 5’-CTCGCTTCGGCAGCACA- 3’, reverse 5’-AACGCTTCACGAATTTGCGT- 3’. Taking U6 as the internal reference, miR-145-5p expression was stimulated by using the equation 2-ΔΔCt.
Statistical analysis:
The data was analyzed by using Statistical Package for the Social Sciences (SPSS) 22.0 software. Data were showed as mean±standard deviation. Comparison of multiple groups was conducted using Analysis of Variance (ANOVA) with Student– Newman–Keuls (SNK)-q test. The difference was statistically significant as results p<0.05.
Results and Discussion
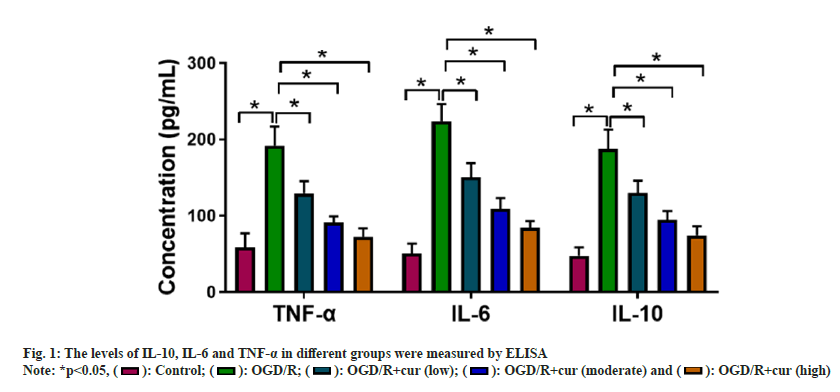
TWe first constructed OGD/R-stimulated HT22 cell models to mimic CI/R injury in vitro. ELISA showed that OGD/R stimulation enhanced IL- 10, IL-6 and TNF-α levels in neurons HT22 in comparison with the control group. However, cur treatment asymptotically lightened OGD/Rmediated promoting effect on IL-10, IL-6, and TNF-α levels in neurons HT22 with the elevation concentrations of cur (fig. 1), suggesting that cur could reduce OGD/R-prompted HT22 cell inflammatory response in a dose-dependent manner.
Next, we further discussed the effect of cur on OGD/ R-prompted oxidative stress in neurons HT22. After OGD/R stimulation, an overt decrease in SOD and CAT activities and a remarkable elevation in cellular MDA content were detected in HT22 cells. As expected, cur treatment substantially weakened OGD/R-mediated changes regarding cellular MDA content, and SOD and CAT activities in neurons HT22 in a concentration-dependent manner (fig. 2A-fig. 2C), indicating that cur could mitigate OGD/R-prompted HT22 cell oxidative stress.
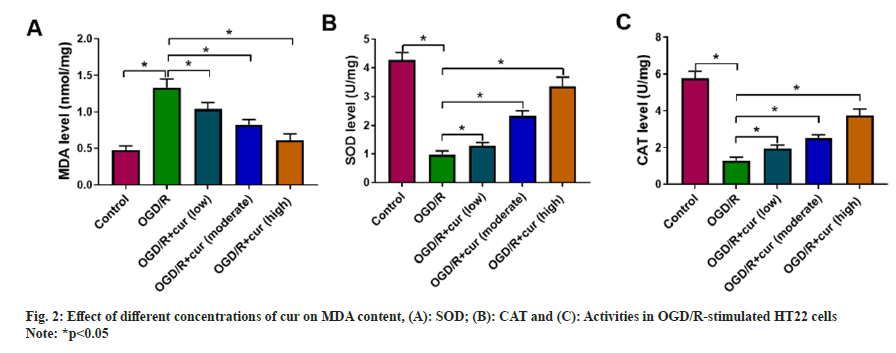
Whether cur can mediate miR-145-5p levels was further assessed. Upregulation of miR-145- 5p was discovered in neurons HT22 stimulated with OGD/R compared to the control group. Cur treatment gradually reduced miR-145-5p levels in neurons HT22 with stimulation, accompanied by an increase in its concentration as shown in fig. 3.
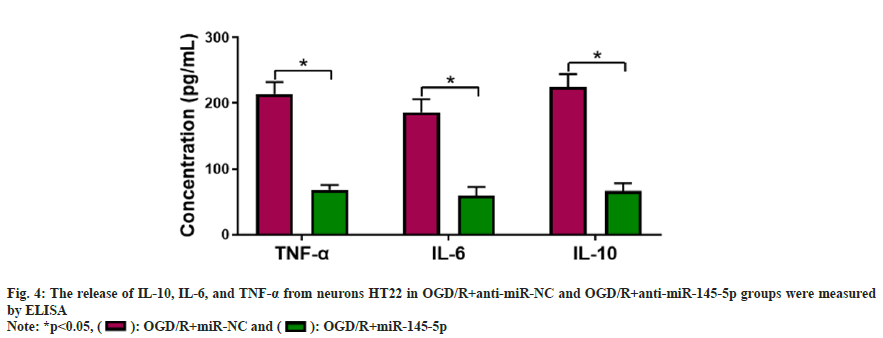
Further experiments were carried to analyze if miR-145-5p participates in OGD/R-urged HT22 cell damage. miR-145-5p silencing markedly decreased IL-10, TNF-α, and IL-6 levels in neurons HT22 stimulated with OGD/R compared with the control group (fig. 4). Furthermore, inhibition of miR-145-5p elevated SOD and CAT activities, and reduced cellular MDA content in OGD/R-induced neurons HT22 (fig. 5). These findings manifested that miR-145-5p knockdown eased OGD/Rimpelled damage in neurons HT22.
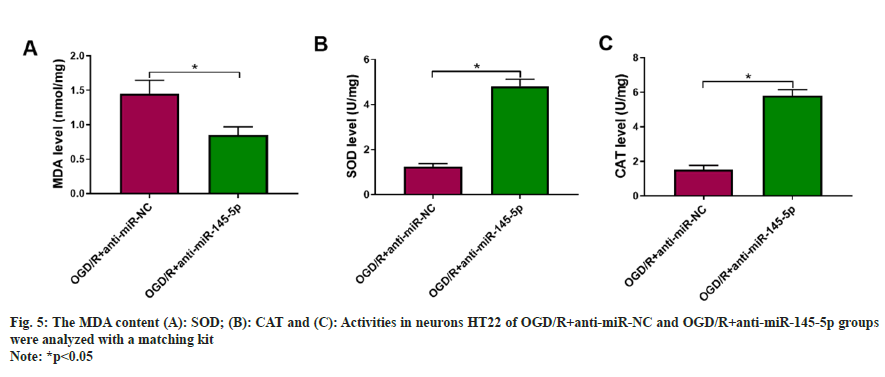
We observed that miR-145-5p overexpression partly overthrew cur-mediated changes regarding pro-inflammatory factors (fig. 6A), as well as cellular MDA content, and SOD and CAT activities in OGD/R-stimulated neurons HT22 (fig. 6B-fig. 6D), manifesting that cur weakened OGD/Rstimulated HT22 cell damage by downregulating miR-145-5p.
There being restored blood supply after a certain period of cerebral ischemia, brain function, however, even more serious impairments, which is called CI/R[22]. Many studies have confirmed that the aggravated inflammatory response after cerebral ischemic injury is an important cause of disease progression, and the CI/R-related inflammatory response is involved in the early stages of progressive cerebral ischemic injury[23]. CI/R injury triggers an inflammatory response, including the release of oxygen radicals and immunomodulation associated with cytotoxicity and brain injury. Studies have shown that antiinflammatory therapy to curb the volume expansion of cerebral infarction following ischemic stroke is beneficial for neuronal protection. Therefore, inhibiting the inflammatory response after cerebral ischemic injury to promote the recovery of neurological function is a new research direction for the treatment of ischemic stroke. Cur was first separated as a low relative molecular weight polyphenol compound from Curcuma longa L. in 1870, which has a certain effect on brain damage[24,25]. Currently, the mechanism by which cur plays a protective role in CI/R injury has not been fully elucidated.
Our data displayed that OGD/R stimulation overtly increased IL-10, IL-6 and TNF-α levels, but cur treatment lightened OGD/R-mediated changes regarding IL-10, TNF-α, and IL-6, indicating that cur could decrease OGD/R-impelled neuronal inflammatory response. Under normal conditions, the oxidation and antioxidant defense mechanisms in the body are in a state of equilibrium, and once out of balance, it will lead to oxidative stress response[26]. In general, oxidative stress states can be assessed by assessing the markers of oxidative stress CAT, SOD and MDA. MDA is a product of lipid peroxides[27]. CAT catalyzes the decomposition of hydrogen peroxide in cells preventing it from further producing highly toxic hydrogen and oxygen free radicals, thereby weakening the oxidation system[28]. SOD prevents tissue damage caused by reactive oxygen species[29]. Increased evidence confirmed that cur can reduce CI/R injury by reducing oxidative stress[25,30]. Here, cur treatment substantially weakened OGD/ R-mediated changes regarding cellular MDA content, and SOD and CAT activities in neurons HT22 in a concentration-dependent manner, indicating that cur could mitigate OGD/R-impelled neuronal oxidative stress. There have been similar studies reported that cur exerted a significant neuroprotective effect by reducing the release of inflammatory cytokines after cerebral ischemia[31]. Besides, cur pre-treatment can protect CI/R injury markedly[11]. Cur can improve CI/R injury in rat models[32,33]. Cur can alleviate cerebral ischemiaprompted pathological damage, and its mechanism may be related to inhibiting the infiltration of white blood cells in neurons and improving the permeability of the blood-brain barrier in brain tissue[34].
According to previous reports, miR-145-5p has confirmed to be associated with various diseases. For example, miR-145-5p could target osteoplastic, exacerbating bone loss in collagenimpelled arthritis[35]. Downregulation of miR- 145-5p enhanced mature cell viability in human oocytes[36]. Instead, based on its cell-damaging effects, miR-145-5p exerts tumor-inhibiting effects in diverse cancers[37-39]. miR-145-5p knockdown caused an overt reduction of infarct volume[21]. Moreover, miR-145-5p was overexpressed in OGD/R-stimulated neuronal models, and miR- 145-5p knockdown could repress oxidative stress in OGD/R-stimulated neurons[40]. Consistent with the predecessors[40], miR-145-5p levels were elevated in the OGD/R-stimulated neuronal model in our data, and miR-145-5p silencing decreased OGD/R-impelled damage in neurons. Elevated miR-145-5p expression impaired the effects of cur on the damage of OGD/R-induced neurons. All results altogether indicated that cur reduced OGD/R-impelled neuronal damage by restraining miR-145-5p expression.
The research revealed a novel mechanism by which cur reduced OGD/R-impelled neuronal damage through repressing miR-145-5p expression. The research provided a theoretical basis and scientific data for the treatment of neurological disorders and played a certain role in preventing neurological disorders.
Conflict of interests:
The authors declared no conflict of interests.
References
- Neri S, Gasparini S, Pascarella A, Santangelo D, Cianci V, Mammì A, et al. Epilepsy in cerebrovascular diseases: A narrative review. Curr Neuropharmacol 2023;21(8):1634-45.
[Crossref] [Google Scholar] [PubMed]
- Liu L, Chen W, Zhou H, Duan W, Li S, Huo X, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol 2020;5(2):159-76.
[Crossref] [Google Scholar] [PubMed]
- Yuan Q, Yuan Y, Zheng Y, Sheng R, Liu L, Xie F, et al. Anti-cerebral ischemia reperfusion injury of polysaccharides: A review of the mechanisms. Biomed Pharmacother 2021;137:111303.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zhang Z, Wang J, Zhang X, Zhao J, Bai N, et al. Scutellarin alleviates cerebral ischemia/reperfusion by suppressing oxidative stress and inflammatory responses via MAPK/NF-κB pathways in rats. Env Toxicol 2022;37(12):2889-96.
[Crossref] [Google Scholar] [PubMed]
- Min X, Zhao L, Shi Y, Wang J, Lv H, Song X, et al. Gomisin J attenuates cerebral ischemia/reperfusion injury by inducing anti-apoptotic, anti-inflammatory and antioxidant effects in rats. Bioengineered 2022;13(3):6908-18.
[Crossref] [Google Scholar] [PubMed]
- Ferrari J, Krebs S, Sykora M. Intravenous thrombolysis and mechanical thrombectomy in patients with minor or rapidly improving neurological deficits. Curr Opin Neurol 2019;32(1):13-8.
[Crossref] [Google Scholar] [PubMed]
- Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin: Miniperspective. J Med Chem 2017;60(5):1620-37.
- Priyadarsini KI. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014;19(12):20091-112.
[Crossref] [Google Scholar] [PubMed]
- Li W, Suwanwela NC, Patumraj S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res 2016;106:117-27.
[Crossref] [Google Scholar] [PubMed]
- Huang L, Chen C, Zhang X, Li X, Chen Z, Yang C, et al. Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J Mol Neurosci 2018;64(1):129-39.
[Crossref] [Google Scholar] [PubMed]
- He R, Jiang Y, Shi Y, Liang J, Zhao L. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater Sci Eng C 2020;117:111314.
[Crossref] [Google Scholar] [PubMed]
- Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics and analysis methods. J Cell Physiol 2019;234(5):5451-65.
[Crossref] [Google Scholar] [PubMed]
- Groot M, Lee H. Sorting mechanisms for microRNAs into extracellular vesicles and their associated diseases. Cells 2020;9(4):1044.
[Crossref] [Google Scholar] [PubMed]
- Zheng L, Tang X, Lu M, Sun S, Xie S, Cai J, et al. microRNA-421-3p prevents inflammatory response in cerebral ischemia/reperfusion injury through targeting m6A reader YTHDF1 to inhibit p65 mRNA translation. Int Immunopharmacol 2020;88:106937.
[Crossref] [Google Scholar] [PubMed]
- Zhou F, Wang YK, Zhang CG, Wu BY. miR-19a/b-3p promotes inflammation during cerebral ischemia/reperfusion injury via SIRT1/FoxO3/SPHK1 pathway. J Neuroinflammation 2021;18(1):122.
- Liang Z, Chi YJ, Lin GQ, Luo SH, Jiang QY, Chen YK. miRNA-26a promotes angiogenesis in a rat model of cerebral infarction via PI3K/AKT and MAPK/ERK pathway. Eur Rev Med Pharmacol Sci 2018;22(11):3485-92.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Wang X, Chen W, Yang Y, Wang Y, Wan H, et al. Danhong injection alleviates cerebral ischemia-reperfusion injury by inhibiting autophagy through miRNA-132-3p/ATG12 signal axis. J Ethnopharmacol 2023;300:115724.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Zhang ZG, Lu M, Zhang Y, Shang X, Chopp M. miR-146a promotes oligodendrocyte progenitor cell differentiation and enhances remyelination in a model of experimental autoimmune encephalomyelitis. Neurobiol Dis 2019;125:154-62.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Chen S, Ni J, Cheng J, Jia J, Zhen X. miRNA-3473b contributes to neuroinflammation following cerebral ischemia. Cell Death Dis 2018;9(1):11.
- Kadkhoda S, Ghafouri-Fard S. Function of miRNA-145–5p in the pathogenesis of human disorders. Pathol Res Pract 2022;231:153780.
[Crossref] [Google Scholar] [PubMed]
- Xie X, Peng L, Zhu J, Zhou Y, Li L, Chen Y, et al. miR-145-5p/Nurr1/TNF-α signaling-induced microglia activation regulates neuron injury of acute cerebral ischemic/reperfusion in rats. Front Mol Neurosci 2017;10:383.
[Crossref] [Google Scholar] [PubMed]
- Yapca OE, Borekci B, Suleyman H. Ischemia-reperfusion damage. Eurasian J Med 2013;45(2):126.
- Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem 2018;46(4):1650-67.
[Crossref] [Google Scholar] [PubMed]
- Mo Y, Yue E, Shi N, Liu K. The protective effects of curcumin in cerebral ischemia and reperfusion injury through PKC-θ signaling. Cell Cycle 2021;20(5-6):550-60.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Han Y, Wu X, Chen B, Liu S, Huang J, et al. Research progress on the mechanism of curcumin in cerebral ischemia/reperfusion injury: A narrative review. Apoptosis 2023;28(9-10):1-9.
[Crossref] [Google Scholar] [PubMed]
- Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 2015;97:55-74.
[Crossref] [Google Scholar] [PubMed]
- Gawe? S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek 2004;57(9-10):453-5.
[Google Scholar] [PubMed]
- Hao M, Liu R. Molecular mechanism of CAT and SOD activity change under MPA-CdTe quantum dots induced oxidative stress in the mouse primary hepatocytes. Spectrochim Acta Part A Mol Biomol Spectrosc 2020;220:117104.
[Crossref] [Google Scholar] [PubMed]
- McCord JM, Edeas MA. SOD, oxidative stress and human pathologies: A brief history and a future vision. Biomed Pharmacother 2005;59(4):139-42.
[Crossref] [Google Scholar] [PubMed]
- Lu C. Reversal of oxidative stress in neural cells by an injectable curcumin/thermosensitive hydrogel. Curr Drug Deliv 2016;13(5):682-7.
[Crossref] [Google Scholar] [PubMed]
- Yu CC, Hu H, Wang XD, Cao H, Ji B, Li J. Effect of curcumin on the injury in hippocampal neurons and the expression of RANTES in hippocamp during cerebral ischemia/reperfusion in spontaneously hypertensive rats SHR. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2014;30(4):360-4.
[Google Scholar] [PubMed]
- Kakkar V, Muppu SK, Chopra K, Kaur IP. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biopharm 2013;85(3):339-45.
[Crossref] [Google Scholar] [PubMed]
- Yavari N, Sharifi ZN, Rekabdar Y, Movassaghi S. Protective effect of curcumin on CA1 region of hippocampus in rat model of ischemia/reperfusion injury. Galen Med J 2022;11:e1062.
- Fan F, Lei M. Mechanisms underlying curcumin-induced neuroprotection in cerebral ischemia. Front Pharmacol 2022;13:893118.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Wang X, Yang M, Ruan W, Wei W, Gu D, et al. miR-145-5p increases osteoclast numbers in vitro and aggravates bone erosion in collagen-induced arthritis by targeting osteoprotegerin. Med Sci Monit 2018;24:5292.
[Crossref] [Google Scholar] [PubMed]
- Cui L, Fang L, Mao X, Chang HM, Leung PC, Ye Y. GDNF-induced downregulation of miR-145-5p enhances human oocyte maturation and cumulus cell viability. J Clin Endocrinol Metab 2018;103(7):2510-21.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Chen T, Zhu Y, Li Y, Zhang Y, Wang Y, et al. circPTN sponges miR-145-5p/miR-330-5p to promote proliferation and stem ness in glioma. J Exp Clin Cancer Res 2019;38(1):398.
[Crossref] [Google Scholar] [PubMed]
- Rajarajan D, Kaur B, Penta D, Natesh J, Meeran SM. miR-145–5p as a predictive biomarker for breast cancer stemness by computational clinical investigation. Comput Biol Med 2021;135:104601.
[Crossref] [Google Scholar] [PubMed]
- Zhou K, Song B, Wei M, Fang J, Xu Y. miR-145-5p suppresses the proliferation, migration and invasion of gastric cancer epithelial cells via the ANGPT2/NOD_LIKE_RECEPTOR axis. Cancer Cell Int 2020;20:416.
- Yan HQ, Yue XJ, Ren RF. miR-145-5p targets FGF5 to mediate ischemia/reperfusion injury-induced apoptosis and oxidative stress in neurons. Acta Univ Med Anhui 2019;54(6):7.