- *Corresponding Author:
- Jun Li
Department of Gynaecology, Gongli Hospital of Pudong, Pudong New, Shanghai 200136, China
E-mail: fangtuizhuo1@163.com
| Date of Received | 19 July 2021 |
| Date of Revision | 04 June 2022 |
| Date of Acceptance | 17 November 2022 |
| Indian J Pharm Sci 2022;84(6):1514-1519 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this study was to analyze the mechanism of curcumin inhibiting the growth and inducing apoptosis of human endometrial carcinoma cell line. Human endometrial carcinoma cell line was cultured in vitro and all cells were divided into control group, curcumin low, medium and high treatment group, the concentrations were 5 mg/l, 10 mg/l and 20 mg/l, respectively. Compared with the non-medicated control group, the proliferation of human endometrial carcinoma cell treated with curcumin at different concentrations for 24, 48 and 72 h was significantly inhibited and with the increase of drug concentration, the inhibition rate was significantly increased in a dose-dependent manner (p<0.05). After treatment of human endometrial carcinoma cell with curcumin 5 mg/l, 10 mg/l and 20 mg/l, the ratio of growth 0/ growth 1 was significantly lower than that of the control group, the ratio of growth 2/mitosis phase was significantly higher than that of the control group and the ratio of growth 2/mitosis phase was gradually increased with the increase of drug concentration, which was dose-dependent (p<0.05). After treatment of human endometrial carcinoma cells with curcumin 5 mg/l, 10 mg/l and 20 mg/l, the apoptosis rate gradually increased and with the increase of drug concentration, the apoptosis rate increased sequentially (p<0.05). After treatment of human endometrial carcinoma cell with curcumin 5 mg/l, 10 mg/l and 20 mg/l, compared with the control group, the protein level of apoptosis factor Livin was significantly decreased and the protein level of caspase-3 was significantly increased in each group (p<0.05). The levels of Livin protein and caspase-3 protein were dose-dependent with curcumin concentration. It was concluded that curcumin has obvious inhibitory effect on human endometrial cancer cells and can induce cell cycle arrest, promote apoptosis, which is concentration dependent. The related reason may be through inhibiting Livin protein expression and increasing caspase-3 protein level.
Keywords
Curcumin, human endometrial carcinoma cell, proliferation, apoptosis, tumor
Endometrial cancer is a primary epithelial malignant tumor in the endometrium. It is one of the three common malignant tumors in the female reproductive tract. Its clinical manifestations are mainly vaginal bleeding, pain in the lower abdomen and so on. In the late stage, there may be signs of enlargement of the uterus and occasional tumor tissue prolapse in the cervical canal[1]. In recent years, the incidence trend of endometrial cancer has increased significantly in the world. The number of new cases accounts for 3.9 % of female malignant tumors every year. Nearly 50% of newly diagnosed female reproductive system tumors in developed countries are endometrial cancer[2]. Its etiology and pathogenesis are not clear. According to its biological characteristics, it can be divided into endometrial and non-endometrial carcinoma. Non endometriosis carcinoma is highly malignant and incidence rate and easy to metastases, resulting in poor prognosis[3]. At present, the effect of surgery or chemotherapy is good in the treatment of endometrial cancer, but the prognosis of patients in the late stage is poor and some patients often have side effects and drug resistance at the same time of chemotherapy, which has become the main reason to affect the clinical effect. Curcumin is a chemical component extracted from the rhizome of Zingiberaceae, which has many pharmacological effects such as anti-inflammatory, lipid regulating, anti-infection, anti-tumor and anti-atherosclerosis[4]. In recent years, some scholars have found that curcumin can inhibit the proliferation of tumor cells by inducing apoptosis of tumor cells and its antitumor effect has become a research hotspot[5]. Therefore, this study further analyzes the mechanism of curcumin inhibiting the growth of cancer cells and inducing their apoptosis by cultivating Human Endometrial Cancer Cells (HEC-1B).
Materials and Methods
Experimental reagents and instruments:
HEC-1B are provided by Chinese Academy of Medical Sciences; curcumin is provided by Shanghai biotechnology Service Co., Ltd.; Roswell Park Memorial Institute (RPMI) 1640 culture medium and collagenase IV are provided by sigma company of the United States; Fetal Bovine Serum (FBS) is provided by Hangzhou Sijiqing Biotechnology Co., Ltd.; 3-(4,5-Dimethylthiazolyl- 2)-2,5-Diphenyltetrazolium Bromide (MTT) cell proliferation kit is provided by Wuhan PhD Bioengineering Co., Ltd.
The optical microscope is provided by Olympus company of Japan; the constant temperature incubator of Carbon dioxide ( CO2) is provided by Asheville NC company of the United States; the low- speed centrifuge is provided by Eppendorf abend China Co., Ltd.; the inverted microscope is provided by Shanghai Optical Instrument Factory; the flow cytometer (FACSCaliburTM type) is provided by BD company of the United States.
Cell culture and grouping:
The HEC-1B was cultured on RPMI-1640 medium containing 10 % FBS at 37° and 5 % CO2. The fluid was changed every day. The logarithmic growth phase cells were selected for the experiment. All cells were divided into control group (curcumin concentration 0 mg/l), curcumin low, medium and high treatment group, the concentrations were 5 mg/l, 10 mg/l and 20 mg/l respectively and stored in refrigerator at -20° for standby.
Experimental method:
Observation of cell morphology: Add curcumin of 5 mg/l, 10 mg/l and 20 mg/l respectively to the logarithmic growth HEC-1B cells for 24 h and observe the change of cell morphology after curcumin treatment for 72 h under inverted microscope. RPMI1640 culture medium was used as the blank control group.
MTT colorimetry to detect change of cell proliferation level: HEC-1B cells were inoculated on 96 well culture plate, cultured in 37°, 50 ml CO2 incubator for 24 h, 100 µl per well. Curcumin of different concentrations was added to the culture medium for 48 h, each group had 5 wells. MTT (5 g/l) was added to culture at 37° for 4 h before culture termination. The control group was non-drug intervention group and the blank control group was non-cell intervention group. After incubation, the supernatant was discarded and Dimethyl Sulfoxide (DMSO) 150 µl/well was added to each well. The purple crystal disappeared completely after the shaking of the flat rocker.
Calculation of cell proliferation inhibition rate: Cell proliferation inhibition rate (%)=1-Optical Density (OD) value of experimental group/OD value of control group.
Changes of cell cycle: The HEC-1B cell lines were treated with curcumin 5 mg/l, 10 mg/l and 20 mg/l respectively. The cultured normal saline was used as the reference control group. After 48 h, the cells were collected to detect the changes of cell cycle by flow cytometry.
Changes of apoptosis level: The cells of curcumin 5 mg/l, 10 mg/l and 20 mg/l treated group and control group were taken and the apoptosis rate was detected by the attachment INV-Fluorescein Isothiocyanate (FITC) and Propidium Iodide (PI) double label flow cytometry.
Expression of Livin and caspase-3 protein: The cells treated with curcumin 20 mg/l and the control group were used to detect the expression of Livin and caspase-3 protein by Western blot.
Statistical methods:
Statistical Package for the Social Sciences (SPSS) 20.0 software package was used to analyze the data of this study, the mean±standard deviation (x±s) was used to measure the data, the single factor analysis of variance was used to compare the multiple groups, the χ2 test was used to count the data and p<0.05 was regarded as the difference with statistical significance.
Results and Discussion
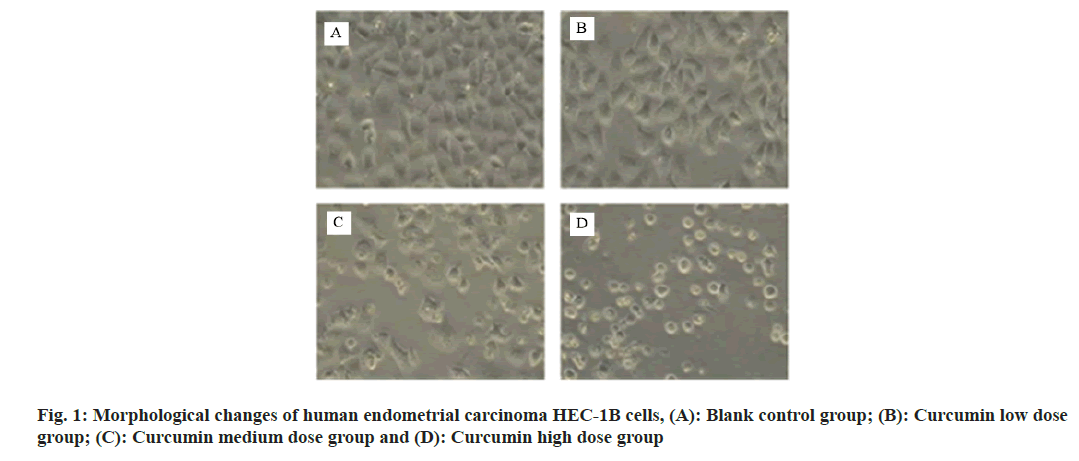
Under the inverted microscope, the cells in the control group grew well on the wall, with uniform and full shape. The cytoplasm of the low dose curcumin group was transparent and the cell morphology was good, but the growth speed was slow. The number of cells in the middle dose curcumin group decreased and the growth was inhibited. The number of adhesion cells in the high dose curcumin group decreased, the cell spacing increased and the refractive index decreased as shown in fig. 1.
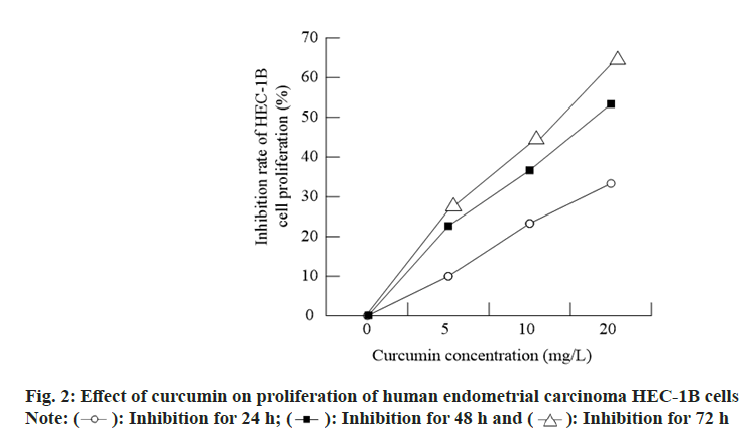
Compared with the control group, the proliferation of HEC-1B cells treated with curcumin at different concentrations for 24, 48 and 72 h was significantly inhibited and with the increase of drug concentration, the inhibition rate was significantly increased in a dose-dependent manner (p<0.05; fig. 2).
After treatment of HEC-1B cells with curcumin 5 mg/l, 10 mg/l and 20 mg/l, the ratio of Growth (G) G0/G1 was significantly lower than that of the control group, the ratio of G2/Mitosis (M) phase was significantly higher than that of the control group and the ratio of G2/M phase was gradually increased with the increase of drug concentration, which was dose-dependent (p<0.05; Table 1).
| Group | Concentration (mg/l) | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|---|
| Control group | - | 67.68±1.23 | 21.27±1.58 | 8.72±0.82 |
| Curcumin low dose group | 5 | 61.37±1.14* | 23.75±1.28* | 12.55±0.57* |
| Curcumin medium dose group | 10 | 55.20±2.53*# | 26.54±3.11*# | 15.13±0.47*# |
| Curcumin high dose group | 20 | 50.05±1.13*#△ | 31.25±1.50*#△ | 18.37±0.88*#△ |
Note: (*): represents compared with the control group, p<0.05; (#): represents compared with the low dose group of curcumin, p<0.05 and (△): represents compared with the medium dose group of curcumin, p<0.05
Table 1: Effect of Curcumin on Cell Cycle of Human Endometrial Carcinoma HEC-1B (x±s)
The apoptotic rate of HEC-1B cells treated with curcumin 5 mg/l, 10 mg/l and 20 mg/l increased gradually and with the increase of drug concentration. The apoptosis rate increased sequentially (p<0.05; Table 2).
| Group | Concentration (mg/l) | Apoptotic rate (%) |
|---|---|---|
| Control group | - | 6.53±0.27 |
| Curcumin low dose group | 5 | 6.81±0.28 |
| Curcumin medium dose group | 10 | 35.31±1.21 |
| Curcumin high dose group | 20 | 40.72±1.98 |
Table 2: The Effect of Curcumin on Multiple Side Effects and Drug-Resistant Apoptosis in Human Endometrial Carcinoma (x±s)
After curcumin 5 mg/l, 10 mg/l and 20 mg/l treated HEC-1B cells, compared with the control group, the level of apoptotic factor Livin protein was significantly decreased in each group and the protein level of caspase-3 was significantly higher (p<0.05). The levels of Livin protein and caspase-3 protein were dose-dependent with curcumin concentration as shown in Table 3.
| Group | Concentration (mg/l) | Livin | Caspase-3 |
|---|---|---|---|
| Control group | - | 1.372±0.016 | 0.858±0.004 |
| Curcumin low dose group | 5 | 1.033±0.017* | 1.268±0.029* |
| Curcumin medium dose group | 10 | 1.001±0.002* | 1.399±0.020* |
| Curcumin high dose group | 20 | 0.947±0.008* | 1.402±0.002* |
Table 3: Expression of Livin and Caspase-3 by Western Blot
The incidence of endometrial cancer is increasing year by year and it is also a common gynecological malignant tumor. The number of deaths per year accounts for 1.7 % of female malignant tumors. In some countries, the incidence is higher than cervical cancer and the 5 y survival rate is 67%[6]. Its pathogenesis is not yet clear. Clinically, hypertension, obesity and diabetes are generally considered to be triads of endometrial cancer. Because the three may be the result of hypothalamic, pituitary, adrenal dysfunction or metabolic abnormalities, abnormalities of the gonadotropic function of pituitary can make the ovary unable to secrete progesterone and lose ovulation function. In addition, obesity itself is easily accompanied by luteal progesterone secretion insufficiency and irregular menstrual cycle, which increases the risk of endometrial cancer[7,8]. In recent years, studies have shown that[9], the occurrence and development of malignant tumors are closely related to the abnormal expression of cytokines and the relationship between apoptosis and tumors has attracted much attention. Livin is a member of the apoptosis suppressor protein family. It is highly expressed in breast cancer, colon cancer, prostate cancer and other tumors. Livin expression has obvious tissue specificity. It can play an anti-apoptosis role by combining with caspase family[10]. Caspase-3 is a key protease in caspase family, which carries out apoptosis and induces apoptosis after activation. Its main function is to be activated by apoptosis pathway and inhibit tumor cell apoptosis[11].
Curcumin is the main component of turmeric. It has stable color and low toxicity. It is widely used in food coloring agents. In recent years, its anti- inflammatory, anti-coagulation, anti-rheumatic, especially anti-tumor effects have attracted clinical attention. It is considered by oncologists as a potential 3rd generation anti-cancer drug, which has the advantages of broad anti-cancer spectrum and small side effects[12,13]. Studies have shown that curcumin can produce anti-tumor effects through multiple pathways, can inhibit the proliferation of colon cancer and liver cancer and can significantly reduce the number of tumor cells and the size of the tumor. At present, chemotherapy is an effective method to treat endometrial cancer, but because patients are prone to toxic side effects and drug resistance, the therapeutic effect is quite different from people’s expectation[14]. Curcumin can not only enhance the killing effect of chemotherapy drugs on tumor cells, but also reduce the incidence of adverse reactions and drug resistance[15]. In this study, the effects of curcumin on the proliferation and inhibition of HEC-1B cell line were observed by MTT and flow cytometry. The results showed that the proliferation of HEC-1B cell line treated with curcumin at different concentrations for 24, 48 and 72 h was significantly inhibited compared with the control group (p<0.05). It was found that the ratio of G0/G1 and G2/M of HEC-1B cells treated with curcumin at different concentrations was significantly lower than that of the control group and the ratio of G2/M phase was significantly higher than that of the control group. Further detection of the apoptosis rate showed that the apoptosis rate of HEC-1B cells treated with curcumin at different concentrations increased gradually and with the increase of drug concentration, the apoptosis rate increased from the normal 6.53 %±0.27 % to 40.72 %±1.98 % (p<0.05). It is suggested that curcumin can inhibit the growth of HEC-1B and inhibit its apoptosis by inducing cell cycle arrest. In order to further explore the mechanism of curcumin, this study used Western blot to detect the expression of apoptotic factors Livin and caspase-3 protein. Compared with the control group, the level of apoptotic factor Livin protein in each treatment group of curcumin was significantly reduced and caspase-3 protein levels increased significantly (p<0.05), so this study speculates that the changes in Livin and caspase-3 protein levels may be closely related to their induction of apoptosis. There have already been reports of the biological effects of turmeric[16-22].
Apoptosis is a type of cell death or cell death during the process of programmed cell death that occurs in extracellular organisms[23-26]. The mechanism of programmed cell death of binuclear cells that occurs following intracellular mechanisms, especially fragmentation of cells, is called apoptosis or programmed cell death[27-29]. Apoptosis is a physiological and biological process for active and natural development as well as maintaining homeostasis. In cases where the survival of a cell endangers the existence of a living organism, the cell commits suicide with programmed death. When a cell is exposed to various environmental or even internal factors such as ionizing radiation, cell-killing drugs (in the treatment of cancers), hyperthermia, glucocorticoid hormones, etc., its structure, including deoxyribonucleic acid, undergoes changes that can lead to its survival if left unchecked. Severe, including cell cancer other factors such as some intracellular pathogenic bacteria such as Salmonella, Shigella, Listeria, Legionella, etc., can also be effective in directing cells to this particular type of death by altering their infectivity by modifying certain intracellular metabolic and biochemical pathways[30-34]. This study still has certain limitations. Although the effect of curcumin on HEC-1B has been clarified, the specific mechanism is still unclear. The differential expression of related genes should be clarified by high-throughput sequencing and the corresponding pathways should be studied in more detail. Furthermore, this study only did relevant research at the cellular level and the next step is to clarify the role of curcumin in tumor-bearing mice.
In conclusion, curcumin has obvious inhibitory effect on HEC-1B and can induce cell cycle arrest, promote apoptosis, which is concentration dependent. The related reason may be through inhibiting Livin protein expression and increasing caspase-3 protein level.
Conflict of interests:
The authors declared no conflict of interests.
References
- Bi Q, Chen Y, Wu K, Wang J, Zhao Y, Wang B, et al. The diagnostic value of MRI for preoperative staging in patients with endometrial cancer: A meta-analysis. Acad Radiol 2020;27(7):960-8.
[Crossref] [Google Scholar] [PubMed]
- Papadia A, Gasparri ML, Radan AP, Stämpfli CA, Rau TT, Mueller MD. Retrospective validation of the laparoscopic ICG SLN mapping in patients with grade 3 endometrial cancer. J Cancer Res Clin Oncol 2018;144(7):1385-93.
[Crossref] [Google Scholar] [PubMed]
- Plotti F, Guzzo F, Schirò T, Terranova C, Nardone CD, Montera R, et al. Role of human epididymis protein 4 (HE4) in detecting recurrence in CA125 negative ovarian cancer patients. Int J Gynecol Cancer 2019;29(4):211.
[Crossref] [Google Scholar] [PubMed]
- Sivalingam VN, Kitson S, McVey R, Roberts C, Pemberton P, Gilmour K, et al. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br J Cancer 2016;114(3):281-9.
[Crossref] [Google Scholar] [PubMed]
- Mirzaei H, Naseri G, Rezaee R, Mohammadi M, Banikazemi Z, Mirzaei HR, et al. Curcumin: A new candidate for melanoma therapy? Int J Cancer 2016;139(8):1683-95.
[Crossref] [Google Scholar] [PubMed]
- Papadia A, Gasparri ML, Buda A, Mueller MD. Sentinel lymph node mapping in endometrial cancer: Comparison of fluorescence dye with traditional radiocolloid and blue. J Cancer Res Clin Oncol 2017;143(10):2039-48.
[Crossref] [Google Scholar] [PubMed]
- Swica Y, Warren MP, Manson JE, Aragaki AK, Bassuk SS, Shimbo D, et al. Effects of oral conjugated equine estrogens with or without medroxyprogesterone acetate on incident hypertension in the Women’s Health Initiative hormone therapy trials. Menopause 2018;25(7):753.
[Crossref] [Google Scholar] [PubMed]
- Hoffman J, Fejerman L, Hu D, Huntsman S, Li M, John EM, et al. Identification of novel common breast cancer risk variants at the 6q25 locus among Latinas. Breast Cancer Res 2019;21(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Zhao J, Lyu C, Gao J, Du L, Shan B, Zhang H, et al. Dietary fat intake and endometrial cancer risk: A dose response meta-analysis. Medicine 2016;95(27):e4121.
[Crossref] [Google Scholar] [PubMed]
- Jin L, Sui YX, Chen Z, Li YH, Wang LP. Expression and clinical significance of apoptosis-related proteins Livin and caspase-3 in diffuse large B-cell lymphoma. Chin J Exp Hematol 2017;25:1664-9.
- Xu Y. Expression and clinical significance of Livin, caspase-3 and ARID in cervical squamous cell carcinoma. J Chronic Dis 2017;4:412-4.
- Song X, Zhang M, Dai E, Luo Y. Molecular targets of curcumin in breast cancer. Mol Med Rep 2019;19(1):23-9.
[Crossref] [Google Scholar] [PubMed]
- Ohnishi Y, Sakamoto T, Zhengguang L, Yasui H, Hamada H, Kubo H, et al. Curcumin inhibits epithelial-mesenchymal transition in oral cancer cells via c-Met blockade. Oncol Lett 2020;19(6):4177-82.
[Crossref] [Google Scholar] [PubMed]
- Shakeri A, Cicero AF, Panahi Y, Mohajeri M, Sahebkar A. Curcumin: A naturally occurring autophagy modulator. J Cell Physiol 2019;234(5):5643-54.
[Crossref] [Google Scholar] [PubMed]
- Huo X, Zhang Y, Jin X, Li Y, Zhang L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer's disease. J Photochem Photobiol B 2019;190:98-102.
[Crossref] [Google Scholar] [PubMed]
- Seyithanoğlu MH, Abdallah A, Kitiş S, Güler EM, Koçyiğit A, Dündar TT, et al. Investigation of cytotoxic, genotoxic and apoptotic effects of curcumin on glioma cells. Cell Mol Biol 2019;65(3):101-8.
[Crossref] [Google Scholar] [PubMed]
- Cortés H, Reyes-Hernández OD, Gonzalez-Torres M, Vizcaino-Dorado PA, Del Prado-Audelo ML, Alcalá-Alcalá S, et al. Curcumin for parkinson s disease: Potential therapeutic effects, molecular mechanisms and nanoformulations to enhance its efficacy. Cell Mol Biol 2021;67(1):101-5.
[Crossref] [Google Scholar] [PubMed]
- Zhou GZ, Wang QQ, Wang PB, Wang ZC, Sun GC. One novel curcumin derivative ZYX01 induces autophagy of human non-small lung cancer cells A549 through AMPK/ULK1/Beclin-1 signaling pathway. Cell Mol Biol 2019;65(2):1-6.
[Crossref] [Google Scholar] [PubMed]
- Alavi M, Adulrahman NA, Haleem AA, Al-Râwanduzi AD, Khusro A, Abdelgawad MA, et al. Nanoformulations of curcumin and quercetin with silver nanoparticles for inactivation of bacteria. Cell Mol Biol 2021;67(5):151-6.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Ma Y, Li W. Curcumin reduces inflammation in mice with the psoriasis model by inhibiting NLRP3 inflammatory bodies. Cell Mol Biol 2021;67(6):48-54.
[Crossref] [Google Scholar] [PubMed]
- Bilal I, Xie S, Elburki MS, Aziziaram Z, Ahmed SM, Jalal Balaky ST. Cytotoxic effect of diferuloylmethane, a derivative of turmeric on different human glioblastoma cell lines. Cell Mol Biomed Rep 2021;1(1):14-22.
- Aziziaram Z, Bilal I, Zhong Y, Mahmod AK, Roshandel MR. Protective effects of curcumin against naproxen-induced mitochondrial dysfunction in rat kidney tissue. Cell Mol Biomed Rep 2021;1(1):23-32.
- Cheng H, Chen L, Fang Z, Wan Q, Du Z, Ma N, et al. The effect of miR-138 on the proliferation and apoptosis of breast cancer cells through the NF-κB/VEGF signaling pathway. Cell Mol Biol 2022;68(2):132-7.
[Crossref] [Google Scholar] [PubMed]
- Liu G, Tian R, Mao H, Ren Y. Effect of lncRNA SNHG15 on LPS-induced vascular endothelial cell apoptosis, inflammatory factor expression and oxidative stress by targeting miR-362-3p. Cell Mol Biol 2021;67(6):220-7.
[Crossref] [Google Scholar] [PubMed]
- Hu W, Ying Y, Jin L, Chen L, Zhou T, Jin X. PMN apoptosis induced by SNHG11 through the inhibition of endotoxin-induced acute lung injury NF-κB pathway. Cell Mol Biol 2022;68(2):145-52.
[Crossref] [Google Scholar] [PubMed]
- Bai Y, Guo N, Chen Q, Chen Y, Bi Z. Ibuprofen on proliferation and apoptosis of sarcoma cells via PI3K/Akt/mTOR signaling pathway. Cell Mol Biol 2021;67(5).
[Crossref] [Google Scholar] [PubMed]
- Abudureyimu M, Zhao L, Luo X, Wang X, Liu H. Influences of ALDH2 on cardiomyocyte apoptosis in heart failure rats through regulating PINK1-parkin signaling pathway-mediated mitophagy. Cell Mol Biol 2022;68(2):94-102.
[Crossref] [Google Scholar] [PubMed]
- Yang Q, Fei Z, Huang C. Betulin terpenoid targets OVCAR-3 human ovarian carcinoma cells by inducing mitochondrial mediated apoptosis, G2/M phase cell cycle arrest, inhibition of cell migration and invasion and modulating mTOR/PI3K/AKT signalling pathway. Cell Mol Biol 2021;67(2):14-9.
[Crossref] [Google Scholar] [PubMed]
- Jiang X, Zhou X, Zhang L, Chen G, Li S, Cao Y. Long-stranded non-coding RNA HCG11 regulates glioma cell proliferation, apoptosis and drug resistance via the sponge MicroRNA-144COX-2 axis. Cell Mol Biol 2021;67(6):62-7.
[Crossref] [Google Scholar] [PubMed]
- Lin R, Guan Z, Zhou Q, Zhong J, Zheng C, Zhang Z. Effects of 7, 12-Dimethylbenz (a) anthracene on apoptosis of breast cancer cells through regulating expressions of FASL and Bcl-2. Cell Mol Biol 2022;68(1):201-8.
[Crossref] [Google Scholar] [PubMed]
- Zhang Q, Pei L, Fu J, Zhao R. The role of CTRP9 on inhibition the high-glucose-induced apoptosis of myocardial cells via Wnt/β-catenin signal pathway. Cell Mol Biol 2022;68(1):59-66.
[Crossref] [Google Scholar] [PubMed]
- Liang C, Wang S, Zhao L, Han Y, Zhang M. Effects of miR-145-5p on cardiomyocyte proliferation and apoptosis, GIGYF1 expression and oxidative stress response in rats with myocardial ischemia-reperfusion. Cell Mol Biol 2022;68(1):147-59.
- Zhao H, Li S, Wang L, Luo D, Hu S, Li D, et al. Long Chain Non Coding RNA targeting miR signal axis regulates the mechanism of apoptosis and invasion and migration of Glioma U251 cells. Cell Mol Biol 2021;67(6):149-54.
[Crossref] [Google Scholar] [PubMed]
- Shan Y, Kong W, Zhu A, Li J, Jin H, Zhu W. Knockdown of EIF3H inhibits the development and progression of pancreatic cancer by regulating cell proliferation and apoptosis in vitro. Cell Mol Biol 2021;67(4):83-90.
[Crossref] [Google Scholar] [PubMed]
 Inhibition for 72 h
Inhibition for 72 h



