- *Corresponding Author:
- Si Chen
Department of Pharmacy, School of Medicine, Shanghai University, Shanghai, Baoshan 200444, China
E-mail: sisichen@shu.edu.cn
| This article was originally published in a special issue, “Clinical Advancements in Life Sciences and Pharmaceutical Research” |
| Indian J Pharm Sci 2024:86(5) Spl Issue “1-9” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Due to the absence of approved treatments, liver fibrosis remains a leading cause of liver cirrhosis and liver failure. Our previous study demonstrated that lipophilic constituents of Salvia miltiorrhiza Bunge can treat hepatic fibrosis by inhibiting hepatic stellate cells activation and regulating the janus kinase 1/signal transducer and the activator of the transcription 3 signaling pathway. However, specific active compounds in Salvia miltiorrhiza Labiatae sp that exert anti-fibrotic effects through this mechanism of action are yet to be identified. In this study, we treated and analyzed transforming growth factor beta-1-induced LX-2 and HSC-T6 cells with purchasable compounds in Salvia miltiorrhiza Labiatae sp via cell viability assays, which identified cryptotanshinone as an effective representative of Salvia miltiorrhiza Labiatae sp. Subsequent flow cytometry and western blot experiments revealed that cryptotanshinone could inhibit hepatic stellate cells activation by promoting apoptosis and reducing the expression of fibrosis markers such as transforming growth factor beta-1 and alpha-smooth muscle actin, along with downregulating the levels of janus kinase 1, phosphorylated-janus kinase 1 and signal transducer and the activator of the transcription 3 (p<0.05). Molecular docking studies further confirmed its binding affinity with janus kinase 1 and signal transducer and the activator of the transcription 3. This study provides a scientific basis for the application of Salvia miltiorrhiza Labiatae sp in the treatment of hepatic fibrosis and partially explains the pharmacological mechanism of cryptotanshinone against liver fibrosis.
Keywords
Cryptotanshinone, liver fibrosis, hepatic stellate cells, Janus kinase 1/Signal transducer and the activator of the transcription 3 signaling pathway, molecular docking
Liver fibrosis or hepatic fibrosis, is usually the outcome of a sustained healing process that occurs in response to chronic liver damage. This damage can arise from various factors, including viral infections, autoimmune reactions, drug-induced injuries, cholestasis, and metabolic disorders[1]. Hepatic fibrosis is marked by the significant release of inflammatory cytokines and the excessive accumulation of extracellular matrix proteins following stimulation, leading to scar tissue formation. While hepatic fibrosis is generally reversible, if not treated and allowed to progress, it can develop into cirrhosis, causing irreversible damage to the liver[2]. It is disappointing that, because of its complex pathogenesis, no drugs have yet been approved for the treatment of hepatic fibrosis in clinical practice[3]. Thus, discovering effective drugs for hepatic fibrosis treatment of is urgently needed.
Salvia miltiorrhiza Bunge, commonly known as Danshen is a well-known traditional Chinese medicine widely utilized in clinical practice for its therapeutic effects on promoting blood circulation and dissolving blood stasis[4-6]. Recently, reports have suggested that Salvia miltiorrhiza also possesses anti-hepatic fibrotic properties. For example, Peng et al.[7] have shown through both in vivo and in vitro experiments that Salvia miltiorrhiza exerts anti-fibrotic effects in liver fibrosis and cirrhosis. This is achieved by increasing the abundance and activity of Natural Killer (NK) cells while inhibiting the activity of Hepatic Stellate Cells (HSCs). Hou et al.[8] reported that cryptotanshinone regulates endoplasmic reticulum stress to trigger apoptosis of HSCs. Jiang et al.[9] provided evidence from in vitro and in vivo experiments that salvianolic acid B mitigates liver fibrosis by modulating the Transforming Growth Factor-Beta (TGF-β)/Small mothers against decapentaplegic (Smad) and Mitogen-Activated Protein Kinase (MAPK) signaling pathways. Wang et al.[10] reported that tanshinol inhibits Carbon tetrachloride (CCl4)-induced liver fibrosis by regulating the Nuclear factor erythroid 2-related factor 2 (Nrf2) and Nuclear Factor Kappa-Beta (NF-κB) signaling pathways, leading to the suppression of oxidative stress and inflammation. In China, Salvia miltiorrhiza is the key component in Fuzheng-huayu tablets which aimed at treating conditions like hepatitis B-induced liver fibrosis and other related ailments[11]. However, the precise pharmacological mechanisms by which Salvia miltiorrhiza exerts its anti-hepatic fibrosis effects which have not completely been explained yet.
In our previous study, we used network pharmacology to gain initial insights into the anti-fibrotic effects and mechanisms of the lipophilic constituents of Salvia miltiorrhiza Labiatae sp. (LS)[12]. The results indicated that LS exerted its effects by regulating the Janus Kinase 1/Signal Transducer and the Activator of the Transcription 3 (JAK1/STAT3) signaling pathway to inhibit HSCs activation. However, the active compounds responsible for these anti-fibrotic effects through this mechanism remain unclear. In this study, we have initially acquired seven commercially available LS compounds. Following in vitro experiments were conducted to explore their effects on HSCs inhibition and potential impact on the JAK1/STAT3 signaling pathway. Finally, the molecular docking simulations were performed to examine the binding interactions of the ligands with their target proteins.
Materials and Methods
Cell-based validation experiments:
Reagents: Tanshinone IIA, dihydrotanshinone, cryptotanshinone, asiatic acid, oleanolic acid, ursolic acid and β-sitosterol were purchased from NatureStandard (Shanghai, China). The purity of all the purchased Chinese herbal monomer standards were >97 %. Sorafenib was purchased from Topscience (Shanghai, China). Dulbecco's Modified Eagle Medium (DMEM) high glucose medium, trypsin, fetal bovine serum and penicillin/streptomycin were all purchased from Gibco (Carlsbad, California, United States of America (USA)). Cell Counting Kit-8 (CCK-8) and Nonidet P-40 (NP-40) lysis buffer were purchased from Beyotime (Shanghai, China). Annexin V-Fluorescein Isothiocyante (FITC) cell apoptosis kit was purchased from Biolegend (San Diego, California, USA). Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), Alpha-Smooth Muscle Actin (α-SMA), STAT3, phosphorylated (p)-STAT, TGFβ1 and Janus Kinase 1 (JAK1) antibodies were all purchased from Abcam (Boston, Massachusetts, USA). p-JAK1 antibody was purchased from Invitrogen (Carlsbad, California, USA). IRDye® 800CW goat anti-rabbit Immunoglobin G (IgG) was purchased from LI-COR (Lincoln, Nebraska, USA). Protease Inhibitor Cocktail (100X) Ethylenediamine Tetraacetic Acid (EDTA)-free was purchased from Roche (Basel, Switzerland). Tris/Glycine/SDS Electrophoresis Buffer (10×) and Omni-Flash™ Western Blot Rapid Transfer Buffer (10×) was purchased from Epizyme (Shanghai, China). 1 min Protein-Free blocking buffer was purchased from Visual protein (Shanghai, China).
Cell culture and treatment:
Cells, including human HSC-LX-2 and rat HSC-T6 cell lines, were purchased from Shanghai Fudan Institute for Basic Science (IBS) Cell Resource Center. They were cultured in a DMEM high-glucose medium supplemented with 10 % fetal bovine serum and 1 % penicillin/streptomycin. The culture was maintained in an incubator at 37° with 5 % Carbon dioxide (CO2). Following 12 h of cell culture, a 16 h starvation period and a subsequent 2 h treatment with the corresponding concentrations of drugs, the cells were induced for 22 h with TGFβ1 at a concentration of 10 ng/ml. Sorafenib was selected as the positive control drug due to its reported effects in improving hepatic fibrosis through the reduction of α-SMA, TGFβ1 and p-STAT3 expression[13].
Cell viability assay:
Cells were divided into 3 groups namely, blank group (without cells), control group (without treatment) and drug treatment group. After 24 h treatment, a 10 % Cell Counting Kit-8 (CCK-8) solution was added in the absence of light and incubated in the constant-temperature incubator for 1-4 h. The microplate reader measured the absorbance at 450 nm (A450) when the color of the control group reached the desired level. Cell viability was calculated using the formula-
ODdrug group-ODblank group/ODcontrol group-ODblank group×100 %
Western blot:
A mixture of NP-40 lysate, phosphatase inhibitor and protease inhibitor was prepared in proportion of 98:1:1. After cell lysis, protein extraction was performed by adding 5× loading buffer to the mixture, followed by boiling it for approximately 5 min. The protein concentration was determined using a Bicinchoninic Acid (BCA) protein assay. We used an electrophoresis buffer (composed of 10 % 1× electrophoresis buffer and 90 % deionized water) for electrophoresis and a transfer buffer (comprising of 10 % 10× rapid transfer buffer, 80 % deionized water and 10 % anhydrous ethanol) for membrane transfer. Membrane washing was performed using Tris-Buffered Saline with Tween (TBST) solution, which was prepared by dissolving 12.1 g Tris-base, 8.7 g Sodium chloride (NaCl) and 1 ml of Tween in 1l of ultra-pure water. The pH of the TBST solution was adjusted to 7.4 using Hydrochloric acid (HCl). Equivalent amounts of protein were separated by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS/PAGE) and transferred to a Polyvinylidene Fluoride (PVDF) membrane. The protein bands on the membrane were scanned and visualized by an Odyssey® DLx infrared fluorescence imaging system. Image J was used to analyze the grayscale intensity of the Western blot bands.
Flow cytometry:
The experiment involved a control group that was activated with TGFβ1, as well as three different drug groups exposed to TGFβ1 in combination with cryptotanshinone at concentrations of 3 μM, 6 μM and 12 μM, respectively. After 24 h of treatment, the supernatant was collected and digested with trypsin for 1-3 min. Subsequently, it was centrifuged at 1500 ×g for 3 min. The cells were washed with Phosphate-Buffered Saline (PBS) after discarding the supernatant. Subsequently, a mixture of 300 μl binding buffer, 2 μl Annexin V and 2 μl Propidium Iodide (PI) solution were added to the re-suspended cells. The solution was thoroughly mixed and incubated at room temperature in the dark for 15 min before analysis by flow cytometry. In the flow cytometry results, the Upper Left (UL) quadrant represents cell necrosis, the Lower Left (LL) quadrant represents cell survival, the Lower Right (LR) quadrant corresponds to early apoptosis and the Upper Right (UR) quadrant signifies late apoptosis. Therefore, the total apoptosis is calculated as the sum of LR and UR.
Molecular docking validation experiments:
Small molecular structure of the drug was obtained from PubChem database (https://pubchem.ncbi.nlm.nih.gov/), while the structure of the target protein was obtained from Protein Data Bank (PDB) and Research Collaboratory for Structural Bioinformatics (RCSB) PDB (https://www.rcsb.org/) databases. Molecular docking was performed using Discovery Studio 2019 software and the docking process involved several steps. Primarily protein and ligand preparation was included where protein preparation included tasks such as hydrogenation, loop modeling, protonation and water removal while ligand preparation involved adjustments like ionization state correction, valency correction and the generation of tautomers and isomers. Next, binding site definition where the binding site was defined based on the original ligand site, with a default radius for the binding site sphere. The original ligand was subsequently removed from the binding site. Subsequently, LibDock was used to calculate the molecular docking results. The docking tolerance was set to 0.5 and docking preferences were configured to "High Quality." The conformation method was set to "Best," and the minimization algorithm was set to "Smart Minimizer." The LibDock score was employed to evaluate the binding affinity between the drug and the target protein, providing a measure of their binding capacity. Finally, PyMOL (version 2.5.5) was used for visualization purpose.
Statistical analysis:
Adobe Photoshop 2020 was used for image compilation, ImageJ was used for the analysis of Western blot grayscale levels and GraphPad Prism (8.0.2 version) software was used for visualization of statistical data. Statistical analysis included t-tests for comparing two sample groups and One-Way Analysis of Variance (ANOVA) for comparing multiple samples. The pairwise comparative analysis of samples were conducted using Turkey test where p<0.05 is referred as statistically significant value. The statistical results are showed as mean±Standard Error of Mean (SEM).
Results and Discussion
Identification of compounds from the LS that inhibit the viability of HSCs was studied. Our prior research demonstrated that LS possesses an anti-hepatic fibrosis effect through the inhibition of HSCs activation by down-regulating the JAK1/STAT3 signaling pathway[12]. However, the specific compounds responsible for this effect through this mechanism remained unclear. To address this issue, 7 compounds (Tanshinone IIA, cryptotanshinone, dihydrotanshinone I, asiatic acid, oleanolic acid, ursolic acid and β-Sitosterol) were selected for further experimental investigation, based on their availability for purchase.
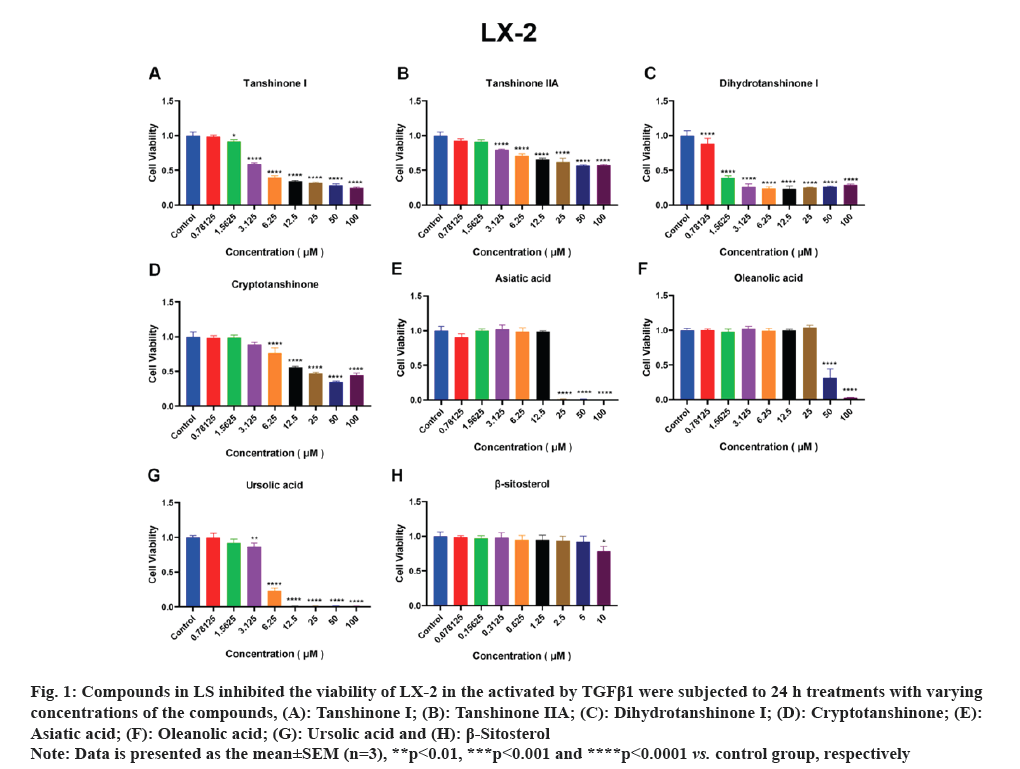
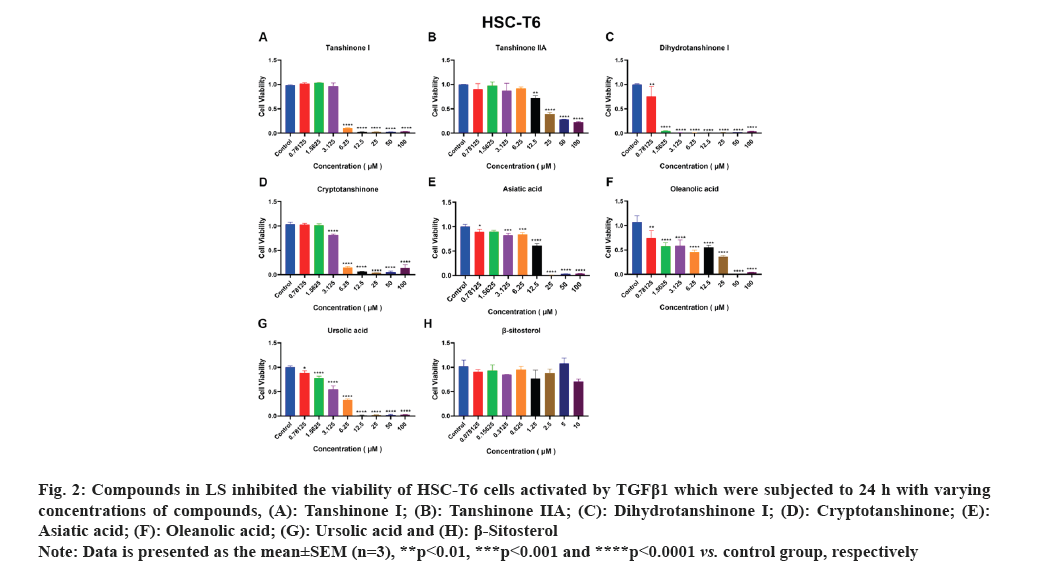
HSCs are the primary source of liver Extracellular Matrix (ECM) and the enzymes predominantly responsible for ECM degradation are found in HSCs. Therefore, the activation of HSCs is a crucial factor in the development of liver fibrosis. Accordingly, we employed activated HSCs as an in vitro model and conducted viability assays using the CCK-8 kit to test the effects of the seven compounds on LX-2 and HSC-T6 cell lines viability. As depicted in fig. 1A-fig. 1F, tanshinone iia, dihydrotanshinone i, cryptotanshinone, asiatic acid, oleanolic acid and ursolic acid exhibited significant inhibition of LX-2 cell viability. Notably, due to its low solubility, β-sitosterol was tested at a concentration of 10 μM and as shown in fig. 1G, it significantly inhibited LX-2 cell viability only at this concentration. In fig. 2, it is evident that all the compounds, except β-sitosterol, significantly inhibited HSC-T6 cell viability. The corresponding half maximal Inhibitory Concentration (IC50) values for these compounds are summarized in Table 1, which indicated that only dihydrotanshinone I and cryptotanshinone exhibited IC50 values <10 μM in both LX-2 and HSC-T6. Given that dihydrotanshinone I had been previously reported to inhibit hepatocellular carcinoma (a disease related to liver fibrosis) by suppressing the JAK2/STAT3 Pathway[14], here we selected cryptotanshinone for further experiments.
Fig. 1: Compounds in LS inhibited the viability of LX-2 in the activated by TGFβ1 were subjected to 24 h treatments with varying
concentrations of the compounds, (A): Tanshinone I; (B): Tanshinone IIA; (C): Dihydrotanshinone I; (D): Cryptotanshinone; (E):
Asiatic acid; (F): Oleanolic acid; (G): Ursolic acid and (H): β-Sitosterol.
Note: Data is presented as the mean±SEM (n=3), **p<0.01, ***p<0.001 and ****p<0.0001 vs. control group, respectively.
Fig. 2: Compounds in LS inhibited the viability of HSC-T6 cells activated by TGFβ1 which were subjected to 24 h with varying
concentrations of compounds, (A): Tanshinone I; (B): Tanshinone IIA; (C): Dihydrotanshinone I; (D): Cryptotanshinone; (E):
Asiatic acid; (F): Oleanolic acid; (G): Ursolic acid and (H): β-Sitosterol.
Note: Data is presented as the mean±SEM (n=3), **p<0.01, ***p<0.001 and ****p<0.0001 vs. control group, respectively.
| Components | IC50 LX-2 | IC50 HSC-T6 |
|---|---|---|
| Tanshinone I | 2.803 | 4.463 |
| Tanshinone IIA | 3.928 | 16.77 |
| Dihydrotanshinone I | 1.164 | 1.224 |
| Cryptotanshinone | 7.727 | 3.942 |
| Asiatic acid | 15.99 | 14.57 |
| Oleanolic acid | 48.06 | 28.81 |
| Ursolic acid | 15.09 | 4.333 |
| β-sitosterol | >10 | >10 |
Table 1: The IC50 values of active compounds in HSC-T6 and LX-2 cell lines (μm).
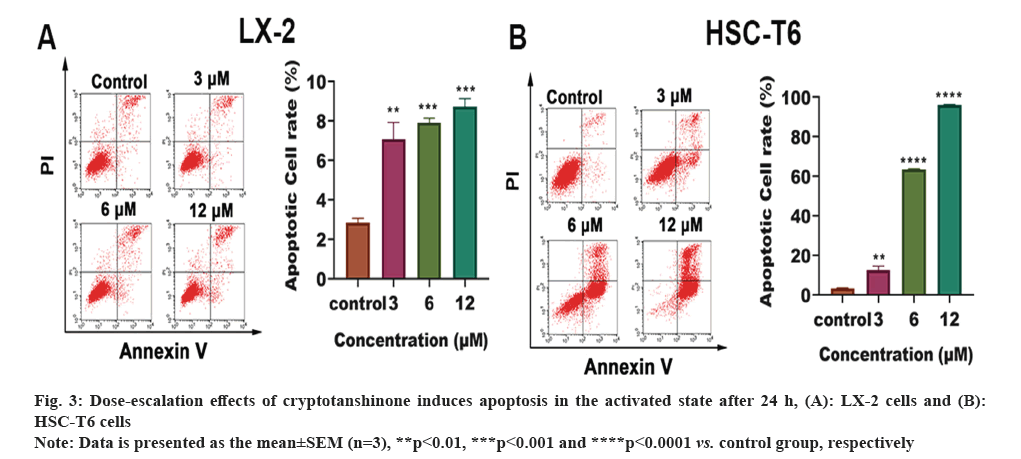
Effect of cryptotanshinone on apoptosis of HSC-T6 and LX-2 was studied. Flow cytometry was employed to evaluate the drug's impact on apoptosis. As depicted in fig. 3A and fig. 3B, treatment of both LX-2 and HSC-T6 cells with cryptotanshinone at concentrations of 0, 3, 6 and 12 μM resulted in a dose-dependent increase in the number of apoptotic cells. These results suggest that cryptotanshinone effectively inhibits the HSC activation by inducing apoptosis.
Further, effect of cryptotanshinone on the JAK1/STAT3 signaling pathway in HSCs was studied. HSC-T6 and LX-2 cells were utilized to explore the effect of cryptotanshinone on HSC activation and JAK1/STAT3 signaling pathway. During their activation phase, HSCs demonstrate an increased expression of α-SMA and other ECM proteins[15]. TGFβ1-induced activation leads to HSCs transdifferentiation into myofibroblasts, triggering enhanced ECM production while inhibiting degradation[16]. Therefore, TGFβ1 is a significant fibrogenic factor and serves as a direct marker for the assessment of hepatic fibrosis, whereas α-SMA serves as a reliable marker for HSC activation[17,18]. Importantly, previous studies have reported that JAK1 mediated TGFβ-induced STAT3 phosphorylation and the knockout of STAT3 in HSCs results in decreased α-SMA expression and the formation of fibrosis[19]. These findings demonstrate that JAK1 and STAT3 can serve as potential target proteins for liver fibrosis.
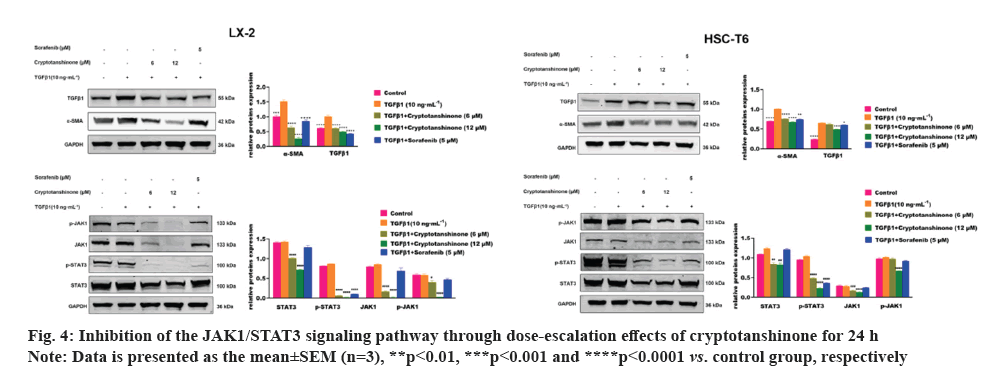
After 24 h of TGFβ1 treatment, there is a significant increase in the expression of α-SMA and TGFβ1 compared to the control group, as demonstrated by Western blot (fig. 4). This finding suggests further activation of HSCs induced by TGFβ1. Subsequent sorafenib treatment resulted in a marked reduction in the expression of α-SMA, TGFβ1 and p-STAT3, indicating effective inhibition of HSC activation by sorafenib. This supports the validity of the experimental design.
As depicted in fig. 4, treatment with 6 μM and 12 μM cryptotanshinone subsequently led to a progressive down-regulation of α-SMA and TGFβ1 expression in both LX-2 and HSC-T6 cells, compared to the TGFβ1 group. This suggests that cryptotanshinone could inhibit the TGFβ1-induced HSCs activation by reducing the expression of α-SMA and TGFβ1. We subsequently examined the impact of cryptotanshinone on the JAK1/STAT3 signaling pathway. TGFβ1 had no notable impact on the expression of p-STAT3, STAT3, JAK1 and p-JAK1 (fig. 4). Following cryptotanshinone treatment, there was a significant dose-dependent reduction in the expression of p-STAT3, STAT3, JAK1 and p-JAK1. These findings indicated that cryptotanshinone could inhibit HSCs activation by inhibiting JAK1/STAT3 signaling pathway.
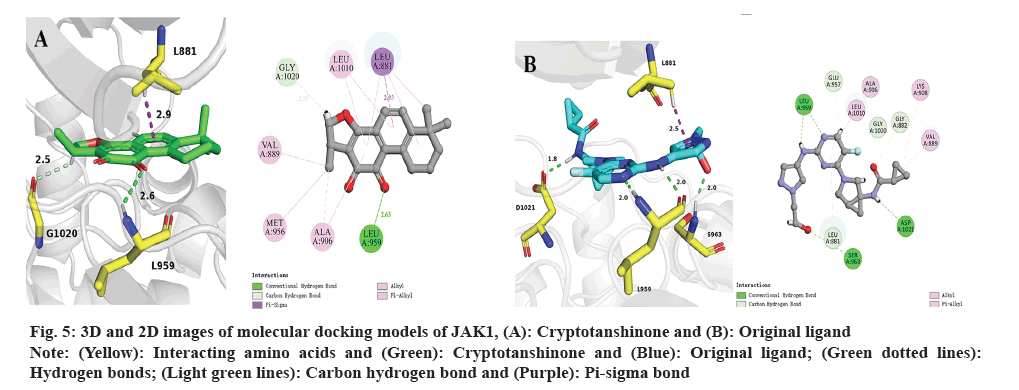
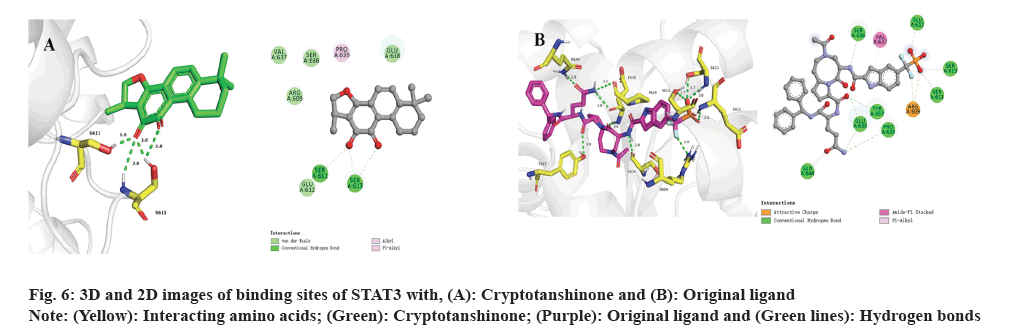
Subsequently, molecular docking results were analyzed. Following the biological experiments described above, molecular docking was used to predict the binding patterns between cryptotanshinone ligand and specific proteins, namely JAK1 (PDB: 6W8L) and STAT3 (PDB: 6NUQ). Similar to the positive ligand, cryptotanshinone can be effectively docked into the binding sites of JAK1 and STAT3. As shown in fig. 5A, the docking analysis demonstrates that cryptotanshinone binds well in the hydrophobic pocket of JAK1 by forming a hydrogen bond with residues L959 (2.6 Å) and a carbon hydrogen bond with G1020 (2.5 Å), as well as a pi-sigma bond with L881 (2.9 Å). Additionally, cryptotanshinone engages in pi-alkyl hydrophobic interaction with V889, M956, A906 and L1010, thereby contributing to its stable binding within the hydrophobic pockets of JAK1 (fig. 5A)[20]. In comparison, the original ligand shows similar interactions with JAK1 by forming hydrogen bonds with L959 (2.0 Å and 2.0 Å), S963 (2.0 Å) and D1021 (1.8 Å), as well as a pi-sigma bond with L881 (2.5 Å) (fig. 5B)[21]. As shown in fig. 6A, cryptotanshinone could be located in the Src-Homology 2 (SH2) domain of STAT3. It has been reported that inhibition of the SH2 domain can prevent the dimerization and transcriptional activity of STAT3[22]. Specifically, cryptotanshinone primarily interacts with STAT3 through hydrogen bonds with S611 (1.9 Å) and S613 (2.8 Å, 1.8 Å and 2.8 Å) (fig. 6A). In comparison, the original ligand shows similar interactions with STAT3 by forming hydrogen bonds with R609 (2.6 Å), S611 (2.7 Å), E612 (2.8 Å), S613 (2.7 Å and 3.0 Å), S636 (2.8 Å), E638 (2.8 Å), P639 (3.4 Å), Q644 (2.8 Å) and Y657 (2.8 Å) (fig. 6B). These findings suggest that Cryptotanshinone may regulate the JAK1/STAT3 signaling pathway by interacting with both JAK1 and STAT3 proteins.
Fig. 5: 3D and 2D images of molecular docking models of JAK1, (A): Cryptotanshinone and (B): Original ligand.
Note: (Yellow): Interacting amino acids and (Green): Cryptotanshinone and (Blue): Original ligand; (Green dotted lines):
Hydrogen bonds; (Light green lines): Carbon hydrogen bond and (Purple): Pi-sigma bond.
Danshen, commonly known as Salvia miltiorrhiza Bunge, is a key ingredient in formulas such as Fuzheng Huayu mixture and compound 861, which have been widely applied in the clinical treatment of liver fibrosis[23]. Moreover, clinical research has demonstrated that the LS can enhance liver functionality and decrease markers of hepatitis B-induced liver fibrosis[24]. A thorough research on LS-derived compounds revealed that only 9 compounds have been explicitly reported to exhibit anti-hepatic fibrosis properties. This indicates that only 6 % (9/138) of the LS compounds have been directly validated through experimental evidence for their anti-hepatic fibrosis effects and molecular mechanisms in vitro or/and in vivo. Although a report in 2022 indicated that cryptotanshinone promotes apoptosis of HSCs through modulation of the ERS pathway and alleviates CCl4-induced hepatic fibrosis in mouse models[8], its precise targets and the mechanisms behind its anti-fibrotic effects remain unclear.
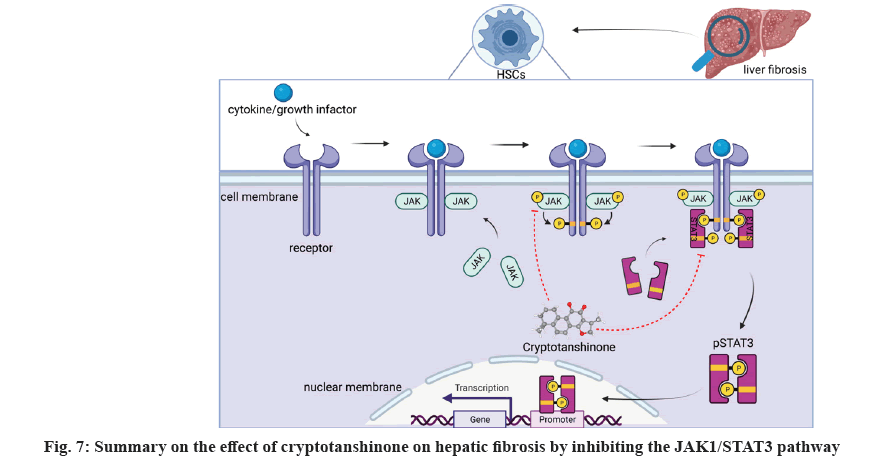
In the previous study, our team reported that LS exhibits anti-hepatic fibrosis effects through regulating JAK1/STAT3 pathway[12]. In this study, we identified cryptotanshinone as a representative compound capable of inhibiting the viability of activated HSCs and promote cell apoptosis through downregulating the hepatic fibrosis markers and suppressing the JAK1/STAT3 signaling pathway (fig. 7). To elucidate the binding affinity of cryptotanshinone with JAK1 and STAT3, molecular docking was employed, indicating its potential to target these proteins and modulate the JAK/STAT signaling pathway in the treatment of liver fibrosis.
Playing a crucial role in various biological processes including cell proliferation, differentiation, apoptosis and immune regulation, the JAK/STAT signaling pathway is a widely expressed intracellular signaling pathway[25]. Upon cytokine receptor binding, JAK proteins are phosphorylated, resulting in the creation of docking sites for cytoplasmic STAT proteins. Through tyrosine phosphorylation binding to the SH2 domain, STAT proteins are recruited into receptor complexes and undergo self-phosphorylation. Phosphorylated STAT proteins eventually initiate gene transcription. JAK1 and STAT3 are the key proteins in JAK1/STAT3 pathway. Many studies have reported that modulating JAK1 or STAT3 proteins can improve liver fibrosis. For instance, Song et al.[26], found that ruxolitinib which is a selective JAK1/JAK2 inhibitor, can reduce liver fibrosis progression, improve cell damage and accelerate liver fibrosis reversal in a CCl4-induced liver fibrosis mouse model. Yu et al.[27], pointed out that Hepatitis B Virus (HBV) sustained activation of STAT3 by down-regulating micro Ribonucleic acid (miR) 340-5p can accelerate HBV induced liver fibrosis and liver cancer. Therefore, JAK1 and STAT3 proteins are closely related to the occurrence and development of liver fibrosis.
TGFβ1 plays a pivotal role in initiating hepatic fibrosis by activating HSCs through paracrine and autocrine mechanisms[28]. However, contrary to existing research findings[29], our results demonstrated that TGFβ1 did not upregulate pSTAT3. This discrepancy can potentially be attributed to short treatment time of TGFβ1 (0.5-8) h, which may lead to pSTAT3 upregulation, whereas longer treatment duration (24 h) fails to induce pSTAT3 upregulation. Nonetheless, this finding doesn't affect our conclusion that cryptotanshinone can inhibit JAK1 and STAT3, and their phosphorylation.
In conclusion, 7 compounds of LS were selected for experimental validation. Cell-based experiments demonstrated that cryptotanshinone emerged as a representative compound of LS, with activity values <10 μM, that inhibited HSCs activation by down-regulating the JAK1/STAT3 signalling pathway. Additionally, molecular docking results indicated that cryptotanshinone could bind with both JAK1 and STAT3. These findings not only highlight JAK1 and STAT3 as potential hepatic fibrosis targets but also elucidate cryptotanshinone's molecular action, positioning it as a promising liver fibrosis treatment.
Authors contributions:
Si Chen, Xin Dong and Liang Zhao worked on conceptualization of the paper. Fen Tang worked on methodology of the study. Huizhong Dong, Yaxin Tang and Si Chen contributed towards software for the study. Huizhong Dong and Yaxin Tang contributed to validation of the study. Huizhong Dong and Fen Tang conducted formal analysis. Si Chen and Yaxin Tang investigated the study. Si Chen worked collected all the resources; Yaxin Tang was involved in data curation. Huizhong Dong, Yaxin Tang and Si Chen were involved in writing and prepared the original draft. Si Chen and Xin Dong were involved in project administration. Si Chen was involved in funding acquisition. All authors reviewed the results and approved the final version of the manuscript.
Funding:
This research was supported by the fund of Shanghai Sailing Program (Grant no: 20YF1414300).
Conflict of interests:
The authors declared no conflict of interests.
References
- Friedman SL. Liver fibrosis-from bench to bedside. J Hepatol 2003;38:S38-53.
[Crossref] [Google Scholar] [PubMed]
- Gines P, Cardenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2024;350(16):1646-54.
[Crossref] [Google Scholar] [PubMed]
- Friedman SL, Pinzani M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology 2022;75(2):473-88.
[Crossref] [Google Scholar] [PubMed]
- Ren J, Fu L, Nile SH, Zhang J, Kai G. Salvia miltiorrhiza in treating cardiovascular diseases: A review on its pharmacological and clinical applications. Front Pharmacol 2019;10:1-15.
[Crossref] [Google Scholar] [PubMed]
- Shi M, Huang F, Deng C, Wang Y, Kai G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit Rev Food Sci Nutr 2019;59(6):953-64.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Morris‐Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of tanshen. Med Res Rev 2007;27(1):133-48.
[Crossref] [Google Scholar] [PubMed]
- Peng Y, Yang T, Huang K, Shen L, Tao Y, Liu C. Salvia miltiorrhiza ameliorates liver fibrosis by activating hepatic natural killer cells in vivo and in vitro. Front Pharmacol 2018;9:762.
[Crossref] [Google Scholar] [PubMed]
- Hou XX, Li YW, Song JL, Zhang W, Liu R, Yuan H, et al. Cryptotanshinone induces apoptosis of activated hepatic stellate cells via modulating endoplasmic reticulum stress. World J Gastroenterol 2023;29(17):2616-27.
[Crossref] [Google Scholar] [PubMed]
- Jiang L, Wang J, Ju J, Dai J. Salvianolic acid B and sodium tanshinone II A sulfonate prevent pulmonary fibrosis through anti-inflammatory and anti-fibrotic process. Eur J Pharmacol 2020;883:1-15.
[Crossref] [Google Scholar] [PubMed]
- Wang R, Wang J, Song F, Li S, Yuan Y. Tanshinol ameliorates CCl4-induced liver fibrosis in rats through the regulation of Nrf2/HO-1 and NF-κB/IκBα signaling pathway. Drug Des Devel Ther 2018;12(5):1281-92.
[Crossref] [Google Scholar] [PubMed]
- Yang Z, Qi J, Ping D, Sun X, Tao Y, Liu C, et al. Salvia miltiorrhiza in thorax and abdomainal organ fibrosis: A review of its pharmacology. Front Pharmacol 2022;13:1-12.
[Crossref] [Google Scholar] [PubMed]
- Tang YX, Liu M, Liu L, Zhen BR, Wang TT, Li N, et al. Lipophilic constituents in salvia miltiorrhiza inhibit activation of the hepatic stellate cells by suppressing the JAK1/STAT3 signaling pathway: A network pharmacology study and experimental validation. Front Pharmacol 2022;13:1-10.
[Crossref] [Google Scholar] [PubMed]
- Su TH, Shiau CW, Jao P, Liu CH, Liu CJ, Tai WT, et al. Sorafenib and its derivative SC-1 exhibit antifibrotic effects through signal transducer and activator of transcription 3 inhibition. Proc Natl Acad Sci USA 2015;112(23):7243-8.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Jiao F, Zhang L, Jiang Y. Dihydrotanshinone inhibits hepatocellular carcinoma by suppressing the JAK2/STAT3 pathway. Front Pharmacol 2021;12:1-10.
[Crossref] [Google Scholar] [PubMed]
- Chang J, Lan T, Li C, Ji X, Zheng L, Gou H, et al. Activation of Slit2-Robo1 signaling promotes liver fibrosis. J Hepatol 2015;63(6):1413-20.
[Crossref] [Google Scholar] [PubMed]
- Orasan OH, Ciulei G, Cozma A, Sava M, Dumitrascu DL. Hyaluronic acid as a biomarker of fibrosis in chronic liver diseases of different etiologies. Clujul Med 2016;89(1):1-24.
[Crossref] [Google Scholar] [PubMed]
- Kostallari E, Hirsova P, Prasnicka A, Verma VK, Yaqoob U, Wongjarupong N, et al. Hepatic stellate cell-derived platelet-derived growth factor receptor-alpha-enriched extracellular vesicles promote liver fibrosis in mice through SHP2. Hepatology 2018;68(1):333-48.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Mi X, Wang Z, Zhang M, Hou J, Jiang S, et al. Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell Death Dis 2020;11(6):454.
[Crossref] [Google Scholar] [PubMed]
- Tang LY, Heller M, Meng Z, Yu LR, Tang Y, Zhou M, et al. Transforming Growth Factor-β (TGF-β) directly activates the JAK1-STAT3 axis to induce hepatic fibrosis in coordination with the SMAD pathway. J Biol Chem 2017;292(10):4302-12.
[Crossref] [Google Scholar] [PubMed]
- de Freitas RF, Schapira M. A systematic analysis of atomic protein-ligand interactions in the PDB. Medchemcomm 2017;8(10):1970-81.
[Crossref] [Google Scholar] [PubMed]
- Fensome A, Ambler CM, Arnold E, Banker ME, Clark JD, Dowty ME, et al. Design and optimization of a series of 4-(3-azabicyclo[3.1.0]hexan-3-yl) pyrimidin-2-amines: Dual inhibitors of TYK2 and JAK1. Bioorg Med Chem 2020;28(10):115481.
[Crossref] [Google Scholar] [PubMed]
- Bai L, Zhou H, Xu R, Zhao Y, Chinnaswamy K, McEachern D, et al. A potent and selective small-molecule degrader of STAT3 achieves complete tumor regression in vivo. Cancer Cell 2019;36(5):498-511.
[Crossref] [Google Scholar] [PubMed]
- Xing X, Chen S, Li L, Cao Y, Chen L, Wang X, et al. The active components of Fuzheng Huayu formula and their potential mechanism of action in inhibiting the hepatic stellate cells viability: A network pharmacology and transcriptomics approach. Front Pharmacol 2018;9:1-13.
[Crossref] [Google Scholar] [PubMed]
- Liang Z. Effect of tanshinone capsules combined with entecavir on hbv-dna negative rate and liver function in patients with chronic hepatitis b and liver fibrosis. Strait Pharmaceutical Journal 2018;30(6):119-20
- Philips RL, Wang Y, Cheon H, Kanno Y, Gadina M, Sartorelli V, et al. The JAK-STAT pathway at 30: Much learned, much more to do. Cell 2022;185(21):3857-76.
[Crossref] [Google Scholar] [PubMed]
- Song Z, Liu X, Zhang W, Luo Y, Xiao H, Liu Y, et al. Ruxolitinib suppresses liver fibrosis progression and accelerates fibrosis reversal via selectively targeting Janus kinase 1/2. J Transl Med 2022;20(1):157.
[Crossref] [Google Scholar] [PubMed]
- Zhao J, Qi YF, Yu YR. STAT3: A key regulator in liver fibrosis. Ann Hepatol 2021;21:1-12.
[Crossref] [Google Scholar] [PubMed]
- Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci 2002;7(1):d793-807.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Li J, Xiao W. The STAT3 inhibitor S3I-201 suppresses fibrogenesis and angiogenesis in liver fibrosis. Lab Invest 2018;98(12):1600-13.
[Crossref] [Google Scholar] [PubMed]