- *Corresponding Author:
- R. Badal
Department of Rasashastra and Bhaishajya Kalpana, All India Institute of Ayurveda, New Delhi110076, India
E-mail: mewbadal@gmail.com
| Date of Received | 09 May 2023 |
| Date of Revision | 28 February 2024 |
| Date of Acceptance | 26 August 2024 |
| Indian J Pharm Sci 2024;86(4):1207-1217 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The Food and Drug Administration has mandated that all pharmaceutical products should have an expiration date (or shelf life), mentioned directly on the container label since 1979. The notion of shelf life was introduced to Ayurveda, Siddha, and Unani medications in the year 2005. In view of this, a number of research works have been published regarding the shelf life assessment of varied Ayurvedic formulations and their modified dosage forms. However, lots of variations have been observed in the estimated shelf life of the same dosage form in the different studies undertaken. Owing to this, the presented article aims to compile and critically appraise both published and unpublished literature on shelf life, considering both classical and scientific concepts. An extensive search was performed in various online databases, websites as well as search engines including Google Scholar, PubMed, ScopeMed, Dhara Online, etc., to screen the available published literature. The search criterion was using the words “Shelf life”, “Saveeryata avadhi”, “stability study”, “real-time stability study”, and “expiry date” in relation to the Ayurvedic formulations. On reviewing the accessible literature, it was found that only 49 researches are there that reported the shelf life of Ayurvedic formulations, corresponding with the norms laid down in the recent version of the 2016 Gazette Notification while a majority of the studies do not comply. The calculated shelf life was found less in 54.68 % and more in 35.93 % and exact equal in only 4.68 % formulations in comparison to the prescribed values. Upon reviewing the available literature, the authors believe that determining shelf life for Ayurvedic formulations is a challenging task. Limiting shelf life to certain duration in absence of a range may be misleading. Although this current attempt is a preliminary one, more in-depth research is needed to determine the changes in stability periods and the underlying rationale for these changes due to different attributes. Moreover, there is a lack of indistinct specifications for the shelf life of many dosage forms. Hence, estimation, validation, and then incorporation of the shelf life of new dosage forms to Gazette are also the need of the moment.
Keywords
Shelf life, Saveeryata avadhi, stability study, real-time stability study, Ayurveda, Siddha, Unani
The Food and Drug Administration (FDA) has mandated since 1979 that all pharmaceutical products must have an expiration date (or shelf life) stated directly on the container label. Similar requirements are in effect in the European Union and other parts of the world also. The concept of shelf life was introduced to Ayurveda, Siddha, and Unani (ASU) medications in 2005. Considering this, rule no. 161-B was incorporated into the notification General Statutory Rules (GSR) 764(E) dated October 15, 2009. It was then amended in 2016 by the gazette notification GSR 789 (E) dated August 12. Shelf life is specified as, the time period during which a drug product is expected to remain within the approved specification, provided that it is stored under the conditions defined on the container label[1-10]. It can be determined through different stability studies viz., real-time and accelerated. To establish testing standards for evaluating stability data and estimating shelf life, distinct recommendations are provided by International Council for Harmonisation (ICH) guidelines Q1A and Q1E[11-15]. A brief narration of these requirements is aminimum of three batches should be sampled, the critical attribute(s) must be measured during the storage times periods advised in ICH Q1A, the stability data must be statistically analyzed as described in ICH Q1E, and the shelf life should be calculated as the storage time when a 95 % confidence limit crosses the acceptance threshold. To ensure the quality, safety, and efficacy of the medicine at the consumer end, it becomes important to consider the product’s shelf life being consumed[16-20]. In view of this, a number of research works have been published regarding the shelf life assessment of varied Ayurvedic formulations and their modified dosage forms. However, significant variations have been observed in the estimated shelf life of the same dosage form across different studies. Consequently, it generates the need to find out the underlying reasoning and then further implications. Owing to this, the presented article aims to compile and critically appraise both published and unpublished literature on shelf life, considering both classical and scientific concepts[20-28].
Methods
An extensive literature search was performed in various online databases, websites as well as search engines including Google Scholar, PubMed, ScopeMed, Dhara Online, etc., to screen the available published literature[29-34]. Besides, a manual search was also done in college libraries to gather unpublished data. The search criterion was restricted to the shelf life of Ayurvedic formulations[35].
Study criteria:
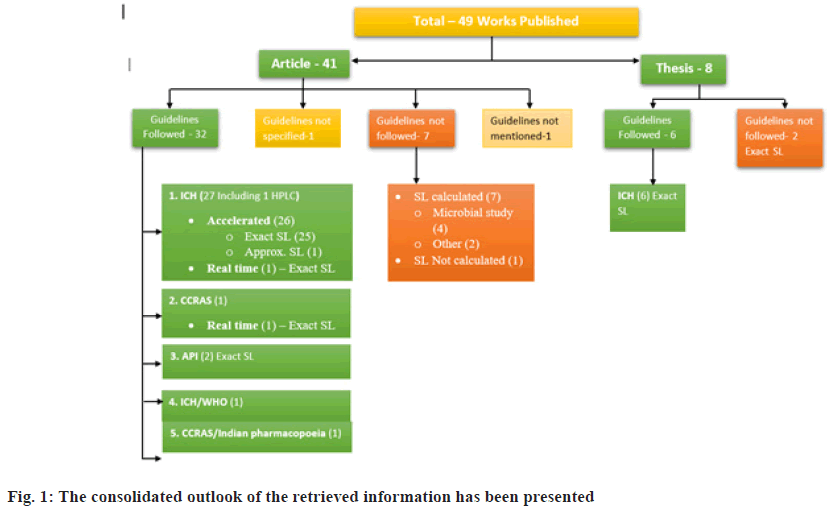
The articles were identified using the words shelf life, Saveeryata avadhi, stability study, real-time stability study, and expiry date in relation to the Ayurvedic formulations and then further verified in terms of reliability[36-40]. The search was limited to English, Hindi, and Sanskrit Languages only. They were not constrained by the place or date of the publication, though. Through this strategy, a total of 70 published articles were retrieved[41]. Among these, the articles which did not meet the screening criteria were excluded from the final analysis. Only 41 articles were found appropriate while checking their suitability, the remaining 29 articles were excluded from the study as they were not related to the topic concerned. In addition, data from 8 thesis work, conducted in different institutes of Ayurveda during the post-graduation curriculum and Ph.D. work, have also been reviewed. These theses are still unpublished. This comprehensive search was conducted from May 2022 to August 2022[42-50].
Study results:
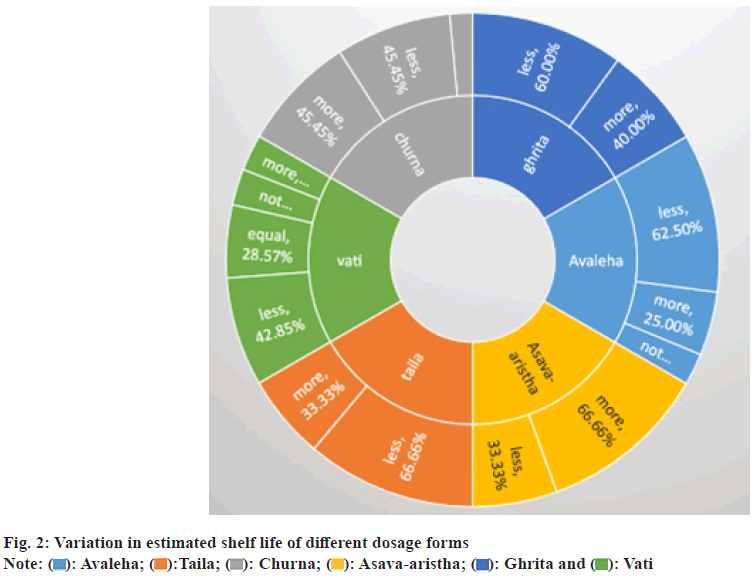
On reviewing the accessible literature, it was found that only 49 studies report the shelf life of Ayurvedic formulations in accordance with the norms outlined in the 2016 gazette notification, while the majority of studies do not comply. The calculated shelf life was less than the prescribed values in 54.68 % of the formulations, more in 35.93 %, and exactly equal in only 4.68 % of the formulations. Out of 43 articles, 33 followed guidelines to evaluate shelf life. These regulations include recommendations from ICH Q1A(R2), the Central Council for Research in Ayurvedic Sciences (CCRAS)[51], Ayurvedic Pharmacopoeia of India (API)[52], and World Health Organization (WHO)[53,54]. Upon closely examining these guidelines, notable similarities were observed. One study did not specify the guidelines used to determine shelf life, and 8 studies did not follow any of the guidelines. Regarding unpublished data, 2 theses were identified that did not follow the standard protocols outlined to date. Additionally, when reviewing the articles based on the type of stability study, it was observed that only 2 studies conducted real-time stability studies, while the others performed accelerated stability studies to calculate shelf life. Furthermore, one article did not estimate the shelf life in the publication[55]. The consolidated overview of this information is presented in fig. 1. Variation in the estimated shelf life of different dosage forms is shown in fig. 2. The list substantiating the calculated shelf life of various dosage forms, such as Avaleha, Churna, Kshaudra, Taila, and Ghrita, is presented in Table 1.
| S. No. | Name of the product | Shelf life | Modified dosage form | Reference |
|---|---|---|---|---|
| AVALEHA | ||||
| 1 | Agastiyaharitaki avaleha | 1 y 6 mo 25 d | 5 y 3 mo 7 d (granules) | [6] |
| 2 | Chitraka haritaki avaleha | 2 y | [7] | |
| 3 | Kanshaharitaki avaleha | 1 y 6 mo | 2 y 3 mo (granules) | [8] |
| 4 | Kanthkari avaleha | 1 y 1 mo 2 d | 1 y 2 mo 25 d (granules) | [9] |
| 5 | Shirish Avaleha | 1 y 4 mo 24 d (water) | 3 y 5 mo 6 d (kanji) | |
| 6 | Shirish ashwagandha avaleha | 8 y 7 mo | [10] | |
| 7 | Vasa Haritaki avaleha | 3 y 3 mo 18 d | 2 y 9 mo 18 d (granules) | [11] |
| 8 | Trivrit avaleha | 1 y 11 mo | [12] | |
| 9 | Vyaghri haritaki avaleha | Not calculated | Not calculated | [13] |
| 10 | Vasavaleha | More than 2 y (no standard) | [14] | |
| KSHAUDRA | ||||
| 11 | Bhallataka kshaudra | 4 y 5 mo 26 d | ||
| OIL | ||||
| 12 | Bhallataka oil | 4 y 10 mo 20 d | [15] | |
| 13 | Panchindra vivradhan oil | 11 mo 13 d | [16] | |
| 14 | Ksheerabala taila | Amurchita-6 mo (stable for 6 mo) | Murchita-6 mo (more stable than Amurchita) | [17] |
| 15 | Arka taila | Stable for 2 mo | [18] | |
| 16 | Lajjalu keram | 3 y 8 mo 6 d | [19] | |
| CHURNA | ||||
| 16 | Constalax powder | 2 y 1 mo 9 d | [20] | |
| 17 | Constac plus powder | 2 y | [21] | |
| 18 | Herbal digestive powder | 1 y 2 mo | [22] | |
| 19 | Hridya yoga churna | 3 y 6 mo 24 d | [23] | |
| 20 | Hutabhugadi churna | 11 mo 12 d | [24] | |
| 21 | Sitopladi churna | 1 y 10 mo 17 d | [25] | |
| 22 | Nisha Amalaki churna | 3 y 7 mo | [26] | |
| 23 | Rasayana churna | 1 y 4 mo 18 d | [27] | |
| 24 | Rasayana churna | 2 y 1 mo 9 d (Abhavita) | 5 y 3 mo 17 d (Bhavita) | [28] |
| 25 | Shatavri churna | 6 mo | [29] | |
| PASTE | ||||
| 26 | Dantashodhana paste toothpaste | 12 y 1 mo 27 d | [30] | |
| Asava-aristha | ||||
| 27 | Devdarviyaristha | 30 y, 20 y, 7 y 1 mo 27 d (market sample) | [31] | |
| Tea | ||||
| 28 | Herbal tea bag | 1 yr 5 mo | [32] | |
| Ghrita | ||||
| 29 | Indukanta ghrita | 1 yr 7 mo | [33] | |
| 30 | Shatdhaut ghrita | 9 mo (Jala siddha) | 6 m (Panchavalkal siddha) | [34] |
| 31 | Saraswata ghrita | 1y 5 mo | [35] | |
| 32 | Kasisaadi ghrita | 4 y 4 mo (agnipaki) | 3 y 8 mo (Suryapaki) | [36] |
| 33 | Ashwagandha ghrita | 3 y 17 d (Naveen) | 2 y 1 mo 27d (Purana) | [37] |
| 34 | Kumkumadi ghrita | 1 y 4 mo (Nagakesara yukta) | 1 y 4 mo (kesra yukta) | [38] |
| Ghantvati | ||||
| 35 | Kusthaghna mahakashaya ghanvati | 6 mo | [39] | |
| 36 | Sarpagandha ghan vati | 1 y 4 mo | [40] | |
| 37 | Vijayasaradi ghan vati | Microbiological study | [41] | |
| Lepa | ||||
| 38 | Marichyadi lepa | 5 mo 15 d (Agnipaki) | 6 mo 7 d (Bhanupaki) | [42] |
| Syrup | ||||
| 39 | Shringyadi syrup | 3 y 8 mo 20 d | [43] | |
| Tablet | ||||
| 40 | Turmocin plus tablet | 3 y (real time) | [44] | |
| 41 | Sanjeevni vati | 4 y 1 mo 6 d | [45] | |
| 42 | Lahsunadi vati | 3 y | [46] | |
| 43 | Bhringraja vati | No growth of microorganisms (bacterial or fungal) was found | [47] | |
| Ointment | ||||
| 44 | Sidhartaka yoga ointment | 5 y 1 mo 6 d | [48] | |
| Khanada/Paka | ||||
| 45 | Amrita bhallataka | 3 y 8 mo | [49] | |
| Rasa | ||||
| 46 | Laghu sootsekhar | 2 y 8 mo | [50] | |
| Kwatha | ||||
| 47 | Gojihwadi kwatha | Kwatha and churna-less than 1 y (stable) | Granules-upto 1 y | [51] |
| Capsule | ||||
| 48 | Herbal capsule | Ashwagandha capsule-upto 2 y | [52] | |
| Shilajeet capsule-upto 2 y | ||||
| Ashwa+shilajeet-upto 2 y | ||||
| 49 | Brahmi extract | No significant change was observed | [53] | |
| Gugglu | ||||
| 50 | Triphala guggul | No growth of microorganisms (bacterial or fungal) was found | [54] | |
Table 1: Estimated Shelf Life for Various Dosage Forms of Ayurvedic Medicines
Avaleha kalpana:
The shelf life of Avaleha is prescribed as 1 y by authoritative treatises[56] and as 3 y according to the latest gazette[7]. A total of 10 Avaleha preparations were assessed for their shelf life. The review revealed a marked variation, with shelf lives ranging from 1.08 y to 8 y and 7 mo. The maximum shelf life of 8 y and 7 mo was observed in Shirisha Ashwagandha Avaleha and the minimum of 1.08 y in Kantkari avaleha[57,58]. Further, attempts have been made to determine the shelf life of some preparations either after modifying their dosage form or some procedure. The Avaleha were modified into granules, which were found to be more stable than the original form in 3 out of 4 studies. It has been stated 1.5 times and 3 times more in Kansaaharitaki granules[11-20] and Agastiyaharitaki granules[59] when compared with their respective Avaleha. Similarly, a greater shelf life was observed for Kantakari avaleha when modified to its granular form. In contrast, the shelf life of Vasa haritaki avaleha was found to be longer than that of its granular form. One study focusing on changes in pharmaceutical procedures requires attention. It found that Shirishavaleha[60-71] showed greater stability when processed with kanji compared to when processed with water. In classical texts, only Shirishtaristha is mentioned[31], but due to its poor bioavailability, Shirisha avaleha was prepared as an alternative.
Asava arishta:
Only one study was found on this dosage form, in which the shelf life of three samples of Devdarvadyarishta was evaluated[32]. These samples varied in age: One was freshly prepared, while the other two were market samples that were 1 y and 6 y old. This study concluded that the older the sample subjected to accelerated circumstances, the lesser will be the expected shelf life. The freshly prepared drug exhibited the longest shelf life of 30 y, which is 3 times of the official specification. Nevertheless, there is a necessity to check their therapeutic effects as well, as they are supposed to have better action owing to their age in accordance with a classical verse[33]. As a result, similar research needs to be conducted for other dosage forms to explore and establish these fundamental concepts.
Capsule:
Although this dosage form is not listed in the gazette for ASU drugs, a study attempted to estimate the shelf life of three capsules viz., Ashwagandha, Shilajeet, and Ashvashila containing their extracts respectively[34]. Little to no variation was observed over 2 y, resulting in a shelf life of 2 y. However, no information was provided about the manufacturing date or the nature of the extract used.
Churna kalpana:
A comprehensive search identified 10 Churna kalpana with documented shelf life estimations. While the shelf life of all types of Churna has been recorded as 2 y, experiments related to this dosage form have shown a range from 6 mo to 3 y. Shatavari churna and Nishamalaki churna possess the shortest and longest shelf life respectively. Additionally, the shelf life of 4 powders is accounted greater than the limits notified in the gazette. One of them is a proprietary medication i.e., Constalax churna, which is comprised of 10 ingredients, reported as an effective laxative. Its shelf life has been determined to be 2 y based on real-time stability studies and 2.11 y based on accelerated stability studies[35]. On the contrary, the shelf life of 4 polyherbal powders was found to be shorter than the assigned duration. One of them is a patented drug, herbal digestive powder[36], while the others are classical medicaments. Additionally, Rasayana churna is a preparation for which two studies are available, each with slight variations
in processing. One of these studies investigated its shelf life, determining it to be 1.38 y through real- time stability testing[37]. Another study evaluated the shelf life of both primary Rasayana churna, as well as the Churna, obtained after Bhavana (levigation) with the decoction of their respective ingredients through accelerated stability[38]. Dissimilarity has been noticed in the outcome of these two experiments owing to differences in the stability study undertaken. Moreover, the potency of Bhavita churna is reported best up to 5 y and 3 mo. Thus, Bhavana could be an essential attribute, responsible for making the product more stable for a longer duration.
Extracts:
The shelf life for different types of ASU extracts is not well-documented. However, a study was conducted to determine the shelf life of dried extracts of Centella asiatica by subjecting them to accelerated stability testing, which included chemical analysis, high performance liquid chromatography, high-performance thin layer chromatography, and assessments of biological activity[39].
Kshaudra:
This dosage form is not specified in the gazette. However, a study determined the shelf life of Bhallataka kshaudra to be 4.49 y[40].
Taila kalpana:
Only 3 research works regarding the shelf life evaluation of Taila kalpana viz., Bhallataka taila[41], Panchendriyavivardhana taila[42] and Lajjalu keram have been published. Moreover, 2 thesis works viz., Arka taila[43] and Ksheerbala taila[44] about their shelf life establishment, have been undertaken. The shelf life of Bhallataka taila and Lajjalu keram has been ascertained as
4.89 y and 3 y 8 mo 6 d respectively, this shelf life exceeds the standard of 3 y. However, the opposite is observed with other Taila preparations. Classical texts reveal that coconut oil is used as a base in Lajjalu keram, while Til taila (sesame oil) is used in the processing of other Taila kalpana. On the other hand, the preparation of Bhallataka taila does not include any liquid media, such as Swarasa (juice) or Kwatha (decoction), which are typically required in Ayurvedic taila formulations. This could result in a low rate of degradation in
Bhallataka taila. Also, the oil is high in tocopherols, which are believed to protect Polyunsaturated Fatty Acids (PUFAs) from peroxidation, rendering increased stability of the oil.
Toothpaste:
Taking this dosage form into consideration, only one study i.e., shelf life assessment of Dantashodhana toothpaste[45] could be screened. In the Drug and Cosmetic Act of 1940 and the 1945 Rules, schedule S and Rule 150-A provide standards only for cosmetics. However, rule 161-B of the same act lacks shelf life data for this dosage form. Based on extrapolation from stability study observations, its shelf life has been reported as 12.16 y, which is significantly longer than the duration specified by the FDA[46].
Ghrita:
The gazette stated the shelf life of all types of Ghrita kalpana as 2 y. However, a discrepancy has been observed in the analysis of previous studies, with reported shelf lives ranging from 6 mo to 4 y and 4 mo. In 4 out of the 6 retrieved articles, the impact of additional factors on the stability of the product was also evaluated. For instance, factors such as the age of the drug, alterations in ingredients, and the manufacturing procedure can influence stability. One thesis determined the shelf life of traditional Shatadhauta ghrita and Panchavalkala siddha, Shatadhauta ghrita to be 9 mo and 6 mo, respectively[47]. However, the thesis did not provide any guidelines or methods for calculating these shelf lives. Besides, an attempt has been made to evaluate the shelf life of a herbomineral preparation, Kasisadya ghritam[48] prepared by two different methods of heating, namely Suryatapi and Agnitapi. The findings suggest that the sample subjected to heat exhibits greater stability over a longer duration compared to the sample exposed to sunlight. Moisture content may be a contributing factor, as it plays a significant role in the biodegradation of products.
Guggulu:
In reference to this important Ayurvedic dosage form, only one study was found that attempted to determine the shelf life of Triphala guggulu using microbial diagnostic methods, including wet mount, fungal culture, gram stain, and aerobic culture tests[49]. No microorganisms (bacterial or fungal) were detected for 268 d from the date of manufacturing. However, this study did not consider other relevant parameters, and the analysis intervals differed from the recommended guidelines.
Kwatha (decoction):
A study with a misleading title was found under this dosage form. The study conducted analyses such as organoleptic evaluation, physicochemical characterization, and microbial contamination checks for Gojhivadi granules only at intervals of 0, 3, and 6 mo, and did not include Gojhivadi kwatha. Thus, the title of the work appears to be a misnomer[50]. Additionally, the study did not use any standard formula to calculate the shelf life, making it unclear how the shelf life was determined.
Malahara/lepa (ointment/gel):
An experiment assessed the shelf life of a gel prepared by incorporating aerosol into its Taila under the name Marichyadi lepa[51]. The study compared the stability profiles of this modified formulation, prepared using two different methods; heating with fire and heating with sunlight. Discrepancies have been noted when compared to observations made previously while reviewing Kasisadya ghritam. Thus, it really felt an arduous task to discern the rationality behind this. Furthermore, a thesis work concerned with the stability study of Siddharthaka yoga ointment has also been undertaken[52]. The product was formulated from Siddharthaka yoga taila, hard paraffin, white soft paraffin, and liquid paraffin in the ratio of 1:1/5:3/4:3/4 respectively. Notably, the recent gazette of India only provides information about the shelf life of Malahara kalpana. To date, no research has been conducted on this dosage form.
Syrup:
A study estimated the shelf life of Shringyadi syrup[53] finding it to be slightly longer than the standard duration. Apart from that, no other research work could be explored on this dosage form.
Vati/Gutika/Ghanavati (pills/tablets):
The Government of India has specified a shelf life of 5 y for Gutika tablet containing Rasa/uparasa/ bhasma (mineral/ metallic content) along with Kasthaushadhi (herbal fraction) and 2 y for Gutika/ tablet containing only Kashthaushadhi. Under this rubric, a study on Laghu Sutshekhar Rasa (LSR) [54] is relevant, as LSR contains a substantial amount of Shunthi (Zingiber officinale Roxb.) and is levigated with Nagawalli swarasa (fresh juice of Piper betel Linn.), which increases its herbal fraction. As a result, its shelf life is reduced to 2 y and 8 mo. Similarly, a recent Post Graduation (PG) thesis on Lashunadi vati[55] found its shelf life to be shorter than the recommended duration. Though it contains gandhaka (sulphur) from the mineral origin, the underlying probable reason could be the presence of Saindhava lavana (rock salt) and its richness in herbal content. On the other hand, this concept seems inaccurate in the case of Sanjivani vati[56], which contains all the 10 ingredients of herbal origin. Its shelf life has been found longest among the tested Vati which is also greater than the classical standards as well as the specifications of gazette. It indicates towards adopting optimal precautions, and good manufacturing as well as storage practices throughout the conduction of the trial. Other than this, the factors considered for the calculation of shelf life could be a possible reason behind this. Additionally, the shelf life of all Ghana vati formulations was observed to be relatively short, indicating variability and the need for more precise guidelines and studies to understand the factors influencing shelf life in Ayurvedic formulations.
Future Prospects
An Ayurvedic medicament is indubitably a highly complex heterogeneous combination that includes alkaloids, flavonoids, terpenoids, and other constituents. As a result, no two products can have the same chemical makeup. From a scientific viewpoint, no two different Ayurvedic products in the same kind of formulation are necessarily expected to have the same shelf life owing to variation in their phytoconstituents. Consequently, the shelf life recommendation needs to be formulated on scientifically planned systematic stability studies of each herbal product in each formulation. Moreover, the therapeutic activity of each stability sample is required to be evaluated to assess the influence of storage conditions on the product’s biological asset. Additionally, the relevant parameters for computing shelf life must be established for each traditional formulation, as the number of parameters can affect the shelf life either by increasing or decreasing it. In addition, testing standards for calculating the shelf life of Ayurvedic drugs need to be developed and implemented to ensure uniformity. Furthermore, it has been observed that most studies have determined the stability period based on only one batch of the test drugs, whereas a minimum of 3 batches should be sampled according to ICH requirements. Therefore, it is important to repeat the experiments a number of times to have more accurate and confirmatory results. Additionally, the intervals for analyzing the drug must be clearly established to avoid variations in the results. Moreover, there is a lack of specific guidelines for the shelf life of many dosage forms. Therefore, it is crucial to estimate, validate, and incorporate the shelf life of new dosage forms such as capsules, syrups, toothpaste, ointments, gels, and extracts- into the gazette. Given the significant variations observed in the estimated shelf life of the same dosage form across different studies, establishing a range rather than a fixed number as the standard norm could enhance the authenticity and reliability of these standards at a global level. This article aims to compile the available literature on the impact of various factors on the shelf life of products. Although this study provides preliminary insights, more in-depth research is required to thoroughly understand the stability period and the underlying reasons for variations. Factors to be explored include the age of the drug, changes in one or more constituents, the presence of ingredients of metal or mineral origin, different manufacturing procedures, the introduction of intermediate methods such as Bhavana, and modifications in the dosage form. This study highlights the need for future research to address these considerations and provide a comprehensive understanding of their implications.
Conclusion
Upon reviewing articles on shelf life based on classical references and gazette notifications, it was found that only 49 studies reported the shelf life of Ayurvedic formulations in accordance with the norms outlined in the 2016 gazette notification. The majority of studies did not adhere to these standards. Among the formulations evaluated, 54.68 % had a calculated shelf life shorter than the prescribed values, 35.93 % had a longer shelf life, and only 4.68 % matched the exact prescribed values. Additionally, only 33 studies adhered to specific guidelines for evaluating shelf life. Thus, the authors identify significant gaps in the standardization, scientific validation, and regulatory enforcement of documented shelf life for Ayurvedic formulations. Addressing these issues through enhanced research, improved regulations, integration of modern technologies, educational initiatives, sustainable practices, and adaptation to global markets can greatly improve the credibility and reliability of Ayurvedic products. Determining the shelf life of Ayurvedic formulations is indeed a complex challenge. Fixing a specific duration without considering a range may be misleading. Both industry and academia should contribute their data on shelf life, ensuring thorough recording and documentation. Variations in manufacturing practices and references used by different pharmacies can lead to non-compliance with gazette notifications. Additionally, the parameters for calculating shelf life vary, and there are no established guidelines. Therefore, it is recommended that more studies be conducted on the shelf life of ASU formulations and that regulatory authorities develop specific guidelines.
Conflict of interests:
The authors declare no conflict of interests.
References
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Q1A(R2): Stability testing of new drug substances and products; 2003.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Q1E evaluation of stability data; 2004.
- Central Council for Research in Ayurvedic Sciences. General guideline series. New Delhi: Ministry of AYUSH, Government of India.
- Anonymous, Government of India Ministry of Health and Family Welfare Department of Indian System of Medicine and Homoeopathy. The Ayurvedic Formulary of India. Part I. Vol. 8. New Delhi: Government of India Ministry of Health and Family Welfare Department of Indian System of Medicine and Homoeopathy; 2010. p. 228-32.
- World Health Organization. WHO guidelines on stability testing of pharmaceutical products containing well-established drug substances in conventional dosage forms. WHO guidelines on stability testing of pharmaceutical products containing well-established drug substances in conventional dosage forms. 1994:13.
- Sharangadhara. Samhita Madhyama Khanda with Dipika of Adhamalla and Gudarthadipika of Kasiramavaidya. Chaukambha Orientalia; 2000(8)3:210.
- Das G. Bhaisajya Ratnawali. Commentary by Sidhi Nandan Mishra, choukhambha publication: Varanasi, Nasaroga Chikitsa Prakaran; 2005. p. 979.
- Government of India Ministry of Health and Family Welfare Department of Indian System of Medicine and Homoeopathy. The Ayurvedic Formulary of India. New Delhi: Government of India Ministry of Health and Family Welfare Department of Indian System of Medicine and Homoeopathy; 2001. p. 40.
- The Ayurvedic Pharmacopoeia of India. Pharmacopoeia Commission for Indian Medicine and Homoeopathy (PCIM&H), Ghaziabad, Ministry of AYUSH, Government of India; 2017. p. 5-6.
- Dave PP, Jadav HR, Vaghela DB, Dhiman KS. Pharmaceutical Standardization And Preliminary Physico-Chemical Profile Of Shirisha Ashwagandhadi Avaleha-A Herbo-Mineral Compound Formulation. Glob J Res Med Plant Indigenous Med 2015;4(10):209.
- Q1E evaluation of stability data
- Gupta KA, editor. Commentary vidhyotini on astanga hridayam, kalpasthana; Varanasi: Chaukhambha samskrita samsthana; 2005. p. 509.
- Government of India, Ministry of Health and Family Welfare Department of Indian System of Medicine and Homoeopathy. The Ayurvedic Formulary of India. New Delhi: Government of India, Ministry of Health of Family Welfare; 2007. p.35.
- Mishra SN, editor. Bhaishajya ratnavali of Govind das sen, chikitsasthana; Varanasi: chaukhambha surbharti prakashan; 2011. p. 408.
- Acharya YT, editor. Charaka samhita with Ayurveda dipika commentary of chakrapanidutt. Varanasi: Chaukhamba prakashan; 2011. p. 383.
- Sharma PH. Kashyapa samhita of vrudhdhajivaka, kalpa sthana, shata kalpa, chaukhambha vishvabharati: Varanasi; 2015. p. 279.
- Aarathi TS. Pharmaceutical standardization of ksheerbala taila with special reference to the concept of taila murcchana and its shelf life study, IPGT&RA, GAU, Jamnagar; 2005.
- Sharangdhar. Sharangdhar samhita. Addhyamalla and kashiram the commentator. Dipika and gudarth dipika commentary. Madhyam khand; Varanasi: Chaukhambha orientalia; 2000. p 227.
- Athar M, Musthaba S, Al-Asmari A, Baboota S, Ali J, Ahmad S. Quality control of an antipsoriatic ayurvedic herbal formulation: Lajjalu keram. Drug Dev Ther 2016;7(1):43.
- Bhagwat GB, Kadam SS, Hivarale M. The accelerated stability study of constalax churna-an ayurvedic formulation. Int J Ayurveda Pharma Res 2017;5(8):10-15.
- Bhagwat GB, Bhowate S, Hivarale M, Porwal A. Stability study of improved constac plus powder an ayurvedic polyherbal formulation used for constipation. World J Pharm Med Res 2018;4(8):191-5.
- Shamim N, Paul V, Paul A. The accelerated stability study of herbal digestive powder- an Ayurvedic formulation. AIAAS 2020:292-296.
[Crossref]
- Unnikrishnan V, Nishteswar K, Patel BR. Shelf life evaluation and comparative HPTLC profile of hridya yoga churna. Pharmacogn J 2016;8(3).
- Government of India Ministry of Health and Family Welfare Department of Indian System of Medicine and Homoeopathy. The Ayurvedic Formulary of India. New Delhi: Government of India, Ministry of Health of Family Welfare; 2003. p.119.
- Government of India, Ministry of Health and Family Welfare Department of Indian System of Medicine and Homoeopathy. The Ayurvedic Pharmacopoeia of India. New Delhi: Government of India, Ministry of Health of Family Welfare; 2007. p.79-89.
- Agnivesha. Prameha chikitsa (chikitsa sthana). In: Jadavji Trikamji. Charaka Samhita. Varanasi: Chaukhambha Sanskrit Sansthan, 2002. p. 447.
- Vagbhata, Astanga Hridaya, Uttara Sthana, Rasayana Adhyaya, Hindi commentary by Kashinath Shastriand. Krishnadas Academy,Varanasi; 1994. p. 637.
- Sarvangasundara of arunadatta and ayurvedarasayana of hemadri. Uttar sthan, Varanasi: Krishanadas academy; 2000. p. 937
- Rao PM, Gupta KV. A study on shelf life (saviryatha avadhi) of herbal drugs wsr to Shatavari (Asparagus racemosus willd) Churna. J Indian Syst Med 2021;9(2):120-5.
- Prajapati PK. Shelf-life assessment of dantashodhana paste (a polyherbal toothpaste): A preliminary study. Asian J Pharm Clin Res. 2020;13(4):79-83.
- Goyal CH, Joshi NA, Sharma VK, Malik AM, Malik SU, Kumar AS, et al. Influence of storage conditions on physico-chemical properties and shelf-life of Devdarvadyarishta. Int J Pharm Res 2021;13:622-9.
- Shamim N, Paul V, Prasad R, Chattree A. The accelerated stability study of herbal tea Bag-an ayurvedic formulation. UGC Care Group J 2020;10.
- Government of India, Ministry of Health and Family Welfare Department of Indian System of Medicine and Homoeopathy. The Ayurvedic Pharmacopoeia of India. New Delhi: Government of India, Ministry of Health of Family Welfare; 2008. p.96-8.
- Barve M, Galib PP, Ravishankar B, Shukla VJ. Shelf life study on Shatadhauta ghrita and Panchavalkala siddha shatadhauta ghrita wsr to its wound healing activity. IPGT&RA, GAU, Jamnagar; 2011.
- Badal R. Pharmaceutical standardization of Saraswata Ghrita and its effect on Alzheimer’s disease (Smritibhramsha) by oral and nasal routes (a randomized controlled clinical trial). All India Institute of Ayurveda, New Delhi; 2021.
- Yadav PR, Galib R, Prajapati PK, Singh P. Shelf-life evaluation of Kasisadya Ghritam prepared by two different methods: A preliminary evaluation. J Drug Res Ayurvedic Sci 2022;7(1):38-46.
- Damami SK. Stability and efficacy study of ashwagadha ghrita prepared with naveen (fresh) and purana (old) ghrita. All India Institute of Ayurveda, New Delhi; 2019.
- Prajapati PK, Sharma R, Amrutia A, Patgiri BJ. Physicochemical screening and shelf life evaluation of Ku?kum?di Ghrta prepared using kesara and n?gakesara. Anc Sci Life 2017;36(3):129-35.
[Crossref] [Google Scholar] [PubMed]
- Patil AD, Patil DB, Ital SV. Comparative pharmaceutico-Analytical Study of kushthaghna mahakashaya and its ghanavati wsr to evaluate its shelf life. J Ayurveda Integr Med Sci 2017;4:80-5.
- Bajiya M, Aggarwal P, Galib R, Prajapati PK, Yadav PR. Stability Study Of Sarpagandha Ghana Vati-A Preliminary Evaluation. Indian J Ayurveda Integr Med KLEU 2021;2(2):73-7.
- Sharma V, Sharma SK, Cholera MS, Pandya DH, Thakar A. Stability study of Amalakibhavita nisha used in the management of type 2 diabetes (Madhumeha)-with respect to baseline microbial diagnostic modalities. Pharma Innov J 2019;8:811-7.
- Agnive SA. Yadavji Trikamji Acharya, editor. Charaka samhita with commentary of chakrapanidatta. Chaukhamba surbharti prakashan; Varanasi. Chikitsa sthana. 2011. p. 456.
- Sharma U, Yadav Y, Sharma KC. Stability analysis for polyherbal formulation Shringyadi syrup. J Emerg Technol Innov Res 2021;8(7):b32-38.
- Bhagwat GA, Kamble P, Porwal A, Barge V. To evaluate accelerated stability study of a polyherbal formulation-Turmocin plus tablet. Int J Pharm Res 2021;13(3):946-53.
- Kaushik S. Pharmaceutical standardization of Sanjivani vati prepared with Shodhita and Ashodhita Bhallataka and their comparative antipyretic activity in experimental animals. All India Institute of Ayurveda, New Delhi; 2020.
- Archna A. Pharmaceutical standardization of Lashunadi Vati and in vitro studies on its interaction with lipid lowering drugs. All India Institute of Ayurveda; 2022.
- Panigrahi M, Baghel AS, Vyas H, Cholera MS, Mohanty KP. Stability study of Bhringraj Vati and Triphala Gugulu with respect to baseline microbial diagnostic modalities. Res Rev J Microbiol Virol 2020;10(1):16-20.
- Sahoo S. Pharmaceutico-clinical study of Siddhartaka Yoga Taila and ointment and their efficacy in the management of dadru. All India Institute of Ayurveda, New Delhi; 2021.
- Sharma S. Stability profile and anti-cancerous activity of Bhallataka Rasayana in breast cancer cell lines. All India Institute of Ayurveda, New Delhi; 2022.
- Shweta M, Shivshankar R, Vaghela DB. Shelf life evaluation of Laghu Sutashekhara Rasa-a preliminary assessment. J Ayurveda Integr Med 2020;11(3):213-6.
[Crossref] [Google Scholar] [PubMed]
- Neetu N, Harish KS, Suchi M, Khemchand S. A comparative pharmaceutico-chemical study of Gojihvadi kwatha and its granules w.s.r. to stability profile. Ayurpharm Int J Ayur Alli Sci. 2013;2(4):105-119.
- Bankoti K, Rana MS, Bharadwaj MK. Accelerated stability study of herbal capsules. IOSR J Pharm 2012;2(5):1-6.
- Kaur I, Suthar N, Kaur J, Bansal Y, Bansal G. Accelerated stability studies on dried extracts of Centella asiatica through chemical, HPLC, HPTLC, and biological activity analyses. J Evid Based Complementary Altern Med 2016;21(4):NP127-37.
[Crossref] [Google Scholar] [PubMed]
- Kaviraja AG, editor. Commentary vidhyotini on astanga hridayam, Kalpasthana; Varanasi: Chaukhambha samskrita samsthana; 2005. p. 509.
- The Gazette of India. New Delhi: Government of India. Ministry of AYUSH; 2016.
- Senarathna UR, Choudhri S, Patgiri B. Accelerated stability studies of kantakari avaleha and its granules. WJPR 2020;9:892-908.
- Khemuka N, Galib R, Patgiri BJ, Prajapati PK. Shelf-life evaluation of Kamsaharitaki avaleha and its granules: A preliminary study. Anc Sci Life 2015;35(2):96-100.
[Crossref] [Google Scholar] [PubMed]
- Jaluthriya V, Jawanjal P, Goud PK, Patgiri BJ, Bedarkar P. Shelf-life evalution of Agastyharitaki Avaleha and its granule: A preliminary study. BLDE Univ J Health Sci 2020;5(2):132-9.
- Kaur H, Ruknuddin G, Prajapati PK. Shelf life evaluation of Shirishavaleha: A preliminary study. BLDE Univ J Health Sci 2016;1(2):120-4.
- Shastri Ambika Datta, Bhaishajya Ratnavali, Vishachiktsa. Varanasi, India: Chaukhambha Sanskrit Sansthan; 2002. p.765.
- Goyal CH, Joshi NA, Sharma VK, Malik AM, Malik SU, Kumar AS, et al. Influence of storage conditions on physico-chemical properties and shelf-life of Devdarvadyarishta. Int J Pharm Res 2021;13:622-9.
- Sharangadhara. Samhita Pratham Khanda with Dipika of Adhamalla and Gudarthadipika of Kasiramavaidya. Varanasi: Chaukambha orientalia; 2000. p. 13.
- Patgiri B, Soni H, Bhatt S. Evaluation of stability study of Ayurvedic formulation “Rasayana Churna. J Pharmacogn Phytochem 2014;2(5):126-30.
- Verma P, Patgiri B, Prajapati PK. Shelf-life evaluation of Rasayana Churna: A preliminary study. Int Q J Res Ayurveda 2014;35(2):184-6.
[Crossref] [Google Scholar] [PubMed]
- Yadav P. Stability study of Bhallataka Kshaudra: An ayurvedic formulation. Asian J Pharm 2019;13(3).
- Ruhila A, Yadav PR, Galib R, Prajapati PK. Evaluation of shelf life of Bhallataka Taila (Oil). Indian Drugs 2021;58(6):36-41.
- Dobariya M, Cholera MS, Patel KS, Kumar V. Stability Study of Panchendriyavivardhana Taila, used in treatment of cerebral palsy with respect to microbiological study. Int J Pharma Res Health Sci 2020;8(4):3214-9.
- Sharangadhara. Samhita madhyama khanda with Dipika of Adhamalla and Gudarthadipika of Kasiramavaidya. Varanasi: Chaukambha Orientalia; 2000. p. 227.
- Aarathi TS, Chaudhary A. Pharmaceutical standardization of Ksheerbala Taila-Shelf life study, M Pharma Ay (Doctoral dissertation, dissertation. Gujarat, India: Gujarat Ayurveda University) 2005.
- Neetu SH, Mitra S, Sharma K. A comparative pharmaceutico-chemical study of Gojivadikwath and its granules wsr to stability profile. Ayurpharm Int J Ayurveda Allied Sci 2013;2(04).
- Barad S, Bhinde SS, Bedarkar P, Ruknuddin G. Shelf-life evaluation of Marichyadi Lepa prepared with Agnipaki and Bhanupaki method: A preliminary assessment. J Indian Syst Med 2020;8(4):304-7.