- *Corresponding Author:
- Wanping Wang
Department of Clinical Medicine, Shanxi Medical University, Taiyuan, Shanxi Province 030000, China
E-mail: CZSRMYYHXKYJS@163.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “105-114” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The correlation between C-reactive protein-albumin-lymphocyte index and the severity of acute exacerbation among chronic obstructive pulmonary disease patients and its association with the use of phlegm-reducing drugs, glucocorticoids, antibiotics and C-reactive protein-albumin-lymphocyte index was explored. 257 acute exacerbation of chronic obstructive pulmonary disease individuals according to the presence and absence of respiratory failure, were selected and were divided into two groups, acute exacerbation of chronic obstructive pulmonary disease with respiratory failure group (n=130) and acute exacerbation of chronic obstructive pulmonary disease without respiratory failure group (n=127). Logistic regression was used to analyze the relationship between C-reactive protein-albumin-lymphocyte index and acute exacerbation of chronic obstructive pulmonary disease severity. To study the predictive ability of C-reactive protein-albumin-lymphocyte index on the severity of acute exacerbation of chronic obstructive pulmonary disease, receiver operating characteristic curve was drawn and we calculated the critical value of C-reactive protein-albumin-lymphocyte. According to the critical value, C-reactive protein-albumin-lymphocyte index <2.74 group (n=187) and C-reactive protein-albumin-lymphocyte index >2.74 group (n=80) were categorized. Chi-square test was used to analyze the relationship between the use of phlegm-reducing drugs, glucocorticoids, antibiotics and C-reactive protein-albumin-lymphocyte index. C-reactive protein-albumin-lymphocyte index of the acute exacerbation of chronic obstructive pulmonary disease with respiratory failure group was significantly lower than that of the acute exacerbation of chronic obstructive pulmonary disease without respiratory failure group (p<0.001); C-reactive protein-albumin-lymphocyte index in both single-factor logistic and multi-factor logistic regression (p<0.05); area under curve value of C-reactive protein-albumin-lymphocyte index was 0.653 (p<0.01), the critical value is determined to be 2.74 based on the value corresponding to the maximum Youden index. The usage rate of phlegm-reducing drugs, glucocorticoids, and antibiotics in the C-reactive protein-albumin-lymphocyte index <2.74 group are significantly higher than those in the C-reactive protein-albumin-lymphocyte index >2.74 group (p<0.05). C-reactive protein-albumin-lymphocyte index is an independent risk factor for acute exacerbation of chronic obstructive pulmonary disease with respiratory failure and has certain value in predicting the severity of acute exacerbation of chronic obstructive pulmonary disease; level of C-reactive protein-albumin-lymphocyte index is significantly related to the use of phlegm-reducing drugs, glucocorticoids and antibiotics.
Keywords
Chronic obstructive pulmonary disease, C-reactive protein-albumin-lymphocyte index, glucocorticoids, antibiotics, immunity
Chronic Obstructive Pulmonary Disease (COPD) is a heterogeneous lung disease characterized by airway abnormalities (bronchitis, bronchiolitis) and/or alveolar abnormalities (emphysema). Chronic respiratory symptoms (dyspnea, cough, sputum production, and/or exacerbations) leading to persistent, often progressive airflow limitation[1]. COPD is the 3rd leading cause of death worldwide and one of the most important global public health problems[2]. Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD) is an important factor that accelerates the progression of COPD and leads to early death. In clinical practice, it is necessary to pay attention to comprehensively and accurately assess acuteness, which is individualized for COPD patients. Key to assessment and management strategies[3]. Therefore, it is of great clinical significance to find markers that are closely related to the severity of AECOPD.
Objective of the treatment in AECOPD is to minimize the negative impact of the current acute exacerbation and prevent subsequent events. Currently recommended drugs for the treatment of AECOPD are mainly bronchodilators, glucocorticoids and antibiotics[1]. Furthermore, the development of AECOPD is affected by many factors, including inflammation levels[4], nutritional status[5] and immune function[6]. C-Reactive Protein (CRP)-Albumin-Lymphocyte (CALLY) index is an improved inflammation-nutritional-immunity scoring system based on CRP, serum Albumin (ALB) and Lymphocyte (LYM) count[7]. This study provides new clinical evidence for the assessment of the severity of AECOPD patients by exploring the correlation between the CALLY index, severity of AECOPD and the use of phlegm-reducing drugs, glucocorticoids and antibiotics.
Materials and Methods
General information:
257 AECOPD patients admitted to the Department of Respiratory and Critical Care Medicine of Changzhi People's Hospital from August 2022 to December 2023 were selected for this study. According to the presence or absence of respiratory failure, patients were divided into AECOPD with respiratory failure group (n=130) and without respiratory failure group (n=127). Data on the following demographic and clinicopathological characteristics was collected within 48 h after admission.
Inclusion criteria:
Patients who were diagnosed with COPD and had acute exacerbation according to the Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines[1] and patients who have complete clinical data were included in the study.
Exclusion criteria:
Patients whose airflow limitation has been affected by some diseases with known causes or characteristic pathological manifestations, such as bronchiectasis, tuberculosis, fibrosis, severe interstitial lung disease, diffuse panbronchiolitis, obliterative bronchioles and inflammation, etc. and patients with severe heart, brain, liver, kidney dysfunction and malignant tumors were excluded from the study.
The baseline characters included gender, age, height, weight, and smoking status, presence of underlying diseases such as hypertension, coronary heart disease, diabetes and cerebral infarction. Further, blood cell analysis was also studied and the results of the analysis such as count of White Blood Cells (WBC), Red Blood Cells (RBC), Hemoglobin (Hb), Platelets (PLT), Neutrophils (NEU), LYM, Eosinophils (EOS) and Basophils (BAS) were studied. Further, serum total protein, ALB, CRP and blood gas analysis like Partial pressure of Oxygen (PO2) and Partial pressure Carbon dioxide (PCO2) were also analyzed.
CALLY index=ALB×LYM/(CRP×10)
Statistical analysis:
Statistical Package for Social Studies (SPSS) version 26.0 was used to analyze the collected data. Normally distributed measurement data was expressed as mean±standard deviation, and independent sample t-test was used for data comparison between groups; non-normally distributed measurement data was expressed as median (25th percentile (P25) and 75th percentile (P75). Rank sum test (Mann-Whitney U) was used for comparison between groups and the count data was expressed in terms of cases (%). Chi-square (χ²) test was used for comparison of data between the groups. Factors with p<0.05 in single-factor analysis were used as independent variables and were included in multi-factor logistic regression analysis. Receiver Operating Characteristic (ROC) curve was constructed and the predictive value of the severity of COPD through the Area Under Curve (AUC), was evaluated which was determined based on the value corresponding to the maximum value of the Youden index; p<0.05 was found to be statistically significant.
Results and Discussion
Clinical characteristics of the patients in both the groups were analyzed. The sample for this study included 257 patients diagnosed with AECOPD. Table 1 summarizes the demographic and clinical characteristics of the patients. The mean age of the patients was (70.2±10.6) y, ranging from (35-98) y. Among 257 patients diagnosed with AECOPD, 130 (50.6 %) were classified into the AECOPD with respiratory failure group and the remaining 127 (49.4 %) were classified into the AECOPD without respiratory failure group. Compared with the group without respiratory failure, patients in the AECOPD group with respiratory failure had a higher incidence of hypertension, higher levels of RBCs, Hb, CRP, low Platelet (PLT) count, LYM and EOS. Furthermore, the CALLY index was significantly lower in the AECOPD group with respiratory failure compared with the AECOPD group without respiratory failure 0.99 (0.15 and 4.70) vs. 0.25 (0.06 and 1.45) (p<0.001).
| Variable | AECOPD without respiratory failure | AECOPD with respiratory failure | Overall | p |
|---|---|---|---|---|
| Age (y) | 69.5±10.8 | 71.0±10.4 | 70.2±10.6 | 0.240 |
| Gender, n (%) | 0.272 | |||
| Male | 106 (83.5 %) | 101 (77.7 %) | 207 (80.5 %) | |
| Female | 21 (16.5 %) | 29 (22.3 %) | 50 (19.5 %) | |
| Body Mass Index (BMI) (kg/m²) | 22.04 (19.03, 25.00) | 21.97 (19.36, 25.50) | 22.00 (19.24, 25.20) | 0.702 |
| Smoke, n (%) | 97 (76.4 %) | 93 (71.5 %) | 190 (73.9 %) | 0.397 |
| Comorbidities, n (%) | ||||
| Hypertension | 52 (40.9 %) | 72 (55.4 %) | 124 (48.2 %) | 0.025 |
| Coronary heart disease | 13 (10.2 %) | 17 (13.1 %) | 30 (11.7 %) | 0.562 |
| Diabetes | 14 (11.0 %) | 14 (10.8 %) | 28 (10.9 %) | 1 |
| Cerebral infarction | 27 (21.3 %) | 16 (12.3 %) | 43 (16.7 %) | 0.066 |
| Biochemical characteristics | ||||
| WBC×109/l | 6.85 (5.28, 8.91) | 6.70 (5.48, 9.60) | 6.82 (5.42, 8.94) | 0.781 |
| RBC×1012/l | 4.53 (4.18, 4.95) | 4.77 (4.25, 5.29) | 4.61 (4.20, 5.17) | 0.013 |
| Hb (g/l) | 142 (131, 154) | 151 (135, 164) | 146 (133, 159) | 0.003 |
| PLT×109/l | 202 (164, 274) | 173 (138, 226) | 188 (153, 241) | 0.005 |
| NEU×109/l | 4.69 (3.47, 6.43) | 5.18 (3.86, 7.46) | 5.04 (3.58, 6.97) | 0.106 |
| LYM×109/l | 1.24 (0.91, 1.73) | 0.88 (0.65, 1.28) | 1.11 (0.73, 1.55) | <0.001 |
| MON×109/l | 0.68 (0.56, 0.93) | 0.88 (0.65, 1.28) | 0.54 (0.40, 0.73) | 0.755 |
| EOS×109/l | 0.06 (0.03, 0.15) | 0.03 (0.00, 0.93) | 0.05 (0.01, 0.12) | 0.001 |
| BAS×109/l | 0.02 (0.01, 0.04) | 0.02 (0.01, 0.04) | 0.02 (0.01, 0.04) | 0.840 |
| TP (g/l) | 65.40 (62.30, 71.00) | 66.40 (61.95, 71.58) | 65.80 (62.1, 71.2) | 0.305 |
| ALB (g/l) | 41.80 (38.70, 44.30) | 40.45(37.00, 44.00) | 40.90 (37.90, 44.10) | 0.155 |
| CRP (mg/l ) | 5.53 (1.41, 29.54) | 16.88 (3.14, 56.94) | 9.76 (1.88, 43.95) | 0.001 |
| CALLY index | 0.99 (0.15, 4.70) | 0.25 (0.06, 1.45) | 0.44 (0.08, 2.89) | <0.001 |
Table 1: Clinical characteristics stratified by the AECOPD patients.
Relationship between the CALLY index and AECOPD respiratory failure and non-respiratory failure was studied. Univariate logistic regression analysis showed that CALLY index was significantly associated with AECOPD accompanied by respiratory failure (Odd’s Ratio (OR)=0.847 and p<0.001) (Table 2). Furthermore, serum CRP levels and lymphocyte count (components of the CALLY index) were strongly associated with AECOPD with respiratory failure (p<0.01). Other indicators include high blood pressure, RBC count and EOS count.
| Clinical variables | OR | 95 % CI | p |
|---|---|---|---|
| Age | 1.014 | 0.991-1.038 | 0.24 |
| Gender | 1.449 | 0.776-2.706 | 0.244 |
| Smoking | 0.777 | 0.444-1.36 | 0.377 |
| Hypertension | 1.79 | 1.092-2.936 | 0.021 |
| Coronary heart disease | 1.319 | 0.612-2.842 | 0.479 |
| Diabetes | 0.974 | 0.444-2.135 | 0.948 |
| Cerebral infarction | 0.52 | 0.265-1.02 | 0.057 |
| WBC | 1.047 | 0.976-1.123 | 0.2 |
| RBC | 1.639 | 1.139-2.36 | 0.008 |
| Hb | 1.017 | 1.004-1.029 | 0.007 |
| PLT | 0.997 | 0.994-1 | 0.089 |
| NEU | 1.011 | 0.966-1.059 | 0.63 |
| LYM | 0.357 | 0.221-0.577 | <0.01 |
| MON | 0.947 | 0.757-1.185 | 0.633 |
| EOS | 0.14 | 0.028-0.695 | 0.016 |
| BAS | 0.271 | 0.005-13.852 | 0.515 |
| TP | 1.019 | 0.984-1.056 | 0.291 |
| ALB | 0.964 | 0.915-1.016 | 0.176 |
| CRP | 1.008 | 1.003-1.014 | 0.002 |
| CALLY index | 0.847 | 0.774-0.926 | <0.001 |
Table 2: Univariate logistic regression analysis for AECOPD patients.
After adjusting confounding variables, multivariable regression analysis results showed that serum CRP levels and lymphocyte count were significantly independent predictors of AECOPD with respiratory failure. In addition, when included in the multivariate analysis of model 2, the CALLY index was still an independent predictor of AECOPD with respiratory failure, and the OR of the CALLY index was 0.810 (95 % Confidence Interval (CI): 0.763-0.925 and p<0.001) (Table 3). In addition, hypertension and RBC count were also identified as independent factors associated with respiratory failure in AECOPD.
| Clinical variables | OR | 95 % CI | p |
|---|---|---|---|
| Model-1 | |||
| Hypertension | 2.215 | 1.278-3.837 | 0.005 |
| RBC | 1.932 | 1.282-2.911 | 0.002 |
| EOS | 0.393 | 0.080-1.924 | 0.249 |
| LYM | 0.425 | 0.252-0.717 | 0.001 |
| CRP | 1.007 | 1.001-1.013 | 0.014 |
| Model-2 | |||
| Hypertension | 2.341 | 1.357-4.039 | 0.002 |
| RBC | 1.891 | 1.266-2.823 | 0.002 |
| EOS | 0.236 | 0.047-1.182 | 0.079 |
| CALLY index | 0.81 | 0.763-0.925 | <0.001 |
Table 3: Multivariate logistic regression analysis for AECOPD patients.
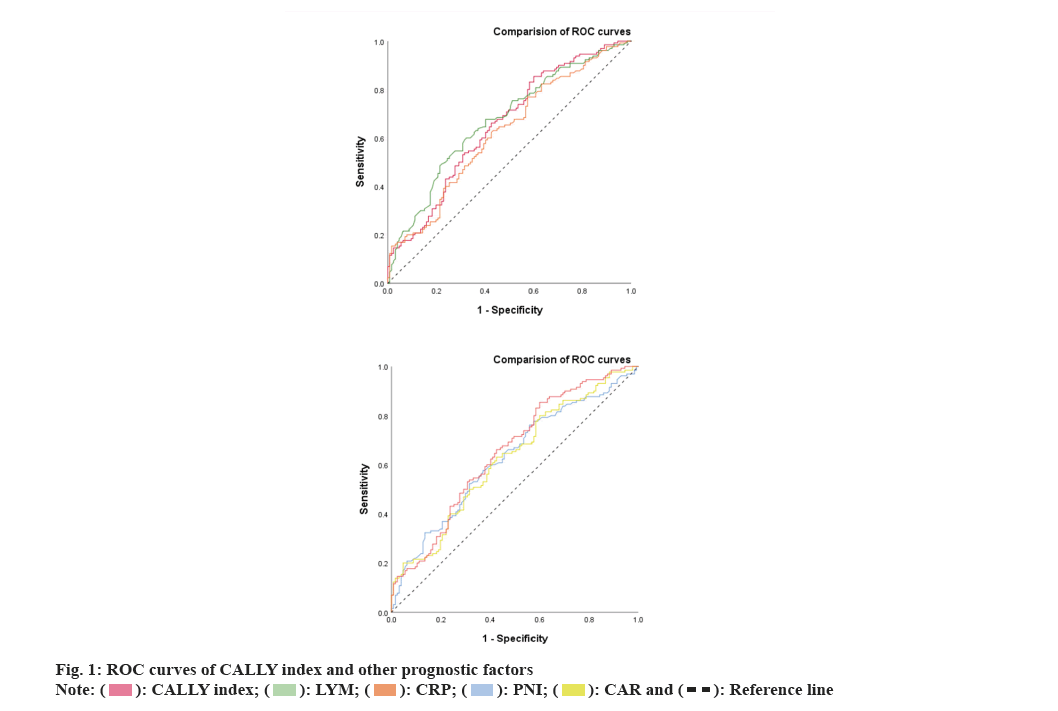
Further, the CALLY index and its components were compared with the other prognostic factors. We investigated the predictive ability of the CALLY index and its important components such as LYM and CRP for AECOPD with respiratory failure by using the AUC curve (fig. 1).
AUC values of CALLY index, LYM counts and serum CRP level were 0.653 (95 % CI: 0.586-0.719), 0.671 (95 % CI: 0.606-0.737) and 0.622 (95 % CI: 0.554-0.690) respectively. Furthermore, the AUC of the CALLY index was significantly higher than serum CRP level but not higher than lymphocyte counts. However, combining serum CRP level with lymphocyte count may improve the prediction of AECOPD with respiratory failure.
CALLY index <2.47 is the best threshold for predicting AECOPD with respiratory failure, with a sensitivity of 85.4 % and a specificity of 40.2 %. The accuracy in predicting AECOPD with respiratory failure is significantly higher than that of Prognostic Nutritional Index (PNI) (AUC: 0.626 and p<0.01) and CAR (AUC: 0.624 and p<0.01) (Table 4).
| Variables | AUC | p | 95 % CI |
|---|---|---|---|
| CALLY index | 0.653 | <0.01 | 0.586-0.719 |
| LYM | 0.671 | <0.01 | 0.606-0.737 |
| CRP | 0.622 | <0.01 | 0.554-0.69 |
| PNI | 0.626 | <0.01 | 0.558-0.694 |
| CAR | 0.624 | <0.01 | 0.556-0.692 |
Table 4: AUC analysis of the patients.
CALLY index, other clinical parameters and treatments were discussed. The clinical characteristics of AECOPD patients were stratified according to the critical value of CALLY index level on admission (Table 5). Patients with CALLY index <2.47 had higher WBC, NEU and Monocyte (MON) count including serum CRP level while Hb, LYM, EOS and ALB are low. Patients with lower CALLY index had significantly higher incidence of respiratory failure than patients with higher CALLY index. In terms of treatment, the patients with lower CALLY index used significantly higher rates of antibiotics, phlegm-reducing drugs and glucocorticoids than patients with higher CALLY index.
| Clinical characteristics | CALLY index <2.74 (n=187) | CALLY index >2.74 (n=70) | p |
|---|---|---|---|
| Age (y) | 70.76±10.619 | 68.87±10.551 | 0.24 |
| BMI (kg/m²) | 22.31 (19.00, 25.11) | 22.87 (20.16, 25.65) | 0.564 |
| Gender | 0.2 | ||
| Male | 147 (78.6 %) | 60 (85.7 %) | |
| Female | 40 (21.4 %) | 10 (14.3 %) | |
| Smoke | 135 (72.2 %) | 55 (78.6 %) | 0.3 |
| Hypertension | 87 (46.5 %) | 37 (52.9 %) | 0.366 |
| Coronary heart disease | 21 (11.2 %) | 9 (12.9 %) | 0.718 |
| Diabetes | 22 (11.8 %) | 6 (8.6 %) | 0.465 |
| Cerebral infarction | 32 (17.1 %) | 11 (15.7 %) | 0.789 |
| WBC×109/l | 8.28 (5.92, 9.77) | 6.43 (5.02, 8.54) | <0.01 |
| RBC×1012/l | 4.65±0.81 | 4.71±0.49 | 0.504 |
| Hb (g/l) | 144.71 (131.0, 159.0) | 147.24 (136.0, 159.6) | <0.01 |
| PLT×109/l | 202.39 (149, 247) | 197.37 (158. 5, 229) | 0.813 |
| NEU×109/l | 6.83 (4.14, 7.87) | 4.20 (2.86, 4.81) | <0.01 |
| LYM×109/l | 1.06 (0.66, 1.36) | 2.75 (1.15, 1.93) | <0.01 |
| MON×109/l | 0.75 (0.45, 081) | 0.51 (0.35, 0.61) | <0.01 |
| EOS×109/l | 0.09 (0.01, 0.09) | 0.20 (0.04, 0.22) | <0.01 |
| BAS×109/l | 0.03 (0.01, 0.04) | 0.07 (0.02, 0.05) | 0.095 |
| TP (g/l) | 65.89±7.47 | 66.47±5.64 | 0.503 |
| ALB (g/l) | 40.05±5.03 | 42.49±3.30 | <0.01 |
| CRP (mg/l) | 49.83 (6.73, 65.96) | 1.29 (0.61, 1.62) | <0.01 |
| AECOPD with respiratory failure, n (%) | 111 (59.4 %) | 19 (27.1 %) | <0.01 |
| Length of stay (d) | 9.27 (7.0, 10.0) | 8.33 (5.75, 12.0) | 0.064 |
| Antibiotic therapy | 165 (88.2 %) | 46 (65.7 %) | <0.01 |
| Mucus therapy | 136 (72.7 %) | 41 (58.6 %) | 0.029 |
| Glucocorticoid therapy | 99 (52.9 %) | 27 (38.6 %) | 0.04 |
| Glucocorticoid therapy of days (d) | 3.22 (0.0, 6.0) | 2.10 (0.0, 3.25) | 0.017 |
| Antibiotic+phlegm+glucocorticoid therapy | 75 (40.1 %) | 9 (12.9 %) | <0.01 |
Table 5: Clinical characteristics stratified by CALLY index level at admission.
COPD is a common chronic respiratory disease characterized by progressive worsening and worsening of symptoms[8]. According to the 2019 Global Health Estimates released by the World Health Organization (WHO), COPD is the 3rd most common cause of death worldwide[9]. The frequency and severity of AECOPD play an important role in determining the management and overall efficacy of COPD[10]. AECOPD is an independent variable associated with decreased lung function[10,11]. 2024 GOLD guidelines define AECOPD as an event characterized by worsening dyspnea and/or cough and sputum production for >14 d, often associated with increased local and systemic inflammation caused by airway infection, pollution or other lung injury[1,12].
This study demonstrates a clear correlation between inflammation, nutritional and immunity status as determined by the CALLY index and severity of AECOPD during the admission. AECOPD is characterized by a persistent inflammatory response in the airways, lung parenchyma and blood vessels. This inflammatory response is initiated and regulated by a variety of cytokines produced by different cell types, including macrophages, NEU and T-LYM. The presence of these cytokines and inflammatory factors leads to the progressive development of airflow limitation and structural deterioration of the airway wall and lung parenchyma[13,14].
CALLY index is an improved inflammation, nutritional and immunity scoring system based on serum CRP and ALB levels, and lymphocyte count[7]. CALLY index has been proven to be a good predictor of hepatocellular carcinoma, ovarian cancer, oral cancer, lung cancer and other diseases[7,15-17]. To our knowledge, none of the studies have been previously conducted to determine the correlation between the CALLY index and AECOPD severity.
CRP is a nonspecific acute-phase reactant that is induced in the liver by cytokines such as Interleukin-1 (IL-1), IL-6 and Tumor Necrosis Factor-Alpha (TNF-α). CRP levels are significantly elevated under various pathological conditions such as injury, infection and inflammation[18,19]. CRP is not only a highly effective biomarker of inflammation but it also actively participates in pathological mechanisms. For example, CRP has the ability to establish variety of complexes by binding to Capsular (C) polysaccharides on bacterial cell walls. This activates the complement system to promote the clearance of pathogens and necrotic cells[18-21]. Several articles have clearly stated that serum CRP levels can be used as a highly sensitive indicator of bacterial infection in AECOPD, thereby playing a key role in the diagnosis and treatment of AECOPD[13,22].
LYM are the markers of inflammation and different subpopulations of LYM that play different roles in the progression of different stages of COPD[23]. COPD induces the activation of the body's adaptive immune system, causing LYM to infiltrate small airways of the lungs, which may lead to a decrease in circulating LYM[24,25]. In the study by Yang et al.[26], a positive correlation between reduced lymphocyte numbers and increased susceptibility to infection was confirmed, thereby increasing the risk of death in AECOPD patients. At the same time, in previous studies on Coronavirus Disease-2019 (COVID-19), lymphopenia was a notable feature observed in severe COVID-19 patients. The reason is that Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‑CoV‑2) particles target the cytoplasm of LYM, leading to their destruction[27].
ALB levels are often used as a marker of disease presence, persistence or remission[28]. It is significantly reduced during inflammation and malnutrition. ALB is synthesized by the liver maintains circulation and enters the interstitium and tissues, where it functions as an antioxidant and free radical scavenger. Eventually, the liver reduces or resynthesizes ALB to restore its antioxidant activity, depending on the severity of inflammation, ALB synthesis and anti-inflammatory functions are upregulated. However, ALB breakdown is also increased in inflammatory regions, leading to hypoalbuminemia[29,30]. Previous studies have shown that AECOPD patients who died during hospitalization had lower ALB levels than survivors[31]. However, this study had no relevant evidence to support the association between ALB levels and AECOPD severity upon admission, but after grouping AECOPD patients according to CALLY index, the serum ALB levels of patients with lower CALLY index were significantly lower than those of patients with higher CALLY index (p<0.01).
According to the 2024 GOLD guidelines, it is recommended that AECOPD patients inhale short-acting Beta (β)-agonists[1] because short-acting β-agonists are routine medications for AECOPD patients when they are admitted to hospital, so they are not included in this study.
Relevant studies have shown that systemic glucocorticoids cannot only shorten recovery time and improve lung function, i.e., Forced Expiratory Volume 1 s (FEV1) during COPD exacerbations, but also improve oxygenation and reduce the risk of early relapse, treatment failure, and length of hospitalization[32-35]. 2024 GOLD guidelines recommend a dose of 40 milligrams of prednisone equivalent/day for 5 d. In this study, the use rate and number of days of glucocorticoid use in AECOPD patients with lower CALLY were significantly higher than those in AECOPD patients with higher CALLY.
Respiratory viruses and bacterial infections are closely related to AECOPD[36]. Therefore, antimicrobial treatment is an essential element of routine treatment of AECOPD[37]. 2024 GOLD guidelines point out that the use of antibiotics in AECOPD patients is currently controversial, but it can be based on clinical symptoms. Such as the use of antibiotics based on sputum characteristics[1]. Previous studies have shown that elevated serum CRP levels are highly sensitive indicators of bacterial infection in AECOPD and can guide the use of antibiotics[13,22]. In this study, the CALLY index was relatively. Antibiotic usage rate of AECOPD patients in the low group was significantly higher than that of AECOPD patients in the higher CALLY index group.
Another finding is that the use rate of phlegm-reducing drugs in AECOPD patients in the lower CALLY index group was significantly higher than that in AECOPD patients in the higher CALLY index group. An obvious clinical feature of AECOPD patients is an increase in sputum and pus, an increase in sputum volume, and difficulty in expelling sputum, which will aggravate symptoms such as dyspnea. Studies have shown that chronic sputum production is related to a decrease in lung function or a greater decrease in FEV1 in COPD patients[1,38-40]. It is also pointed out in the 2024 GOLD guidelines that the use of mucolytic agents in COPD patients can reduce the number of acute exacerbations[1,41].
Taken together, we can infer that the CALLY index is able to describe the nutritional, inflammatory and immunity status of AECOPD patients. We verified for the first time that the CALLY index is a useful biomarker independently associated with whether AECOPD is accompanied by respiratory failure. We also divided AECOPD patients into 2 groups based on the cut-off value of CALLY. In the treatment of patients in the AECOPD group with lower CALLY, the use rate of glucocorticoids, antibiotics, phlegm-reducing drugs and the combination of the three was significantly higher than that of CALLY. For patients in the higher AECOPD group, it is recommended to take treatment measures based on the CALLY index on admission, and to take more comprehensive treatment in the AECOPD group with lower early CALLY.
This study has several limitations. Primarily, the retrospective design of this study did not allow us to obtain laboratory test measures such as interleukins, ferritin levels, and Procalcitonin (PCT), thus hampering our ability to evaluate the potential significance of these measures in predicting AECOPD severity during data analysis. Secondly, because patients are often lost to follow-up after discharge, it is impossible to obtain the survival time of patients after discharge, so as to evaluate the relationship between medication use during hospitalization and survival, so as to further guide post-admission treatment; finally, this study was only conducted in one center, and the research cohort. The relatively small and limited number of severe cases may affect the generalizability of our findings.
CALLY index is an independent risk factor for AECOPD with respiratory failure, and patients with lower CALLY have higher rate of use of systemic glucocorticoids, antibiotics, and phlegm-reducing drugs during admission, and the number of days of glucocorticoid use is longer. These findings are expected to help clinicians promptly identify patients with respiratory failure in the early stages of hospitalization and adopt more aggressive treatments for AECOPD patients.
Author contributions:
Zhihao Yang and Meng Jin contributed equally to this study
Funding:
This research was funded by the Shanxi Provincial Health Commission’s “Four Batches” Scientific and Technological Medical Innovation Plan (Project No: 2021XM16 and Name: Bronchoalveolar Lavage Fluid Pepsin in Chronic Obstructive Pulmonary Disease Combined with Gastroesophageal Reflux Disease Exploratory Research).
Conflict of interests:
The authors declared no conflict of interests.
References
- Global initiative for chronic Obstructive Lung Disease (GOLD). Global strategy for prevention, diagnosis and management of COPD: 2024 report. 2024.
- Chen S, Kuhn M, Prettner K, Yu F, Yang T, Bärnighausen T, et al. The global economic burden of chronic obstructive pulmonary disease for 204 countries and territories in 2020-50: A health-augmented macroeconomic modelling study. Lancet Glob Health 2023;11(8):e1183-93.
[Crossref] [Google Scholar] [PubMed]
- Expert consensus on the identification and management of patients at high risk for acute exacerbation of chronic obstructive pulmonary disease in China. Int Respir J 2022;42(24):1845-63.
- Oudijk EJ, Lammers JW, Koenderman L. Systemic inflammation in chronic obstructive pulmonary disease. Eur Respir J Suppl 2003;46:5-13.
[Crossref] [Google Scholar] [PubMed]
- Schols AM, Ferreira IM, Franssen FM, Gosker HR, Janssens W, Muscaritoli M, et al. Nutritional assessment and therapy in COPD: A European respiratory society statement. Eur Respir J 2014;44(6):1504-20.
[Crossref] [Google Scholar] [PubMed]
- Man X. Expert consensus on the diagnosis and treatment of acute exacerbation of chronic obstructive pulmonary disease. Int J Respir 2023;43(2):132-49.
- Muller L, Hahn F, Mahringer-Kunz A, Stoehr F, Gairing SJ, Michel M, et al. Immunonutritive scoring for patients with hepatocellular carcinoma undergoing transarterial chemoembolization: Evaluation of the CALLY index. Cancers (Basel) 2021;13(19):1-12.
[Crossref] [Google Scholar] [PubMed]
- Safiri S, Carson-Chahhoud K, Noori M, Nejadghaderi SA, Sullman MJM, Heris AJ, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: Results from the global burden of disease study 2019. BMJ 2022;378:1-13.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. WHO reveals leading causes of death and disability worldwide: 2000-2019. WHO; 2020.
- Hillas G, Perlikos F, Tzanakis N. Acute exacerbation of COPD: Is it the "stroke of the lungs"? Int J Chron Obstruct Pulmon Dis 2016;11:1579-86.
[Crossref] [Google Scholar] [PubMed]
- Kanner RE, Renzetti AD Jr, Klauber MR, Smith CB, Golden CA. Variables associated with changes in spirometry in patients with obstructive lung diseases. Am J Med 1979;67(1):44-50.
[Crossref] [Google Scholar] [PubMed]
- Celli BR, Fabbri LM, Aaron SD, Agusti A, Brook R, Criner GJ, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: The rome proposal. Am J Respir Crit Care Med 2021;204(11):1251-8.
[Crossref] [Google Scholar] [PubMed]
- Jia TG, Zhao JQ, Liu JH. Serum inflammatory factor and cytokines in AECOPD. Asian Pac J Trop Med 2014;7(12):1005-8.
[Crossref] [Google Scholar] [PubMed]
- Moermans C, Heinen V, Nguyen M, Henket M, Sele J, Manise M, et al. Local and systemic cellular inflammation and cytokine release in chronic obstructive pulmonary disease. Cytokine 2011;56(2):298-304.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Gu J, Liu Y, Liu X, Jiang L, Wu C, et al. Pre-treatment CRP-Albumin-Lymphocyte Index (CALLY Index) as a prognostic biomarker of survival in patients with epithelial ovarian cancer. Cancer Manag Res 2022;14:2803-12.
[Crossref] [Google Scholar] [PubMed]
- Tsai YT, Ko CA, Chen HC, Hsu CM, Lai CH, Lee YC, et al. Prognostic value of CRP-Albumin-Lymphocyte (CALLY) index in patients undergoing surgery for oral cavity cancer. J Cancer 2022;13(10):3000-12.
[Crossref] [Google Scholar] [PubMed]
- Liu XY, Zhang X, Zhang Q, Ruan GT, Liu T, Xie HL, et al. The value of CRP-Albumin-Lymphocyte index (CALLY index) as a prognostic biomarker in patients with non-small cell lung cancer. Support Care Cancer 2023;31(9):1-12.
[Crossref] [Google Scholar] [PubMed]
- Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018;9:1-10.
[Crossref] [Google Scholar] [PubMed]
- Strang F, Schunkert H. C-reactive protein and coronary heart disease: All said-is not it? Mediators Inflamm 2014;2014:1-8.
[Crossref] [Google Scholar] [PubMed]
- Luan YY, Yin CH, Yao YM. Update advances on C-Reactive protein in COVID-19 and other viral infections. Front Immunol 2021;12:1-10.
[Crossref] [Google Scholar] [PubMed]
- Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, et al. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid Based Med 2021;26(3):107-8.
[Crossref] [Google Scholar] [PubMed]
- Arinzon Z, Peisakh A, Schrire S, Berner Y. C-Reactive Protein (CRP): An important diagnostic and prognostic tool in nursing-home-associated pneumonia. Arch Gerontol Geriatr 2011;53(3):364-9.
[Crossref] [Google Scholar] [PubMed]
- Baker JR, Donnelly LE. Leukocyte function in COPD: Clinical relevance and potential for drug therapy. Int J Chron Obstruct Pulmon Dis 2021;16:2227-42.
[Crossref] [Google Scholar] [PubMed]
- Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011;378(9795):1015-26.
[Crossref] [Google Scholar] [PubMed]
- Hu Y, Long H, Cao Y, Guo Y. Prognostic value of lymphocyte count for in-hospital mortality in patients with severe AECOPD. BMC Pulm Med 2022;22(1):1-15.
[Crossref] [Google Scholar] [PubMed]
- Cao Y, Xing Z, Long H, Huang Y, Zeng P, Janssens JP, et al. Predictors of mortality in COPD exacerbation cases presenting to the respiratory intensive care unit. Respir Res 2021;22(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 2020;8(5):475-81.
[Crossref] [Google Scholar] [PubMed]
- Sheinenzon A, Shehadeh M, Michelis R, Shaoul E, Ronen O. Serum albumin levels and inflammation. Int J Biol Macromol 2021;184:857-62.
[Crossref] [Google Scholar] [PubMed]
- Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int J Mol Sci 2021;22(9):1-10.
[Crossref] [Google Scholar] [PubMed]
- Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr 2019;43(2):181-93.
[Crossref] [Google Scholar] [PubMed]
- Zeng Z, Ke X, Gong S, Huang X, Liu Q, Huang X, et al. Blood urea nitrogen to serum albumin ratio: A good predictor of in-hospital and 90-d all-cause mortality in patients with acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med 2022;22(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: A prospective randomised controlled trial. Lancet 1999;354(9177):456-60.
[Crossref] [Google Scholar] [PubMed]
- Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1999;340(25):1941-7.
[Crossref] [Google Scholar] [PubMed]
- Alia I, de la Cal MA, Esteban A, Abella A, Ferrer R, Molina FJ, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Arch Intern Med 2011;171(21):1939-46.
[Crossref] [Google Scholar] [PubMed]
- Aaron SD, Vandemheen KL, Hebert P, Dales R, Stiell IG, Ahuja J, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J Med 2003;348(26):2618-25.
[Crossref] [Google Scholar] [PubMed]
- Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011;184(6):662-71.
[Crossref] [Google Scholar] [PubMed]
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106(2):196-204.
[Crossref] [Google Scholar] [PubMed]
- Sherman CB, Xu X, Speizer FE, Ferris BG Jr, Weiss ST, Dockery DW. Longitudinal lung function decline in subjects with respiratory symptoms. Am Rev Respir Dis 1992;146(4):855-9.
[Crossref] [Google Scholar] [PubMed]
- Dowson LJ, Guest PJ, Stockley RA. The relationship of chronic sputum expectoration to physiologic, radiologic, and health status characteristics in alpha (1)-antitrypsin deficiency (PiZ). Chest 2002;122(4):1247-55.
[Crossref] [Google Scholar] [PubMed]
- Stanescu D, Sanna A, Veriter C, Kostianev S, Calcagni PG, Fabbri LM, et al. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax 1996;51(3):267-71.
[Crossref] [Google Scholar] [PubMed]
- Poole P, Chong J, Cates CJ. Mucolytic agents vs. placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;5(5):1-10.
[Crossref] [Google Scholar] [PubMed]
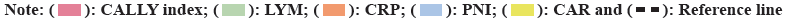 .
.



