- *Corresponding Author:
- Haili Zhang
Department of Otolaryngology-Head and Neck Surgery,
Shanxi Medical University,
Xinjian Taiyuan,
Shanxi 030001,
China
E-mail: zhanghalii@163.com
| This article was originally published in a special issue,“New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “138-145” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To analyze long non-coding RNAs taurine up-regulated gene 1 expression in middle ear cholesteatoma and its correlation with the degree of ossicle destruction. Sixty four patients with middle ear cholesteatoma admitted to our hospital from June 2019 to December 2020 were selected as the research participants for retrospective analysis. Middle ear cholesteatoma tissue and normal skin of the external auditory canal were obtained to determine taurine up-regulated gene 1 expression. Correlations of taurine up-regulated gene 1 with clinicopathological features and ossicle destruction as well as clinical efficacy were discussed. Moreover, patients were followed up for 1 y to observe taurine up-regulated gene 1’s impacts on disease recurrence. Taurine up-regulated gene 1 expression level increased in middle ear cholesteatoma tissue (p<0.001), was strongly linked to the course of disease, history of ear canal diseases and pathological staging (p<0.001). Taurine up-regulated gene 1 increased obviously in patients with complete ossicle destruction and decreased in patients with intact ossicles (p<0.001). Pearson correlation coefficient revealed a positive connection between taurine up-regulated gene 1 and the ossicle destruction score (r=0.791, p<0.001). Taurine up-regulated gene 1 was the lowest in the cured patients and was higher in ineffective patients than in improved patients (p<0.05). Spearman correlation coefficient identified an inverse connection between taurine up-regulated gene 1 and therapeutic efficacy. Patients were given with amoxicillin clavulanate potassium and prednisone after surgery and taurine up-regulated gene 1expression was significantly decreased by these anti-infection drugs. Taurine up-regulated gene 1 also increased in patients with recurrence during prognostic follow-up, with a sensitivity of 81.82 % and specificity of 86.00 % for predicting the 1 y recurrence in patients (p<0.001). Taurine up-regulated gene 1 is elevated in middle ear cholesteatoma and is closely linked to ossicle destruction in patients, which may be a breakthrough in diagnosing and treating middle ear cholesteatoma in the future.
Keywords
Ear canal cholesteatoma, long non-coding RNA taurine up-regulated gene 1, ossicle destruction, pathological features
Middle Ear Cholesteatoma (MEC) is mainly induced by chronic inflammation in the ear canal under long term stimulation, which is more prevalent in the middle-aged and elderly, with a high incidence in clinic[1,2]. Globally, there are about 1 50 000 new cases of MEC each year, representing a 4.5 fold increase in the incidence over the past 20 y[3,4]. The main pathological changes of MEC are accelerated growth of skin basal cells in the ear canal and epithelial keratinization and exfoliation, which gradually compresses and destroys the tissues around the ear, resulting in hearing impairment and headache. For patients with severe illness, MEC may cause irreversible damage to the ossicles due to the growth of MEC, which may further spread to deep brain tissues, causing intracranial infection and endangering the life safety of patients[5]. Therefore, it is clearly pointed out in clinical practice that surgical resection of MEC is the most effective scheme to eliminate organ damage and ensure patients’ life safety[6]. While to achieve this end, improving the early diagnosis rate of MEC is of utmost importance. But as the lesion is deepseated in the ear canal, it is very insidious in the early stage[7]. Given the fact that the pathological changes of MEC and the range of bone destruction can only be confirmed by high-resolution thin-layer Computerized Tomography (CT) of the temporal bone[8], finding a marker that can effectively diagnose the occurrence of MEC and understand the disease progression will be a significant progress in improving the prognosis of patients with MEC.
Modern medicine focuses on Long non-coding RNAs (LncRNAs) in the study of most neoplastic diseases[9,10]. As a kind of molecular substance with cell cycle, cell differentiation and epigenetic regulation, lncRNAs participate in a number of life activities and disease development[11]. In addition, lncRNA per se has high cell regulation ability and can be easily detected in cells, blood, tissues and organs, so it has great potential to become a tumor disease marker, a disease evaluation index and a molecular therapy target[12]. Among them, lncRNA Taurine Up-Regulated Gene 1 (TUG1) is shown to play a vital part in osteosarcoma and also affect the process of inflammatory reaction through downstream molecular axis[13,14]. Besides, it is related to the development of brain cholesteatoma and can promote cyst formation in pancreatic cancer[15,16]. Nonetheless, its connection with MEC remains to be defined.
We speculate that TUG1 may also interfere with MEC progression and clarification regarding this issue will be of great implications for future disease assessment of MEC. Therefore, this study will confirm our above conjecture through experiments, aiming to provide reliable theoretical guidance for future clinical application of TUG1 in MEC treatment.
Materials and Methods
Clinical data:
The internal Ethics Committee approved the study protocol without reserves. Sixty four MEC patients admitted to our hospital from June 2019 to December 2020 were selected as the research participants for retrospective analysis. There were 39 males and 25 females with an age of (32.9±6.5) y and a course of disease of (7.24±2.04) d.
Eligibility criteria:
Inclusion criteria: Patients who met the clinical manifestations of MEC and were diagnosed as MEC after pathological examination in our hospital; patients with unilateral lesions; patients with varying degrees of ossicle destruction by CT examination and patients with complete case data. Informed consent was obtained from patients before enrollment.
Exclusion criteria: This includes other malignant tumors, cardiovascular and cerebrovascular diseases, severe heart, lung and renal dysfunction, cognitive impairment, speech and hearing impairment and inability to follow up patients due to hospital transfer.
Degree of ossicle destruction:
The temporal bone CT and surgical records was used to detect the degree of ossicle destruction of patients, with the parameters set as follows: 140 kV, 200 mA, pitch ratio: 0.531:1 and layer thickness: 0.625 mm. The original axial images were collected and uploaded to the workstation for image Multiplanar Reorganization (MPR), supplemented by tracking function. Then, bone injury was determined by the reconstructed Three Dimensional (3D) auditory ossicle images.
Treatment methods:
After admission, patients received corresponding MEC surgical treatment according to the severity of the disease, patients with stage I underwent MEC resection; stage II patients were treated with MEC resection combined with external auditory meatoplasty. When the external auditory canal defect was large during operation, the skin behind the ear was freed to cover it; stage III patients underwent MEC resection, mastoidectomy and tympanoplasty. All the operations were performed by senior otolaryngologists in our hospital and the MEC tissue and normal skin of the external auditory canal sections were obtained during the operation for subsequent detection. The treatment method is carried out according to the study of Prasad et al.[17]. After surgery, patients were given anti-infective drugs including amoxicillin clavulanate potassium tablet at the dosage of 375 mg/time, 3 times a day (n=32) and prednisone tablet at the dosage of 20 mg/d (n=32).
Detection methods:
Total RNA was extracted from tissues by Trizol and transcribed into complementary DNA for quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) according to the kit instructions. TUC1 expression was calculated by 2-ΔΔCt (internal reference: Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)). The primer sequence (Table 1) was completed by Guangzhou Biosense.
| Gene | Forward | Reverse |
|---|---|---|
| TUG1 | 5'-CTATACTCAGCTTCAGTGTT-3' | 5'-TACTGTATGGCCACCACTCC-3' |
| GAPDH | 5'-GAAGGCTGGGGCTCATTTGCAGGG-3' | 5'-GGTGCAGGAGGCATTGCTGATGAT-3' |
Table 1: Primer Sequences
Efficacy evaluation:
Cured was translated in the disappearance of clinical symptoms such as tinnitus, ear tightness and hearing impairment, improvement of pure tone audiometry by 0-10 dB and no exudation of the external ear for more than 6 mo; improved corresponded to obviously resolved clinical symptoms, increase of pure tone audiometry by 15-25 dB and a small amount of exudation in the external auditory canal, with the presence of a small amount of granulation and failure to meet the above criteria was deemed ineffective.
Follow-up:
MEC patients were followed up for 1 y in the form of hospital revisit.
Endpoints:
Primary endpoints: TUG1 expression in MEC and the association with clinicopathological features and ossicle destruction degree.
Secondary endpoints: Correlations of TUG1 with clinical efficacy, prognosis and recurrence.
Statistical methods:
All the results of this experiment were expressed in the form of (x±s) and the statistical calculation was carried out by Statistical Package for the Social Sciences (SPSS) 26.0 software. Inter-group comparisons employed the independent samples t test (t), intra group comparisons before and after surgery employed paired t test (t) and multi group comparisons employed one way Analysis of Variance (ANOVA) and Student- Newman-Keuls (SNK) post-hoc test (F), with p<0.05 indicating statistically significant differences. Pearson and Spearman correlation coefficients were utilized for correlation analysis (r).
Results and Discussion
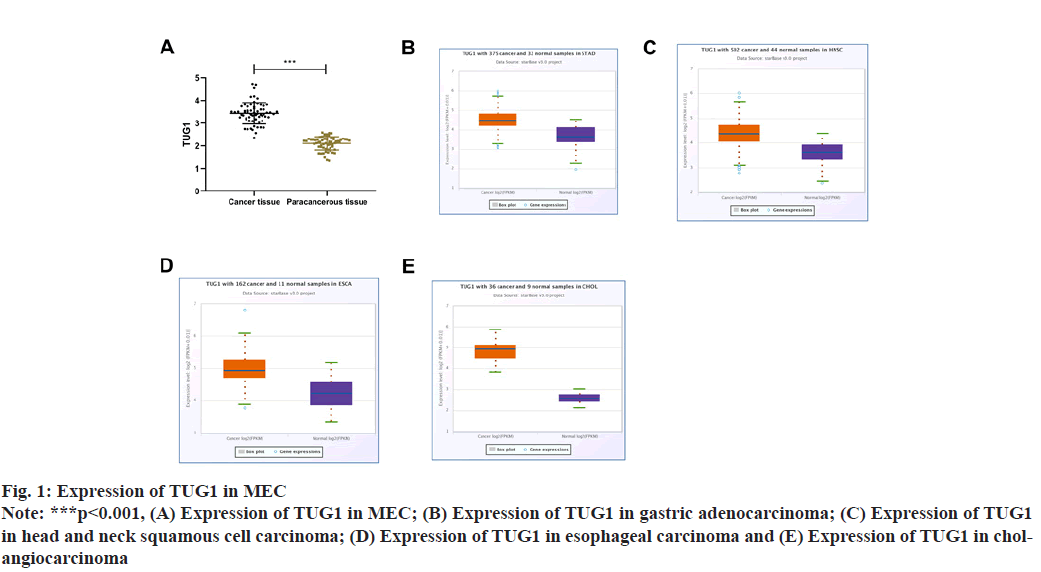
TUG1 in MEC tissue was 3.43±0.47, which was markedly higher than that in normal skin of external auditory canal (p<0.001). We further analyzed TUG1 through the online database ENCORI and also found elevated TUG1 expression in gastric adenocarcinoma, head and neck squamous cell carcinoma, esophageal carcinoma and cholangiocarcinoma, as shown in fig. 1.
TUG1 in MEC had nothing to do with age, gender, etiology and tumor location (p>0.05), but was closely related to course of disease, history of ear canal disease and pathological staging (p<0.001) Table 2.
| n | TUG1 | t or f | p | |
|---|---|---|---|---|
| Age | ||||
| <58 | 27 | 3.45±0.30 | 0.33 | 0.741 |
| ≥58 | 37 | 3.41±0.57 | ||
| Course of disease | ||||
| <5 | 29 | 3.08±0.30 | 7.61 | <0.001 |
| ≥5 | 35 | 3.73±0.37 | ||
| Gender | ||||
| Male | 39 | 3.43±0.44 | 0.16 | 0.875 |
| Female | 25 | 3.45±0.57 | ||
| History of ear canal disease | ||||
| Yes | 43 | 3.64±0.39 | 6.46 | <0.001 |
| No | 21 | 3.01±0.31 | ||
| Etiology | ||||
| Primary | 26 | 3.42±0.54 | 0.17 | 0.868 |
| Secondary | 38 | 3.44±0.42 | ||
| Tumor location | ||||
| Left | 34 | 3.47±0.43 | 0.68 | 0.499 |
| Right | 30 | 3.39±0.51 | ||
| Pathological staging | ||||
| Phase I | 19 | 2.98±0.32 | 47.8 | <0.001 |
| Phase II | 27 | 3.42±0.21* | ||
| Phase III | 18 | 3.94±0.38*# |
Note: *indicates that there is a difference compared with patients with Stage I and #indicates that there is a difference compared with patients with Stage II
Table 2: Relationship Between TUG1 and Clinicopathological Features
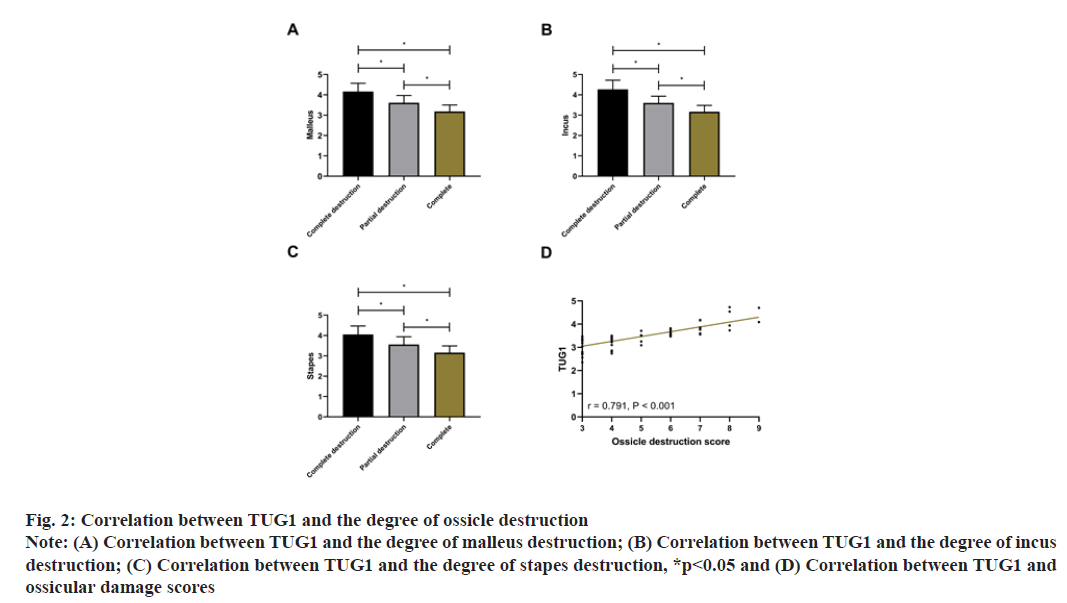
The ossicle destruction degree in MEC patients by CT examination is shown in Table 3. TUG1 was closely bound up with the degree of malleus, incus and stapes destruction (p<0.001). Subsequently, with 3 points for complete destruction, 2 points for partial destruction and 1 point for complete, the score of ossicle destruction was calculated to be (4.84±1.78) points. Pearson correlation coefficient analysis showed that TUG1 was positively correlated with the ossicle destruction score (r=0.791, p<0.001), that is, the higher the expression of TUG1, the more serious the ossicle destruction as shown in fig. 2.
| n | |
|---|---|
| Malleus | |
| Complete destruction | 7 |
| Partial destruction | 22 |
| Complete | 35 |
| Incus | |
| Complete destruction | 6 |
| Partial destruction | 24 |
| Complete | 34 |
| Stapes | |
| Complete destruction | 8 |
| Partial destruction | 26 |
| Complete | 30 |
Table 3: Ossicle Destruction Degree
Fig. 2: Correlation between TUG1 and the degree of ossicle destruction
Note: (A) Correlation between TUG1 and the degree of malleus destruction; (B) Correlation between TUG1 and the degree of incus
destruction; (C) Correlation between TUG1 and the degree of stapes destruction, *p<0.05 and (D) Correlation between TUG1 and
ossicular damage scores
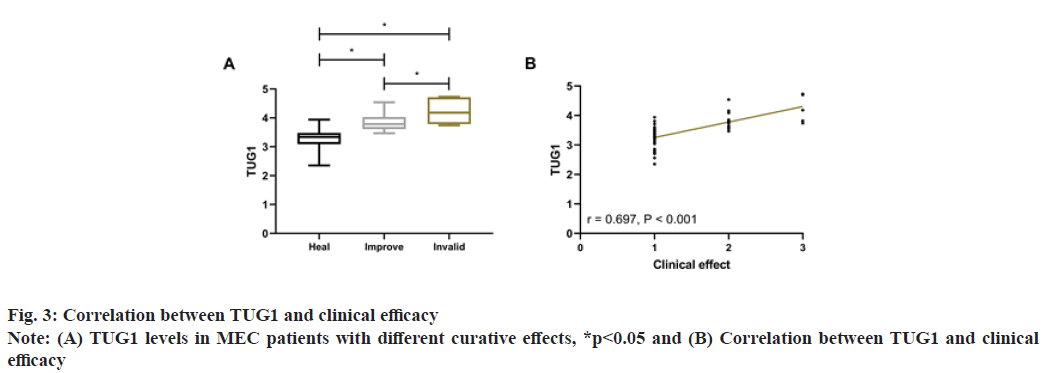
After treatment, 47 patients were cured, 12 were improved and 5 were ineffective. Comparison of TUG1 levels in patients with different curative effects showed that TUG1 was the lowest in cured patients and higher in ineffective patients than in improved patients (p<0.05). The clinical curative effect was defined as 1 point for cured, 2 points for improved and 3 for ineffective for Spearman correlation coefficient analysis. It showed that TUG1 was positively correlated with TUG1 (r=0.697, p<0.001), that is, the higher the TUG1, the worse the curative effect, as shown in fig. 3.
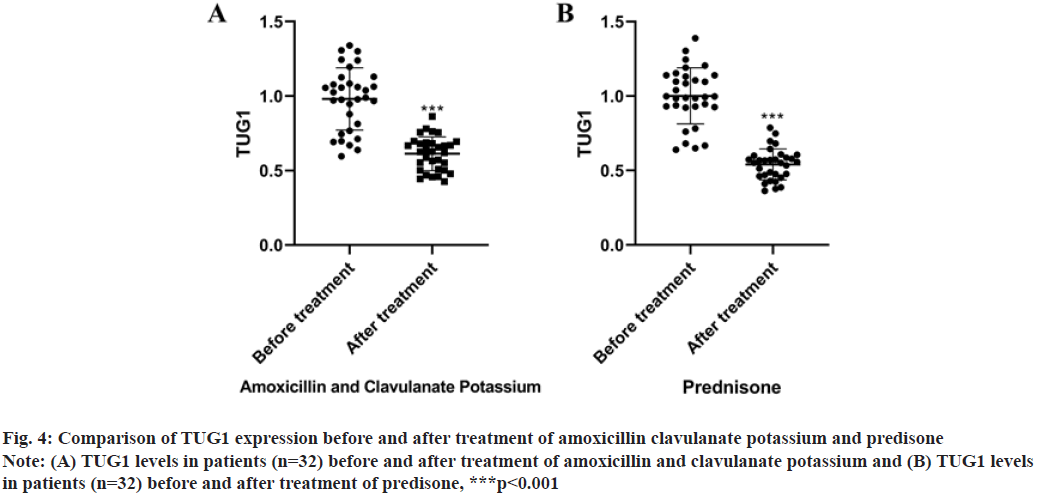
As revealed in fig. 4, TUG1 expression was significantly decreased by amoxicillin clavulanate potassium (t=8.768, p<0.001) and predisone (t=12.11, p<0.001).
Fig. 4: Comparison of TUG1 expression before and after treatment of amoxicillin clavulanate potassium and predisone
Note: (A) TUG1 levels in patients (n=32) before and after treatment of amoxicillin and clavulanate potassium and (B) TUG1 levels
in patients (n=32) before and after treatment of predisone, ***p<0.001
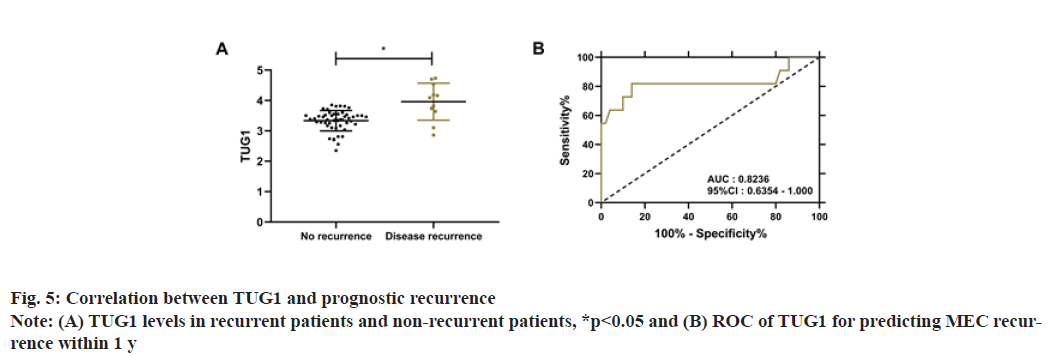
During the 1 y follow-up, 61 MEC patients were successfully followed up, with a success rate of 95.31 %. Eleven patients had disease recurrence, with a 1 y recurrence rate of 18.03 %. Comparison of TUG1 levels between recurrent and non-recurrent patients revealed that TUG1 levels were notably higher in relapsed patients (p<0.05). Receiver Operating Characteristic (ROC) curve analysis identified that when TUG1 >3.62, the sensitivity and specificity were 81.82 % and 86.00 % respectively in predicting the 1 y recurrence of MEC (p<0.001), as shown in fig. 5.
The potential threat of MEC deserves clinical attention given its obviously increased in the past few years[18]. Although MEC is a benign lesion, it can still cause serious consequences such as hearing impairment and intracranial nerve infection if left treated in time and effectively[19]. For MEC, the tumor tissue is not a substantial tumor, so traditional tumor markers cannot screen the occurrence of MEC[20]. And because of it, finding a convenient, rapid and accurate marker is of great significance for the early screening of MEC. This study, by analyzing the clinical implications of TUG1 in MEC, lays a reliable foundation for the future use of TUG1 in the diagnosis and treatment of MEC.
First, we found highly expressed TUG1 in MEC, suggesting that TUG1 may be involved in the onset and progression of MEC. The online database also revealed high TUG1 expression in other tumor diseases such as gastric adenocarcinoma, head and neck squamous cell carcinoma, esophageal cancer and cholangiocarcinoma, which confirmed the consistent expression profiling of the gene. Previous studies have suggested that TUG1 can promote the multiplication of squamous cells and activate epithelial cells through nuclear factor erythroid 2–related factor 2[21,22]. The histology of MEC also showed squamous epithelium proliferation, epithelial keratinization and exfoliation, as well as epithelial clump like aggregation[23], which may be the primary mechanism of TUG1 involvement in MEC. By analyzing the connection between TUG1 and the clinicopathological features of MEC patients, we found that TUG1 was closely related to the course of disease, history of ear canal and pathological staging of patients, which was another reminder of the importance of TUG1 for the development of MEC.
MEC usually develops from the pouch invagination of the tympanic membrane relaxation. The destruction of MEC starts from the superior crypt of the superior tympanic recess between the malleus neck and the flaccid part and develops through the ossicle, mucosal folds and ligaments, causing the destruction of the ossicle and invading the tympanic sinus and mastoid process[24]. So, evaluating the degree of ossicle destruction is also one of the keys to understanding the development of MEC[25]. Therefore, to further confirm the connection between TUG1 and MEC, we need to fully grasp the relationship between TUG1 and ossicle destruction. In this research, we found that TUG1 was higher in patients with serious ossicle destruction and was positively correlated with the ossicle destruction score. Komori proposed that Runt-Related Transcription Factor 2 (RUNX2) would be significantly activated in bone tissues during bone damage and destruction, triggering severe inflammatory damage[26]. TUG1 is involved in the occurrence and development of osteosarcoma by promoting RUNX2[27]. Hence, we hypothesize that the mechanism by which TUG1 affects the ossicle might be related to RUNX2.
Subsequently, through the correlation analysis between the clinical efficacy of patients and TUG1, we observed that the higher TUG1, the worse the curative effect, which also indicated that TUG1 had the potential effect of evaluating MEC. On the other hand, it suggests that we may treat MEC through targeted silencing of TUG1 in the future. At present, molecular targeted therapy is a hot spot in clinical research[28,29]. Whereas the current treatment of MEC has major limitations. Due to the concealed location of MEC, residual cholesteatoma and loss of skin, tympanic membrane and facial nerve of external auditory canal are easy to occur during the surgical resection of tumor tissue, which not only leads to a high recurrence rate, but may cause serious complications such as narrowing of the external auditory canal, deafness and facial paralysis[30]. While molecular targeted therapy can effectively make up for the above shortcomings, which is of great significance for further improving the clinical treatment effect of MEC. During the prognostic follow up we found that the disease recurrence rate reached 18.03 %, demonstrating that there was still much room for improvement in MEC treatment. As for TUG1, it showed excellent effect in predicting patients’ prognosis and recurrence, further verifying its huge implications in MEC.
However, due to the limited experimental conditions, only the tissues of MEC patients were analyzed in this study. In fact, for the diagnosis of MEC, blood samples are more preferable. In addition, due to the lack of support from in vitro experiments, we are still unable to determine the exact mechanism by which TUG1 participates in MEC. We will carry out extended experimental analysis as soon as possible in view of the above shortcomings, to verify the effectiveness of the treatment of MEC with TUG1 as the treatment target we proposed above.
To sum up, TUG1 is elevated in MEC and is closely related to ossicle destruction in patients, which may be a breakthrough in future diagnosis and treatment of MEC.
Acknowledgement:
The study was supported by General National Nature Fund Project (No. 201901D111359).
Conflict of interests:
The authors declared no conflict of interest.
References
- Luntz M, Barzilai R. Middle ear cholesteatoma. Harefuah 2021;160(5):316-22.
- Ayache D, Schmerber S, Lavieille JP, Roger G, Gratacap B. Cholestéatome de l’oreille moyenne. Annales d'Otolaryngologie et de Chirurgie Cervico-faciale 2006;123(3):120-37.
- Kuo CL, Shiao AS, Yung M, Sakagami M, Sudhoff H, Wang CH, et al. Updates and knowledge gaps in cholesteatoma research. BioMed Res Int 2015;2015:854024.
[Crossref] [Google Scholar] [Pub Med]
- Djurhuus BD, Faber CE, Skytthe A. Decreasing incidence rate for surgically treated middle ear cholesteatoma in Denmark 1977-2007. Dan Med Bull 2010;57(10):A4186.
- Song SP, Li WW, Ma XL. Advances in research on apoptosis in the pathogenesis of acquired middle ear cholesteatoma. J Clin Otorhinolaryngol Head Neck Surg 2019;33(6):572-6.
- Nagel J, Wöllner S, Schürmann M, Brotzmann V, Müller J, Greiner JF, et al. Stem cells in middle ear cholesteatoma contribute to its pathogenesis. Sci Rep 2018;8(1):1-0.
[Crossref] [Google Scholar] [Pub Med]
- Feng YS, Si Y, Zhang ZG. The research progress of keratin's roles in the pathology of middle ear cholesteatoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2019;54(11):870-4.
- Künzel J, Raftis F, Hagemann J, Bahr K, Zimmer S, Koutsimpelas D, et al. Ist die routinemäßige histologische untersuchung von mittelohrcholesteatomen nötig? HNO 2019;67(1):30-5.
- Jathar S, Kumar V, Srivastava J, Tripathi V. Technological developments in lncRNA biology. Adv Exp Med Biol 2017:283-323.
[Crossref] [Google Scholar] [Pub Med]
- Qian X, Zhao J, Yeung PY, Zhang QC, Kwok CK. Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem Sci 2019;44(1):33-52.
[Crossref] [Google Scholar] [Pub Med]
- Ferre F, Colantoni A, Helmer-Citterich M. Revealing protein–lncRNA interaction. Brief Bioinform 2016;17(1):106-16.
[Crossref] [Google Scholar] [Pub Med]
- Charles Richard JL, Eichhorn PJ. Platforms for investigating LncRNA functions. SLAS Technol 2018;23(6):493-506.
[Crossref] [Google Scholar] [Pub Med]
- Cao J, Han X, Qi X, Jin X, Li X. TUG1 promotes osteosarcoma tumorigenesis by upregulating EZH2 expression via miR-144-3p. Int J Oncol 2017;51(4):1115-23.
[Crossref] [Google Scholar] [Pub Med]
- Zhang Y, Gu M, Ma Y, Peng Y. LncRNA TUG1 reduces inflammation and enhances insulin sensitivity in white adipose tissue by regulating miR-204/SIRT1 axis in obesity mice. Mol Cell Biochem 2020;475(1):171-83.
[Crossref] [Google Scholar] [Pub Med]
- Gao J, Tang Q, Zhu X, Wang S, Zhang Y, Liu W, et al. Long non coding RNAs show differential expression profiles and display ceRNA potential in cholesteatoma pathogenesis. Oncol Rep 2018;39(5):2091-100.
[Crossref] [Google Scholar] [Pub Med]
- Xu K, Zhang L. Inhibition of TUG1/miRNA-299-3p axis represses pancreatic cancer malignant progression via suppression of the notch1 pathway. Dig Dis Sci 2020;65(6):1748-60.
[Crossref] [Google Scholar] [Pub Med]
- Prasad SC, Giannuzzi A, Nahleh EA, Donato GD, Russo A, Sanna M. Is endoscopic ear surgery an alternative to the modified Bondy technique for limited epitympanic cholesteatoma? Eur Arch Otorhinolaryngol 2016;273(9):2533-40.
[Crossref] [Google Scholar] [Pub Med]
- Khemani S, Singh A, Lingam RK, Kalan A. Imaging of postoperative middle ear cholesteatoma. Clin Radiol 2011;66(8):760-7.
[Crossref] [Google Scholar] [Pub Med]
- Lou Z. Transcanal endoscopic ear surgery to treat middle-ear cholesteatoma should be performed cautiously. Otol Neurotol 2018;39(1):133.
[Crossref] [Google Scholar] [Pub Med]
- Kim KH, Lim HJ, Kim YJ, Kim SW, Kim YS, Tian C, et al. The oncoprotein, gankyrin, is up-regulated in middle ear cholesteatoma. Acta Otolaryngol 2014;134(3):238-43.
[Crossref] [Google Scholar] [Pub Med]
- Zhang Z, Xiong R, Li C, Xu M, Guo M. LncRNA TUG1 promotes cisplatin resistance in esophageal squamous cell carcinoma cells by regulating Nrf2. Acta Biochim Biophys Sin 2019;51(8):826-33.
[Crossref] [Google Scholar] [Pub Med]
- Xu Y, Deng W, Zhang W. Long non-coding RNA TUG1 protects renal tubular epithelial cells against injury induced by lipopolysaccharide via regulating microRNA-223. Biomed Pharmacother 2018;104:509-19.
[Crossref] [Google Scholar] [Pub Med]
- Sun Y, Wang EH, Yu JT, Zhong G, Zhu LX, Wang Y, et al. A novel surgery classification for endoscopic approaches to middle ear cholesteatoma. Curr Med Sci 2020;40(1):9-17.
[Crossref] [Google Scholar] [Pub Med]
- Xie S, Wang X, Ren J, Liu W. The role of bone resorption in the etiopathogenesis of acquired middle ear cholesteatoma. Eur Arch Otorhinolaryngol 2017;274(5):2071-8.
[Crossref] [Google Scholar] [Pub Med]
- Aslıer M, Erdag TK, Sarioglu S, Güneri EA, Ikiz AO, Uzun E, et al. Analysis of histopathological aspects and bone destruction characteristics in acquired middle ear cholesteatoma of pediatric and adult patients. Int J Pediatr Otorhinolaryngol 2016;82:73-7.
[Crossref] [Google Scholar] [Pub Med]
- Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem 2011;112(3):750-5.
[Crossref] [Google Scholar] [Pub Med]
- Sheng K, Li Y. LncRNA TUG1 promotes the development of osteosarcoma through RUNX2. Exp Ther Med 2019;18(4):3002-8.
[Crossref] [Google Scholar] [Pub Med]
- Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol 2018;834:188-96.
[Crossref] [Google Scholar] [Pub Med]
- Guan L, Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discov Med 2018;26(144):219-29.
- Semenov FV, IuV M. Advantages and disadvantages of the application of endoscopic techniques at different stages of tympanoplasty. Vestn Otorinolaringol 2010;(6):48-50.