- *Corresponding Author:
- Rui Zhang
Department of Ophthalmology, Tianjin Prevention and Treatment Center for Occupational Diseases, Heping, Tianjin 300020, China
E-mail: zhangrui21212@126.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “226-233” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Diabetes mellitus can cause a decrease in tear secretion and tear film composition, resulting in a series of pathological changes such as the stability of tear film, the increased risk of inflammation, peripheral neuropathy and impairment of meibomian glands, leading to dry eye. The imbalance of inflammatory mediators in diabetic patients and the injury of ocular surface are the main factors that aggravate dry eye. Nod-like receptor protein 3 inflammasome was related to the occurrence and development of various systemic diseases. Evaluate the difference between nod-like receptor protein 3 inflammasome and diabetic dry eye parameters in diabetic dry eye patients, analyze the trend of correlation and discuss its mechanism. All subjects received teardrop break time, tear Schirmer’s I test, fluorescein staining score and objective scatter index measurement. The level of nod-like receptor protein 3, interleukin-1 beta, interleukin-6, interleukin-10, interleukin-18 in tears meanwhile nod-like receptor protein 3, caspase-1, tumor necrosis factor alpha, interleukin-1β, interleukin-6, interleukin-10, interleukin-18, superoxide dismutase, monoamine oxidase type B in serum was measured by double antibody sandwich enzyme-linked immunosorbent assay. In addition, the promoter gene expression levels of nod-like receptor protein 3, caspase-1, toll-like receptor 4, tumor necrosis factor alpha were determined by real-time quantitative real time polymerase chain reaction. BUT in diabetic dry eye patients was shortened, tear Schirmer’s I test and fluorescein staining scores were reduced and the levels of nod-like receptor protein 3, interleukin- 1β, interleukin-6 and interleukin-18 in tear increased significantly while the change of interleukin-10 was nonstatistical significance. Nod-like receptor protein 3, caspase-1, tumor necrosis factor alpha, interleukin-1β, interleukin-18 in serum were increased significantly and superoxide dismutase, monoamine oxidase type B were decreased statistically, meanwhile, levels of interleukin-6, interleukin-10 has no statistical difference. The results of real-time quantitative real time polymerase chain reaction showed that levels of nod-like receptor protein 3, caspase-1, toll-like receptor 4, tumor necrosis factor alpha were statistically increased also. Pearson correlation analysis showed that there was a strong positive correlation between nod-like receptor protein 3 levels and diabetic dry eye. The level of nod-like receptor protein 3 in diabetic dry eye increased significantly. There was a positive correlation between the clinical parameters and nod-like receptor protein 3 levels in diabetic dry eye, suggesting that regulation of nod-like receptor protein 3 level may provide new ideas for preventing and treating diabetic dry eye.
Keywords
Nod-like receptor protein 3, inflammasome, diabetic, dry eye, correlation
Dry eye is the most common kind of ocular surface diseases. It refers to the abnormality of tear quality and quantity or fluid dynamics caused by any reason, resulting in the decline of tear film stability, accompanied by various ocular discomfort symptoms and ocular surface tissue lesions[1]. With the extension of the course of dry eye, it gradually affects normal work and life. Diabetic patients are becoming the susceptible population of dry eye. Atleast 73.6 % of diabetic patients can develop eye symptoms[2]. The study found that diabetic patients with dry eye symptoms are common and easily overlooked.
Diabetes is one of the most common causes of dry eye[3]. Long term hyperglycemia can aggravate dry eye symptoms[4,5]. In patients with type 2 diabetes, inflammation of the body is a major factor contributing to dry eye symptoms[6,7]. Inflammasome is a kind of multi protein complex in cells, which is mainly composed of receptor platform protein, adapter protein and effector protein[8]. As a response platform to internal and external danger signals, inflammasomes promote the body’s innate immune response and acquired immune response, so as to prevent infection and maintain the body’s homeostasis[9].
Nod-Like Receptor Protein 3 (NLRP3) inflammasome recognizes inflammation, the receptor platform protein is NLRP3 and the effector protein is caspase-1[10,11]. The inflammatory receptor platform NLRP3 is composed of amino terminal Pyrin Domain (PYD), NACHT and terminal Leucine Rich Repeat (LRR). Studies have shown that Nod like receptor NLRP3 is an intracellular pattern recognition receptor, which can be activated by a variety of exogenous and endogenous stimulation signals to form a multi protein complex, NLRP3 inflammasome, which can activate caspase-1 and promote proinflammatory cytokine Interleukin (IL)-1 Beta (β) and IL-18 shear maturation and secretion, causing a series of inflammatory reactions[12,13]. Therefore, NLRP3 inflammasome is closely related to the inflammatory reaction of diabetic dry eye. It was found that the expressions of NLRP3, Activating Signal Cointegrator (ASC), pro-caspase-l and pro-IL-1β were significantly increased in the cytological samples of dry eye patients and the expressions of IL-lβ and IL-18 in tears were also increased[14,15].
The increase of NLRP3 inflammasome related components may become the diagnostic basis of dry eye[16,17]. Blocking the activation of NLRP3 inflammasome may become a new direction of dry eye treatment[18]. However, the relationship between NLRP3 inflammasome and diabetic dry eye has not been further studied.
Materials and Methods
In this study, 65 cases of diabetic dry eye from January 2020 to March 2021 were selected as the observation group and 63 normal people as control group. All patients read and signed the informed consent form and agreed to participate in the clinical trial. The inclusion criteria of diabetic dry eye are type 2 diabetes, including subjective symptoms, dry eye, foreign body sensation, burning sensation, redness, excessive secretion, heavy eyelid, asthenia, photophobia, tears, itching, eye pain, and visual fluctuation. One or more of the above symptoms must occur frequently and persist. Tear film instability includes BUT, <5 s (+) or ≤10 s (+). Tear secretion decreased and the amount of tear secretion was ≤5 mm/5 min (+) or ≤10 mm/5 min (+). Ocular surface damage, including corneal Fluorescein Staining (FLS) score ≥1. Dry eyes can be diagnosed by positive subjective symptoms and strong positive (++) or positive (+) of eye examination (BUT, SIT, FLS). Exclude other diabetic eye diseases and other systemic diseases that affect tear secretion. Corneal contact lens wearers, history of ocular trauma and surgery, history of drug allergy and any ocular and systemic diseases were excluded.
BUT detection method is to touch the sodium fluorescein filter paper strip into the conjunctiva sac of the subject’s lower eyelid, asks the patient to look flat naturally after blinking and observes the subject under the blue excitation light of slit lamp. The time from opening the eyes to the first black spot (rupture point) of the cornea is BUT[19]. FLS detection method is FLS positive, reflecting corneal epithelial defect (discontinuity). After the tear film rupture test, the corneal epithelium staining was observed. The cornea was divided into four quadrants. 0 point were no staining, 1 point were scattered punctate staining or less than 5 staining points, 3 points were high-density punctate staining or flake staining or massive or filamentous objects and 2 points were between the two, a total of 0~12 points. SIT test is to bend the head end of 5 mm×35 mm tear test paper for 5 mm, place it at the junction of the outer and inner 1/3 of the lower eyelid, close the eyes for 5 min, take out the test paper and measure the length of the test paper soaked by tears from the bend. The Enzyme-Linked Immunosorbent Assay (ELISA) test method is to add a drop of sterile normal saline into the conjunctiva sac of the subject, rotate the eyeball to fully mix the tears with the saline, gently place the capillary glass tube at the junction of the fornix conjunctiva and bulbar conjunctiva, gently press it, collect the left and right tears respectively, transfer them to the same micro centrifugal test tube and store the tear samples at -20° for standby. Meanwhile blood taken from the brachial vein to anti-coagulated harvesting vessels and centrifuged 12 000 rpm in 4° as soon as possible and the supernatant was stored at -20° for use. The concentration of NLRP3 inflammasome, IL-1β, IL-6, IL-10, IL-18, caspase-1 in tears and NLRP3, caspase-1, Toll-Like Receptor 4 (TLR-4), Tumour Necrosis Factor Alpha (TNF-α), IL-1β, IL-6, IL-10, IL-18, Superoxide Dismutase (SOD), Monoamine Oxidase Type B (MAO-B) in serum were detected by ELISA. Operate according to the instructions of the kit and determine the absorbance A450 value with an enzyme labeling instrument. Draw the standard curve, the abscissa was the sample concentration and the ordinate was the value. Finally, the concentration of the sample was calculated on the standard curve. Total Ribonucleic Acid (RNA) from serum samples was extracted using Trizol reagent, according to the manufacturer’s instructions. The purity RNA concentration was determined using Qubit® 3.0 fluorimetric analysis. Template complementary Deoxyribonucleic Acid (DNA) was synthesized using the complementary DNA (cDNA) preparation kit. Primer sequences are shown in Table 1.
| Primer | Forward | Reverse |
|---|---|---|
| NLRP3 | 5′-CCTGGGGGACT TTGGAATCAG-3′ | 5′-GATCCTG ACAACAC GCGGA-3′ |
| Caspase-1 | 5′-TTTCCGCAAGGTTC GATTTTCA-3′ | 5′-GGCATCTGCGCTCTAC CATC-3′ |
| TLR-4 | 5′-TGTATCGGTGGTCAGTGTGC-3′ | 5′‐CAGCTCGTTTCTCACCCAGT-3′ |
| TNF-α | 5′-AGCCTCTTCTCCTTCCTGATCGTG-3′ | 5′-GGCTGATTAGAGAGAGGTCCCTGG-3′ |
Table 1: Specific contents of Primer Sequences.
Statistical Package for Social Sciences (SPSS) 22.0 software was used for statistical analysis. The mean±standard deviation was used to express the concentrations of BUT, SIT, FLS, NLRP3 inflammasome, IL-1β and IL-18. The difference was statistically significant by one-way Analysis of Variance (ANOVA) (p<0.05). Correlation analysis between NLRP3 and diabetic dry eye was performed by Pearson test, p<0.05 was correlated, r>0 was positively correlated and r<0 was negatively correlated.
Results and Discussion
65 diabetic dry eye patients were included in the observation group and 63 healthy persons were included in the control group. The demographic data was shown in Table 2. There was no significant difference between the two groups by comparing the number of cases, age, sex, weight and disease time, which means that subsequent studies are based on comparable patient groups.
| Control | Diabetic dry eye | p value | |
|---|---|---|---|
| Number | 63 | 65 | 0.852 |
| Age (year) | 55.12±6.31 | 52.03±8.26 | 0.524 |
| Sex (male/female) | 43/41 | 52/50 | 0.625 |
| Weight (kg) | 58±9 | 55±8 | 0.868 |
| Disease time(month) | 22.1±3.6 | 24.5±6.9 | 0.739 |
Table 2: Demographic Data.
In the study, the ocular disease parameters showed significant statistical differences. Among the above indicators, BUT in diabetic dry eye patients was shortened in statistical, while SIT and FLS scores were reduced also distinguishing as shown in Table 3.
| Control | Diabetic dry eye | p value | |
|---|---|---|---|
| BUT (s) | 12.17±2.31 | 3.54±1.94 | ≤0.000 |
| SIT (mm) | 15.21±3.25 | 7.56±2.87 | ≤0.000 |
| FLS (point) | 3.56±1.93 | 2.41±1.91 | ≤0.034 |
Table 3: Diabetic Dry Eye Examination Parameters.
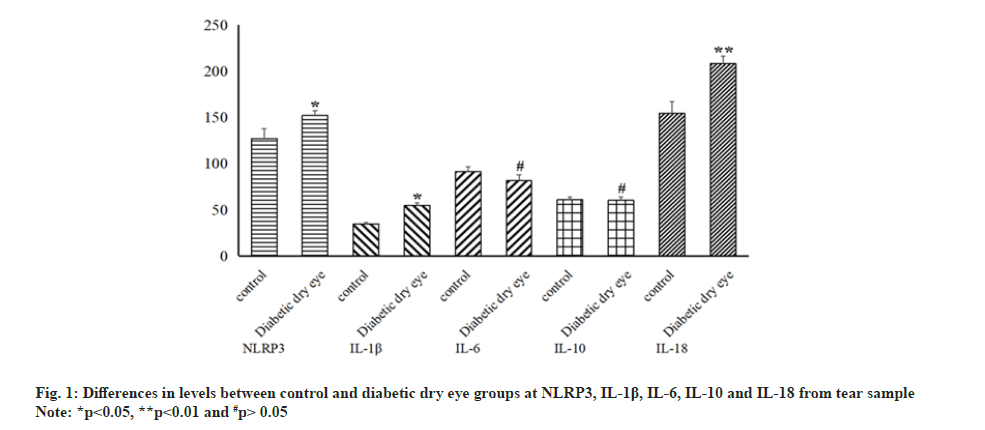
Although significant statistical differences were seen in the above indicators, obvious differences were not always present in the results of the inflammatory indicators of the tear. The levels of NLRP3, IL-1β and IL-18 in tear increased significantly in diabetic dry eye patients compared to control group (p<0.05). At the same time, levels of IL-6 and IL-10 did not show certain statistical distinction between control and diabetic dry eye group (p>0.05) as shown in fig. 1. In the same sample (tear sample), different inflammatory factors and indicators showed distinct statistical differences, which makes us more interested in the relationship between NLRP3 and diabetic dry eye.
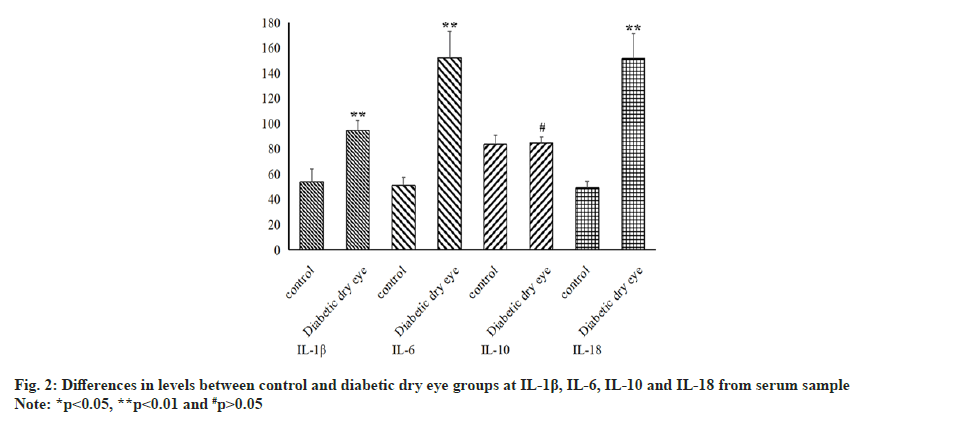
Since the volume of tear fluid was limited and we could not get more useful information from it, we obtained enough blood samples from patients. Also using ELISA, we found that some inflammatory factors showed different statistical results in tear samples and blood samples. As seen in fig. 2, IL-1β and IL-18 demonstrated the same results, the levels of those in diabetic dry eye group were significantly higher than in the control group (p<0.05). Similarly, IL-10 also has the same result between those two groups (p>0.05), but inversely, IL-6 in serum sample has a statistical increase from diabetic dry eye group than control group (p<0.05).
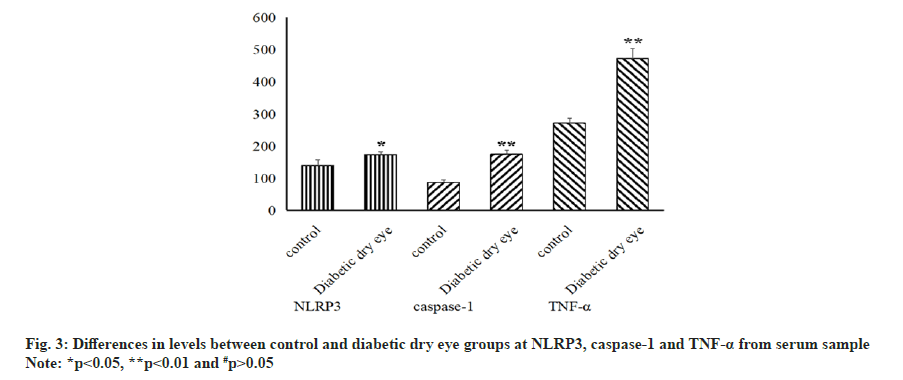
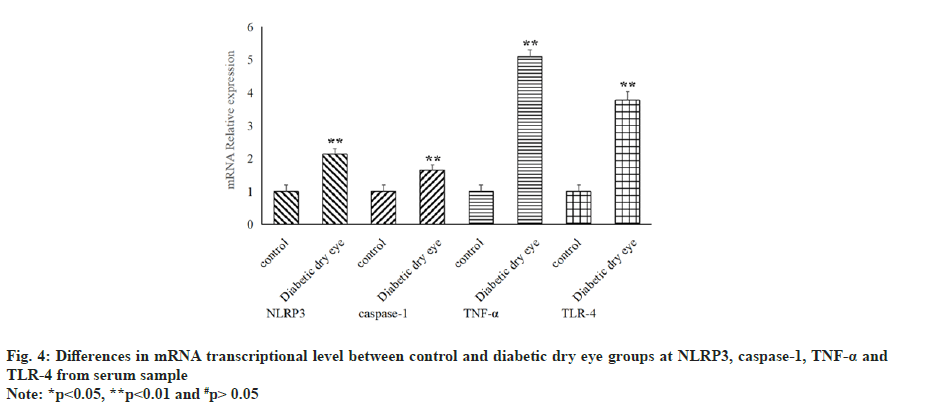
Not only that, the level of NLRP3, caspase-1 and TNF-α in serum samples were also examined and the result showed that all of three indicators in diabetic dry eye groups has a significant increase compared to control group (p<0.05), as shown in fig. 3. The same statistical results that the distinctions existed between the diabetic dry eye groups and the control group were also observed in the messenger RNA (mRNA) transcriptional levels of these indicators, as shown in fig. 4. Hence, the levels of cytokines and mRNA in serum of patients suffered from diabetic dry eye were significantly higher than those of the control group. This is the same as we expected and in some extent, the results explained our doubts in the previous step, that different inflammatory factors showed different levels and the same inflammatory factors also expressed different levels in different parts of sample.
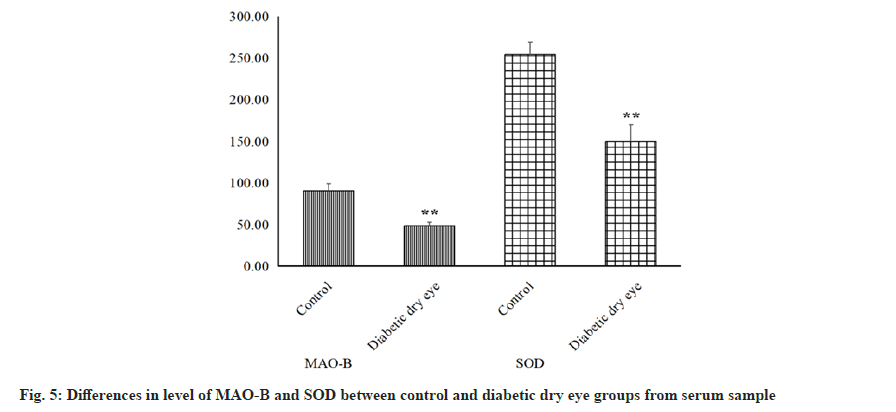
In addition to above inflammatory factors, antioxidant markers like MAO-B and SOD were also measured from serum samples. From the ELISA results as shown in fig. 5, we can intuitively see that both indicators have a significant decrease from diabetic dry eye groups compared to control group (p<0.01). The reduction and decline of oxidative stress capacity in diabetic patients is systemic and oxidative stress and inflammatory responses are interrelated in many ways. Reduced oxidative stress capacity will lead to the production of Reactive Oxygen Species (ROS), which precisely leads to the production and activation of the NLRP3 inflammasome.
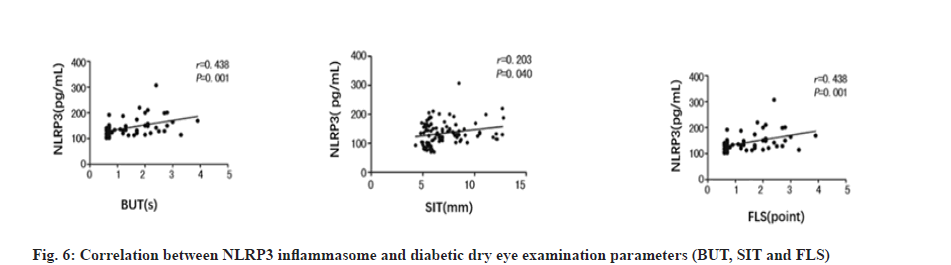
On the other hand, Pearson correlation analysis showed that there existed strong positive correlation between NLRP3 level and diabetic dry eye, from BUT, SIT and FLS, as shown in fig. 6 and Table 4. The magnitude of the r value and p value together support the positive correlation between these two hands, NLPR3 and diabetic dry eye, which mean that the interaction between the interaction of the inflammasome NLRP3 and diabetic dry eye is inseparable.
| Parameters | SE | r | p |
|---|---|---|---|
| BUT | 0.075 | 0.438 | 0.001 |
| SIT | 0.169 | 0.203 | 0.040 |
| FLS | 0.036 | 0.438 | 0.001 |
Table 4: Correlation between NLRP3 and Diabetic Dry Eye Parameters.
The mechanism of dry eye is complex. Inflammation, apoptosis and sex hormone level can cause the occurrence of dry eye[20]. Diabetic patients are susceptible to dry eye. The occurrence of diabetic dry eye is characterized by the imbalance of tear mass and quantity, the development of squamous metaplasia and reduction of goblet cells[21]. With the continuous decrease of the hydrophilic and tear film stability, and the accumulation of inflammatory factors, the stability of ocular surface is constantly damaged, which leads to the occurrence of diabetic dry eye[22]. It is found that conjunctiva goblet cells have the function of maintaining ocular surface function. The tear film stability of diabetic patients is related to the decrease of normal human beings and the decrease of conjunctiva goblet cell density and the inflammatory reaction of ocular surface plays a key role in the damage of ocular surface function[23]. The inflammatory reaction of ocular surface can reduce the number of conjunctiva epithelial cells and conjunctively goblet cells, resulting in the decrease of tear film stability and ocular surface damage. Inflammasome is a kind of pathogenic associated molecular patterns that can recognize intracellular Pathogen-Associated Molecular Patterns (PAMPs) or Damage Associated Molecular Patterns (DAMPs), which mediate interleukin IL-1β and IL-18 and regulate the expression of inflammation related genes[24]. The results showed that NLRP3 inflammasome was related to the occurrence and development of various diseases. Microglia β amyloid abnormal host proteins can mediate neurodegenerative diseases such as Alzheimer’s disease by activating NLRP3 inflammasome[25]. Intestinal Salmonella and Proteus can cause mitochondrial damage through endoplasmic reticulum stress, promote the assembly and activation of NLRP3 inflammasome and mediate intestinal inflammation. The study also found that carrying out NLRP3 related rare mutant genes (such as T348M) have an increased risk of Cryopyrin Associated Periodic Syndrome (CAPS)[26]. In addition, NLRP3 inflammasome also plays an important role in diabetes, gout, obesity, liver disease, atherosclerosis, coronary artery endothelial dysfunction and other metabolic diseases[27].
In this study, the level of NLRP3, IL-1β and IL-18 in the tear as well as NLRP3, IL-1β, IL-6, IL-18, TNF-α, caspase-1 and TLR-4 in the serum of the diabetic dry eye group was significantly higher than that of the control group, suggesting that the activation of NLRP3 and the increase of specific inflammatory responses were related to diabetic dry eye. Existing research suggests that NLRP3 activation occurs in two steps[28], the first is priming and the next is protein complex assembly. The first step of NLRP3 is triggered by pattern recognition receptor signaling which accompanied by activation of TLR-4 and TNF signaling pathway[29]. While, the second step is accompanied by mitochondrial dysfunction and accumulation of ROS[30]. It is noteworthy that the expression of IL-6 in tear was significantly lower than that in serum when compared in diabetic dry eyegroup. As far as we know, the co-expression of IL-6 binding receptor molecule IL-6Rα and signal transducer gp130 on the cell surface is required for the expression of IL-6[31]. However, IL-6Rα and gp130 are not always present at the same time during lacrimal duct and tear production. The expression of IL-10 depends on the Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) signaling pathway, that is the increase of IL-10 expression is accompanied by the increase of STAT3 and STAT3 signaling is widely involved in inflammatory response and oxidative stress[32]. There is evidence that IL-6 production is also mediated and activated by STAT3[33], but from the level of IL-10, we speculate that STAT3 signaling is not up-regulated during this process. Increasing studies have highlighted the IL-6-STAT3 signaling pathway as a potential target for the treatment of chronic inflammatory diseases[34,35], but IL-6 is not well represented in patients with diabetic dry eye because this indicator is not significantly increased in tears. Although STAT3 is the main pathway for IL-6 production, studies have shown that many other factors can also stimulate the up-regulation of IL-6, such as TLR, TNF-α, ROS, etc.,[36,37]. Notably, the levels of both TLR-4 and TNF-α were significantly increased in the serum samples and the antioxidant levels were significantly decreased, suggesting that multiple factors act together on the concentration of IL-6 in the blood.
In this study, we found that the activity of SOD and MAO-B decreased, which means that the increase of ROS and the activation of oxidative stress process, but the increase of IL-10 has no statistical difference, indicating that STAT3 signal does not participate in this process and increase of IL-6 in serum does not due to this. This allows us to further investigate the regulation of inflammatory response and possibly oxidative stress in diabetic dry eye patients.
Meanwhile studies have confirmed that hyperglycemia is a key inducer of IL-1β production and pancreatic NLRP3 inflammasome activation[38]. Therefore, lowering blood glucose and regulating lipid metabolism disorder in diabetic patients can reduce the activation of NLRP3 inflammasome, reduce the levels of IL-1β and IL-18 and thus alleviate the occurrence and development of diabetic dry eye[39]. Ocular inflammatory reaction will lead to temporary or even permanent changes in the structure of visual organs. If not controlled in time, it would lead to vision loss and even blindness. NLRP3 inflammasome plays an important role in inflammation related ocular diseases. It was found that the expression of NLRP3, caspase-1A, IL-1β and IL-18 in retina of Sprague Dawley male rats induced by Streptozotocin (STZ) was significantly up-regulated[40]. It was found that NLRP3 blocker MCC950 can effectively inhibit the disorder of Human Retinal Endothelial Cells (HRECs) in high glucose environment[41]. The results of this study also showed that NLRP3 was positively correlated with diabetic dry eye parameters, indicating that reducing NLRP3 can alleviate the occurrence and development of diabetic dry eye. NLRP3 was a highrisk factor for dry eye in diabetic patients. The reason may be the role of macrophages in promoting ocular inflammation and promoting dry eye formation. Therefore, regulating the level of NLRP3 could effectively prevent and treat diabetic dry eye.
In conclusion, priming pathway and protein complex assembly pathway both contributed the increase of NLRP3 in patients suffered from diabetes mellitus dry eye, which means that the ability of anti-oxidative stress and anti-inflammatory are declining in patients with diabetic dry eye meanwhile the level of NLRP3 inflammatory corpuscle in diabetic dry eye patients was positively correlated with ocular diseases and could predict the severity of cervical diabetes mellitus dry eye. Clinically, we could detect the risk of diabetic dry eye by detecting the level of NLRP3 inflammasome and provide new detection and therapeutic targets for delaying the occurrence and development of diabetic dry eye.
Conflict of interests:
The authors declared no conflict of interests.
References
- Bourne R, Steinmetz JD, Flaxman S. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob Health 2021;9(2):e130-43.
- Liu NN, Liu L, Li J, Sun YZ. Prevalence of and risk factors for dry eye symptom in mainland china: A systematic review and meta-analysis. J Ophthalmol 2014;2014:748654.
[Crossref] [Google Scholar] [PubMed]
- Yong WC, Sanguankeo A, Upala S. Association between primary Sjogren’s syndrome, arterial stiffness and subclinical atherosclerosis: A systematic review and meta-analysis. Clin Rheumatol 2019;38(2):447-55.
[Crossref] [Google Scholar] [PubMed]
- Li JQ, Welchowski T, Schmid M, Letow J, Wolpers C, Pascual-Camps I, et al. Prevalence, incidence and future projection of diabetic eye disease in Europe: A systematic review and meta-analysis. Eur J Epidemiol 2020;35(1):11-23.
[Crossref] [Google Scholar] [PubMed]
- Shih K, Lam KS, Tong L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes 2017;7(3):e251.
[Crossref] [Google Scholar] [PubMed]
- Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016;86:100-9.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013;36(1):166-75.
[Crossref] [Google Scholar] [PubMed]
- Cursiefen C, Cordeiro F, Cunha-Vaz J, Wheeler-Schilling T, Scholl HP. Unmet needs in ophthalmology: A European vision institute-consensus roadmap 2019–2025. Ophthalmic Res 2019;62(3):123-33.
[Crossref] [Google Scholar] [PubMed]
- Sepehri Z, Kiani Z, Afshari M, Kohan F, Dalvand A, Ghavami S. Inflammasomes and type 2 diabetes: An updated systematic review. Immunol Lett 2017;192:97-103.
[Crossref] [Google Scholar] [PubMed]
- Cano Sanchez M, Lancel S, Boulanger E, Neviere R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018;7(8):98.
[Crossref] [Google Scholar] [PubMed]
- Nwadiugwu MC. Inflammatory activities in type 2 diabetes patients with co-morbid angiopathies and exploring beneficial interventions: A systematic review. Front Public Health 2021;8:600427.
[Crossref] [Google Scholar] [PubMed]
- Rheinheimer J, de Souza BM, Cardoso NS, Bauer AC, Crispim D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism 2017;74:1-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Ding L, Shen T, Peng D. HMGB1 involved in stress-induced depression and its neuroinflammatory priming role: A systematic review. General Psychiatr 2019;32(4):e100084.
- Sepehri Z, Kiani Z, Afshari M, Kohan F, Dalvand A, Ghavami S. Inflammasomes and type 2 diabetes: An updated systematic review. Immunol Lett 2017;192:97-103.
[Crossref] [Google Scholar] [PubMed]
- He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 2016;41(12):1012-21.
[Crossref] [Google Scholar] [PubMed]
- Cano Sanchez M, Lancel S, Boulanger E, Neviere R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018;7(8):98.
[Crossref] [Google Scholar] [PubMed]
- Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell MolImmunol 2016;13(2):148-59.
[Crossref] [Google Scholar] [PubMed]
- Alfonso-Loeches S, Ureña-Peralta JR, Morillo-Bargues MJ, Oliver-De La Cruz J, Guerri C. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci 2014;8:216.
[Crossref] [Google Scholar] [PubMed]
- Feldman-Billard S, Larger É, Massin P. Early worsening of diabetic retinopathy after rapid improvement of blood glucose control in patients with diabetes. Diabetes Metab 2018;44(1):4-14.
[Crossref] [Google Scholar] [PubMed]
- Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, et al. Incidence and progression of diabetic retinopathy: A systematic review. Lancet Diabetes Endocrinol 2019;7(2):140-9.
[Crossref] [Google Scholar] [PubMed]
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35(3):556-64.
[Crossref] [Google Scholar] [PubMed]
- Rowsey TG, Karamichos D. The role of lipids in corneal diseases and dystrophies: A systematic review. Clin Transl Med 2017;6(1):30.
[Crossref] [Google Scholar] [PubMed]
- Sherwin JC, Kokavec J, Thornton SN. Hydration, fluid regulation and the eye: In health and disease. Clin Exp Ophthalmol 2015;43(8):749-64.
[Crossref] [Google Scholar] [PubMed]
- Yong WC, Sanguankeo A, Upala S. Association between primary Sjögren’s syndrome, cardiovascular and cerebrovascular disease: A systematic review and meta-analysis. Clin Exp Rheumatol 2018;36(112):190-7.
[Google Scholar] [PubMed]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011;469(7329):221-5.
[Crossref] [Google Scholar] [PubMed]
- Liu Q, Zhang D, Hu D, Zhou X, Zhou Y. The role of mitochondria in NLRP3 inflammasome activation. Mol Immunol 2018;103:115-24.
[Crossref] [Google Scholar] [PubMed]
- Kim JK, Jin HS, Suh HW, Jo EK. Negative regulators and their mechanisms in NLRP3 inflammasome activation and signaling. Immunol Cell Biol 2017;95(7):584-92.
[Crossref] [Google Scholar] [PubMed]
- Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol 2021;18(5):1141-60.
- McKee CM, Coll RC. NLRP3 inflammasome priming: A riddle wrapped in a mystery inside an enigma. J Leukoc Biol 2020;108(3):937-52.
- Meyers AK, Zhu X. The NLRP3 inflammasome: Metabolic regulation and contribution to inflammaging. Cells 2020;9(8):1808.
[Crossref] [Google Scholar] [PubMed]
- Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol 2021;33(3):127-48.
[Crossref] [Google Scholar] [PubMed]
- Nagata K, Nishiyama C. IL-10 in mast cell-mediated immune responses: Anti-inflammatory and proinflammatory roles. Int J Mol Sci 2021;22(9):4972.
[Crossref] [Google Scholar] [PubMed]
- Hirano T, Murakami M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity 2020;52(5):731-3.
[Crossref] [Google Scholar] [PubMed]
- Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16(5):448-57.
[Crossref] [Google Scholar] [PubMed]
- Rébé C, Ghiringhelli F. STAT3, a master regulator of anti-tumor immune response. Cancers 2019;11(9):1280.
[Crossref] [Google Scholar] [PubMed]
- Murakami M, Kamimura D, Hirano T. Pleiotropy and specificity: Insights from the interleukin 6 family of cytokines. Immunity 2019;50(4):812-31.
[Crossref] [Google Scholar] [PubMed]
- Hu L, Yu Y, Huang H, Fan H, Hu L, Yin C, et al. Epigenetic regulation of interleukin 6 by histone acetylation in macrophages and its role in paraquat-induced pulmonary fibrosis. Front Immunol 2017;7:696.
[Crossref] [Google Scholar] [PubMed]
- Budai MM, Varga A, Milesz S, Tőzsér J, Benkő S. Aloe vera down regulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol Immunol 2013;56(4):471-9.
[Crossref] [Google Scholar] [PubMed]
- Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q, et al. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: Possible involvement of NF-κB pathway and NLRP3 inflammasome. Mol Neurobiol 2016;53(5):3462-76.
[Crossref] [Google Scholar] [PubMed]
- Lio CT, Dhanda SK, Bose T. Cluster analysis of dry eye disease models based on immune cell parameters–new insight in to therapeutic perspective. Front Immunol 2020;11:1930.
[Crossref] [Google Scholar] [PubMed]
- Navel V, Sapin V, Henrioux F, Blanchon L, Labbé A, Chiambaretta F, et al. Oxidative and antioxidative stress markers in dry eye disease: A systematic review and meta-analysis. Acta Ophthalmologica 2022;100(1):45-57.
[Crossref] [Google Scholar] [PubMed]