- *Corresponding Author:
- Yingying Zhang
Department of Neurology, Huadong Hospital, Jingan, Shanghai 200063, China
E-mail: Zyy18621554121@163.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “372-379” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
With the acceleration of people’s pace of life, the incidence of cerebrovascular diseases, including ischemic stroke, is on the rise, making it a common clinical condition. The symptoms of cerebrovascular diseases are diverse, significantly impacting the quality of life of those affected. While cerebrovascular collateral circulation has been found to be closely associated with ischemic stroke, there is limited research specifically addressing cerebrovascular collateral stroke and its connection with ischemic stroke. Currently, intravenous thrombolysis within 4.5 h of symptom onset is the most widely recognized therapy for stroke treatment. Additionally, endovascular interventional therapy presents an opportunity for early rehabilitation training for stroke patients due to its minimal invasiveness and convenient hemostasis. However, during interventional therapy, appropriate nursing intervention is crucial to promote limb movement, sensory perception and self-care abilities, ultimately enhancing the quality of life and prognosis of stroke patients. This study included stroke patients diagnosed via transcranial Doppler ultrasound from March 2019 to March 2022. Patients were divided into ischemic stroke and non-ischemic stroke groups in a 1:1 ratio. A total of 100 patients were included, all of whom underwent whole-brain angiography (digital subtraction angiography), digital subtraction angiography intervention and comprehensive care interventions. The incidence of cerebrovascular collateral circulation in the ischemic stroke group was notably higher at 60.00 % compared to 41.82 % in the non-ischemic stroke group (p<0.05). Logistic regression analysis revealed that cerebrovascular collateral circulation, hypertension and hyperlipidemia were significant risk factors for ischemic stroke (p<0.05). Following therapy, stroke patients showed improved psychological status scores, enhanced limb motor function and improved quality of life scores, while neurological function and prognosis scores showed significant improvement (p<0.05). Based on digital subtraction angiography examination, cerebrovascular collateral circulation is identified as a risk factor for ischemic stroke. Implementing appropriate and effective nursing measures can effectively improve mood, limb function, quality of life and prognosis for patients with cerebrovascular diseases and ischemic stroke.
Keywords
Digital subtraction angiography examination, cerebrovascular circuitous, ischemic stroke, correlation, interventional surgery, ministration countermeasures
With the accelerating pace of life, the incidence of cerebrovascular tortuosity and ischemic stroke is on the rise, becoming a prevalent clinical condition that significantly impacts the quality of life for affected individuals. Relevant examination results indicate a close association between cerebrovascular tortuosity and ischemic stroke[1]. As science progresses, Digital Subtraction Angiography (DSA) has become instrumental in diagnosing ischemic stroke. In recent years, the widespread use of DSA in interventional diagnosis and therapy has revealed that many stroke patients exhibit cerebrovascular tortuosity without evidence of vascular stenosis, occlusion, cardiogenic stroke, vascular dissection, or malformation[2]. However, in examining numerous ischemic stroke patients, evidence of cardiogenic stroke, vascular dissection, vascular occlusion, or cerebrovascular tortuosity is sometimes lacking, leading some medical experts to question whether cerebrovascular tortuosity is a risk factor for ischemic stroke. Yet, due to the limited research on cerebrovascular tortuosity, the association with ischemic stroke remains unclear[3].
Intravenous thrombolysis is the most widely accepted therapy for stroke, but its strict time window and low vascular recanalization rate for extensive vascular occlusive strokes limit its clinical benefits. The 2015 update of China’s early endovascular interventional therapy guidelines for acute ischemic stroke recommends early implementation of endovascular interventional therapy, particularly stent thrombectomy, for anterior circulation extensive vascular occlusive strokes within 6 h of onset[4]. Approximately 70 % to 80 % of stroke patients experience varying degrees of limb dysfunction, with limb motor dysfunction being the most common. Endovascular interventional therapy offers opportunities for minimally invasive treatment and convenient hemostasis, facilitating early rehabilitation training for stroke patients[5]. However, during interventional therapy, appropriate nursing interventions are crucial to enhance limb movement and perception, improve self-care abilities and ultimately enhance quality of life and prognosis for stroke patients[6]. Hence, the aim of this study is to investigate the association between cerebrovascular collateral circulation and ischemic stroke, as well as to assess the impact of nursing interventions on the quality of life and prognosis of stroke patients. The objectives include evaluating the incidence of cerebrovascular collateral circulation in ischemic stroke patients, identifying risk factors for ischemic stroke, and assessing the effectiveness of nursing interventions in improving psychological status, limb motor function, quality of life, and prognosis of stroke patients.
Materials and Methods
General information:
Stroke sufferers diagnosed from March 2019 to March 2022 were selected into 1:1 set and 100 cases were divided into ischemic stroke and non-ischemic stroke with 50 cases in each set; 22 males, 28 women, average age (61.76±10.38); 18 drinking alcohol, 22 smoking, 13 diabetes, 27 hypertension and 18 hyperlipidemia in ischemic stroke set. In the non-ischemic stroke set 23 were male and 27 female, mean age (58.36±13.34); 22 drank alcohol, 21 smoking, 8 diabetes, 13 hypertension and 9 hyperlipidemia.
Inclusion criteria: Sufferers with total cerebrovascular imaging; no contraindications for surgery; complete medical records; no other cardiovascular and cerebrovascular ailments; no serious cognitive and communication impairment and signed informed consent.
Exclusion criteria: Iodine allergy of contrast agent; severe bleeding tendency and liver and kidney ailments; sufferers with serious comprehensive neurological defects and cognitive and communication disorders; cerebral hemorrhage caused by various blood ailments and vascular ailments; sufferers with upper gastrointestinal bleeding and coma.
Examination methods:
Detection method: All included sufferers underwent whole cerebral angiography (DSA examination) and the definition of cerebrovascular winding is divided into three categories. Distortion which has arterial winding into “c”, “u” or “s” shape; coil which has artery winding into one or more arterial loop; bending which has arterial winding into sharp angles. According to the results of DSA, the sufferers were divided into circuitous and no stroke sets and recorded the number of ischemic stroke events in 3 sets (circuitous stroke set, circuitous stroke set, non- stroke set); secondly, the vertebral artery, internal carotid artery, vertebral artery, common carotid artery, and subclavian artery respectively according to the different locations of cerebrovascular detour. All sufferers signed an informed consent for surgery prior to performing whole cerebral angiography.
DSA interventional therapy: Comprehensive endotracheal intubation, supine position, give signs monitoring, blood contrast, heparin, whole cerebral angiography, determine the stenosis, clear circulation, catheter, calculation and measurement of stenosis diameter and stenosis length, select stent, through the guide wire, stenosis expansion, stent, using DSA observation stent position to achieve satisfactory effect after release, contrast again, determine whether to expand, postoperative angiography evaluation. The diagnosis was made by two experienced doctors, who observed and recorded the diagnostic data. After discussion, the consistent diagnosis was made, contrast with the sufferer’s symptoms and determined the diagnostic compliance rate of the responsible lesions.
Ministration countermeasures: On the basis of not affecting the rescue measures, should continuously introduce sufferers and their families the development process and cause of the ailment, hospital department layout, doctor name, position, technical level of therapy team, working years, and the recovery of previous sufferers; pay attention to the eye contact with sufferers and their families, guide sufferers to express their psychological feelings, and to answer questions carefully, initially establish a good nurse- sufferer relationship with the sufferers; encourage sufferers to establish the mentality of overcoming the ailment, and actively cooperate with the medical work and carefully check whether the sufferer’s basic information and relevant examinations are complete, such as: blood routine, blood type, blood coagulation 5, biochemical 8, liver function, kidney function, chest X-ray and Electrocardiogram (ECG) results. Report any abnormality to the surgeon in time. Explain the characteristics of the DSA machine during the operation to eliminate the psychological burden. For the sufferers who cannot cooperate, the head and limbs should be used to prevent intraoperative agitation; establish venous channels and pay attention to the smooth rehydration; prepare non-ionic contrast agent, lidocaine, heparin sodium injection, prolinase, rtpA, nimodipine, protamine injection, palavering injection and first-aid supplies. Prepare sterile surgical package, pig tail contrast tube, vertebral artery single curved catheter, Simon contrast tube, Rebra27 micro catheter, micro guide wire and different types of solitaire AB stent; use local or general anesthesia according to the cooperation of the sufferer. When using non-ionic contrast agent, pay attention to whether the sufferer has facial flushing, urticarial, chest tightness, shortness of breath and other allergic manifestations. Monitor ECG, blood pressure, heart rate and blood oxygen outside the operating room. The whole process is heparinized during the operation, record the time of the heparin and the time of the next injection, reminds the doctor, and if any abnormal condition is found, remind and assist the operating surgeon to deal with it.
Evaluation indicators:
To evaluate the psychological status of stroke sufferers before and after the therapy[7], the Self-rating Anxiety Scale (SAS) and Self-rating Depression Scale (SDS) were used to evaluate the sufferer’s psychological state before and after the intervention. Anxiety and depression were positively correlated with the score value.
To evaluate the limb motor function and nerve function of stroke sufferers before and after therapy[8], using the Fugl-Meyer Exercise Scale (FMA), both before and after the intervention, assess the limb motor function in both sets. The higher the score value, the better the limb movement function. Before and after the intervention, the National Institutes of Health Stroke Scale (NIHSS), assess the degree of the neurological deficit, the higher the score value, the more serious the neurological deficit.
Evaluation the quality of life of stroke sufferers before and after therapy[9], the comprehensive quality of life assessment questionnaire-74 (Generic Quality of Life Inventory-74 (GQOL-74)) to assess the quality of life before and after the intervention, including four dimensions: Life, psychological, social and physical, A total of 74 entries, the higher the score value the higher the quality of life; before and after the intervention, the modified Rankin scale. To assess the prognosis in both sets, the lower the score value, the better is the prognosis.
Statistical methods:
The required data of this study are seated and entered into the excel table, Statistical Package for the Social Sciences (SPSS) 26.0 software, normality and variance test are normally distributed data, expressed in (x±s), independent sample t-test for data contrast and paired sample t-test; percentage and χ2 test; influencing factors using logistic regression; when p<0.05.
Results and Discussion
The ischemic stroke set exhibited a higher incidence of cerebrovascular circulatory abnormalities compared to the no-ischemic stroke set, with 64.00 % and 46.00 %, respectively (Table 1). This difference was statistically significant (χ2=8.167, p=0.004), indicating a notable association between ischemic stroke and cerebrovascular circulatory abnormalities.
| Set | Example number | Cerebrovascular roundabout | Non-cerebrovascular circuitous set | Incidence of cerebrovascular circuitous set |
|---|---|---|---|---|
| Ischemic stroke | 50 | 27 | 18 | 64.00 |
| There was no ischemic stroke | 50 | 23 | 32 | 46.00 |
| χ2 | 8.167 | |||
| p | 0.004 |
Table 1: Contrast of the Incidence of Ischemic Stroke between the Ischemic Stroke Set and the No-Ischemic Stroke Set
Table 2, presents the results of a univariate analysis examining clinical factors associated with the occurrence of ischemic stroke. The ischemic stroke set, comprising 50 cases, was compared with a non-ischemic stroke set of equal size. The analysis examined various metrics including age, gender, alcohol consumption, smoking status, presence of cerebrovascular abnormalities, diabetes mellitus, hypertension, and hyperlipidemia. The mean age of individuals in the ischemic stroke set was 61.76 y, slightly higher than that of the non-ischemic stroke set (59.36 y), although this difference was not statistically significant (t=1.420, p=0.157). There were no significant differences observed between the two groups in terms of gender distribution, alcohol consumption, or smoking status (p>0.05 for all). Notably, a higher proportion of individuals in the ischemic stroke set exhibited cerebrovascular circulatory abnormalities compared to the non- ischemic stroke set (χ2=8.167, p=0.004), suggesting a potential association between these abnormalities and ischemic stroke occurrence. Additionally, hypertension and hyperlipidemia were significantly more prevalent in the ischemic stroke set compared to the non-ischemic stroke set (χ2=8.167, p=0.004 and χ2=4.110, p=0.043, respectively), while no significant differences were observed in the prevalence of diabetes mellitus (χ2=1.507, p=0.200). These findings highlight the importance of factors such as cerebrovascular abnormalities, hypertension, and hyperlipidemia in the pathogenesis of ischemic stroke.
| Metric | Ischemic stroke set (n=50) | Non-ischemic stroke set (n=50) | χ2/t | p |
|---|---|---|---|---|
| Age (year) | 61.76±10.38 | 59.36±13.34 | 1.42 | 0.157 |
| Gender (n) | 0.04 | 0.841 | ||
| Male | 22 | 23 | ||
| Female | 28 | 27 | ||
| Drink | 0.667 | 0.414 | ||
| Yes | 18 | 22 | ||
| Deny | 32 | 28 | ||
| Smoke | 0.041 | 0.840 | ||
| Yes | 22 | 21 | ||
| Deny | 28 | 29 | ||
| Cerebrovascular roundabout | 8.167 | 0.004 | ||
| Yes | 27 | 13 | ||
| Deny | 23 | 37 | ||
| Diabetes mellitus | 1.507 | 0.200 | ||
| Yes | 13 | 8 | ||
| Deny | 37 | 42 | ||
| Hypertension | 8.167 | 0.004 | ||
| Yes | 27 | 13 | ||
| Deny | 23 | 37 | ||
| Hyperlipemia | 4.110 | 0.043 | ||
| Yes | 18 | 9 | ||
| Deny | 32 | 41 |
Table 2: Univariate Analysis of the Clinical Factors of Ischemic Stroke Occurrence
Multivariate analysis is shown in Table 3, including the occurrence of ischemic stroke as the dependent variable, taking cerebrovascular detour, hypertension and hyperlipidemia as the independent variable, combined with the actual clinical situation of the model and the results of logistic regression model showed that cerebrovascular detour, hypertension and hyperlipidemia were the occurrence of ischemic stroke.
| Factor | Assignment |
|---|---|
| Dependent variable cerebral arterial thrombosis argument | No occurred=0; occurrence=1 |
| Cerebrovascular roundabout | No=0; Yes=1 |
| Hypertension | No=0; Yes=1 |
| Hyperlipemia | No=0; Yes=1 |
Table 3: Assignment Scale for Multivariate Analysis
The logistic regression analysis conducted to assess the risk factors associated with ischemic stroke revealed significant findings for several metrics (Table 4). Cerebrovascular roundabout, hypertension and hyperlipidemia emerged as influential factors in predicting the occurrence of ischemic stroke. Cerebrovascular roundabout demonstrated a Beta (β) coefficient of 1.352 (SE=0.311), indicating a significant positive association with ischemic stroke (Wald=4.632, Odds Ratio (OR)=1.536, 95 % Confidence Interval (CI)=1.163-7.993, p=0.001). Similarly, hypertension showed a β coefficient of 0.982 (SE=0.263), with a significant positive correlation observed (Wald=7.519, OR=1.337, 95 % CI=1.093–5.613, p=0.009). Hyperlipidemia also displayed a noteworthy association, with a β coefficient of 0.577 (SE=0.189), indicating a positive relationship with ischemic stroke risk (Wald=3.776, OR=1.392, 95 % CI=1.002–6.337, p=0.011). These findings underscore the importance of these risk factors in predicting the likelihood of ischemic stroke occurrence and highlight their potential significance in clinical risk assessment and management strategies.
| Metric | Results of the multivariate analysis | |||||
|---|---|---|---|---|---|---|
| β | SE | Wald | OR | 95 % CI | P price | |
| Cerebrovascular roundabout | 1.352 | 0.311 | 4.632 | 1.536 | 1.163~7.993 | 0.001 |
| Hypertension | 0.982 | 0.263 | 7.519 | 1.337 | 1.093~5.613 | 0.009 |
| Hyperlipemia | 0.577 | 0.189 | 3.776 | 1.392 | 1.002~6.337 | 0.011 |
Table 4: Logistic Regression in Multivariate Analysis Affecting Ischemic Stroke, Sufferer Risk Factors
Table 5, presents the contrast in psychological status among stroke sufferers before and after therapy intervention. The measurements were conducted using the SAS and SDS components. Before the intervention, stroke sufferers exhibited higher levels of anxiety, with a mean SAS score of 72.36±2.63, and depression, with a mean SDS score of 69.58±4.81. However, after the intervention, significant improvements were observed in both anxiety and depression levels. The mean SAS score decreased substantially to 46.19±2.33, while the mean SDS score decreased to 45.29±3.76. Statistical analysis using the t-test revealed highly significant differences between pre and post-intervention scores for both SAS (t=74.481, p<0.001) and SDS (t=39.786, p<0.001). These findings underscore the effectiveness of the therapy intervention in alleviating psychological distress among stroke sufferers, highlighting its potential impact on enhancing overall well-being and mental health outcomes post-stroke.
| Time point | SAS (component) | SDS (component) |
|---|---|---|
| Before the intervention | 72.36±2.63 | 69.58±4.81 |
| After the intervention | 46.19±2.33 | 45.29±3.76 |
| t | 74.481 | 39.786 |
| p | <0.001 | <0.001 |
Table 5: Contrast of Psychological Status Before and After Stroke Sufferer Therapy (n=100)
Table 6, illustrates the comparison of limb motor function and nerve function among stroke sufferers before and after therapy intervention. The assessments were conducted using components related to body motor function and neural function. Before the intervention, stroke sufferers exhibited impaired limb motor function, as evidenced by a mean score of 25.63±3.19, and compromised nerve function, with a mean score of 14.26±2.18. Following the therapy intervention, significant improvements were observed in both motor and nerve function. The mean score for body motor function increased substantially to 40.26±5.78, indicating enhanced motor capabilities. Similarly, the mean score for neural function decreased to 7.06±1.33, reflecting improved nerve functionality. Statistical analysis using the t-test demonstrated highly significant differences between pre and post-intervention scores for both body motor function (t=-22.160, p<0.001) and neural function (t=28.195, p<0.001). These findings underscore the effectiveness of the therapy intervention in promoting motor and nerve recovery among stroke sufferers, highlighting its potential role in facilitating rehabilitation and functional restoration post-stroke.
| Time point | Body motor function (component) | Neural function (component) |
|---|---|---|
| Before the intervention | 25.63±3.19 | 14.26±2.18 |
| After the intervention | 40.26±5.78 | 7.06±1.33 |
| t | -22.160 | 28.195 |
| p | <0.001 | <0.001 |
Table 6: Contrast of Limb Motor Function and Nerve Function Before and After Therapy of Stroke Sufferers (n=100)
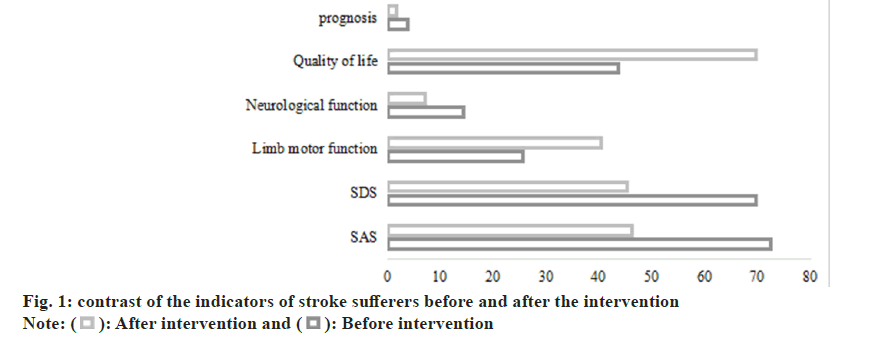
Table 7 and fig. 1, presents a comparison of the quality of life and prognosis among stroke sufferers before and after therapy intervention. Quality of life was assessed using sub-components, while prognosis was evaluated based on specific parameters. Before the intervention, stroke sufferers reported a compromised quality of life, with a mean score of 43.62±10.81, indicating a diminished overall well- being. Additionally, the prognosis was relatively poor, as evidenced by a mean score of 3.83±1.02. Following the therapy intervention, notable improvements were observed in both quality of life and prognosis. The mean score for quality of life significantly increased to 69.55±7.93, reflecting enhanced overall well-being and life satisfaction. Similarly, the prognosis substantially improved, with the mean score decreasing to 1.71±0.59, indicative of a more favorable outlook for recovery and health outcomes. Statistical analysis using the t-test revealed highly significant differences between pre and post- intervention scores for both quality of life (t=-19.341, p<0.001) and prognosis (t=17.991, p<0.001). These findings underscore the positive impact of therapy intervention on enhancing the quality of life and prognosis of stroke sufferers, highlighting its crucial role in promoting holistic recovery and long-term well-being post-stroke.
| Time point | Quality of life (sub) | Prognosis (component) |
|---|---|---|
| Before the intervention | 43.62±10.81 | 3.83±1.02 |
| After the intervention | 69.55±7.93 | 1.71±0.59 |
| t | -19.341 | 17.991 |
| p | <0.001 | <0.001 |
Table 7: Contrast of Quality of Life and Prognosis Before and After Therapy of Stroke Sufferers (n=100)
In the clinical aspect, cerebrovascular circuitous is a common neurological ailment, usually manifested with abnormal vascular shape, including vessel length and dilation[10]. From the current situation, there is a great controversy whether cerebrovascular detour is related to the occurrence of ischemic stroke. Cerebrovascular detour will affect the blood supply to the brain of sufferers. If sufferers have insufficient brain blood supply for a long time, it is likely to lead to ischemic stroke[11]. According to the expert test, vertebral artery detour is the highest incidence of cerebrovascular detour site, located in the middle of the brain. Many medical experts believe that the incidence of cerebral ischemia in sufferers with cerebral artery circuitous sufferers is higher than that of non-circuitous sufferers, and some medical experts believe that the incidence of ischemia in circuitous sufferers is not much different from that of non-circuitous sufferers[12]. Theoretically, the mechanism of cerebrovascular circuitous-induced ischemic stroke is not fully defined. According to the study of the relevant departments, the blood flow of sufferers with cerebrovascular detour is far lower than that of normal sufferers, which is found to detect the terminal pressure of the vertebral artery[13]. After the intraoperative opening of the sufferer’s skull, the lengthening of the abnormal terminal pressure of the vertebral artery can be detected, and the terminal pressure of the vertebral artery is far lower than the normal blood flow pressure of the human body. Therefore, we can think that this may have some relationship to the occurrence of ischemic stroke. However, the current medical experts have proved that the vertebrobasilar artery detour may be a risk factor for stroke in the posterior circulation, so this is the direction of our future examination to analyze the influence of different vascular detour sites on the incidence of stroke. This study found that in the ischemic stroke set incidence of cerebrovascular circuitous set it was 60.00 %, which was notoriously higher than that in the set without ischemic stroke 41.82 %. Moreover, the results of logistic regression model analysis showed that cerebrovascular detour, hypertension and hyperlipidemia were the occurrence of ischemic stroke the risk factors (p<0.05); because the cerebrovascular circuitous is a common abnormal vascular morphology phenomenon in the department of neurology, it is too long, dilated, resulting in distortion, circle, loop and other abnormalities, thus affecting the cerebral blood flow supply, resulting in insufficient cerebral blood supply, severe cases can be accompanied by the occurrence of ischemic stroke. Some studies have pointed out that cerebrovascular circuitous includes single arc, multiple arc, folding and loops. However, in this study, the correlation between morphological changes and ailment occurrence was not explored, so in future studies, the sample size should be further expanded to analyze the correlation of cerebrovascular circuitous morphology and ischemic stroke occurrence[14,15].
In addition, this study through the whole ministration intervention, found that stroke sufferers psychological state scores are enhanced, stroke sufferers body movement function and quality of life score is notoriously enhanced, neurological function and prognosis score is notoriously reduced (p<0.05), the reason is mainly because endovascular interventional therapy care is the vital signs, diet, medication, basic care for sufferers in hospital, aimed to ensure sufferer’s life safety, promote ailment recovery, but poor mental health intervention and routine care intervention is not comprehensive. The whole-process care model is based on sufferer health, including physical, psychological and other aspects. Comprehensive, full-process care from admission to 6 mo after discharge, including psychological intervention, ailment knowledge of different periods, matters needing attention, self- care methods, medication, diet, exercise and other aspects of the explanation, and make an appropriate and timely health guidance plan according to the sufferer’s recovery situation, to guide and supervise a new ministration model of sufferers according to the plan, designed to promote sufferer health behaviors, correct your living habits, increase the mastery of ailment knowledge, enhance the nerve and limb motor function of sufferers, to enhance the prognosis, reduce the occurrence of adverse events, thus improving the sufferer’s quality of life and therapy compliance. Therefore, we combined with the concept of accelerated rehabilitation and after careful examination and evaluation; we carried out the effect study of the rehabilitation training immediately after endovascular interventional surgery in sufferers with acute ischemic stroke. First of all, this study shows that interventional immediate postoperative rehabilitation training can be through earlier stimulation of motor neurons adjust their excitability, thus effectively enhance the limb movement function and daily activity ability, at the same time can enhance sufferers anxiety or depression, that the level of the ability of life directly affect the confidence of sufferers to return to society, sufferer’s happiness and recognition. Secondly, immediate rehabilitation training can reduce sufferer’s psychological stress and physiological stress, reduce their hospital stay, reduce medical costs, avoid excessive medical therapy, and reduce the family and social burden[16,17].
Based on DSA examination, individuals with ischemic cerebrovascular disease are at a heightened risk of ischemic stroke. Therefore, implementing reasonable and effective nursing measures is essential to improve mood, enhance limb movement function, and ultimately enhance the quality of life and prognosis for these patients.
Data availability:
The simulation experiment data used to support the findings of this study are available from the corresponding author upon request.
Author’s contributions:
Nan Cao and Jie Xu contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Wang C, Dong J, Sun J. Silence of lncRNA XIST impairs angiogenesis and aggravates cerebrovascular damage after ischemic stroke. Mol Ther Nucl Acids 2021;26:148-160.
- Jin G, Zhan QL, Chen L. Correlation between carotid artery curvature and cerebral artery stenosis and cerebral ischemic events. J Reg Anat Surg Surg 2019;28(2):125-8.
- Wang Y, Zhang Z, Mei Y. Systematic evaluation of qualitative research on disease experience of patients with urinary incontinence after stroke. Chin J Nur 2020;55(6):932-6
- Wang B, Liu D, Gong Y. Effect of different antiplatelet regimens on complications related to digital subtraction angiography in patients with ischemic cerebrovascular disease. Shenzhen J Integr Tradit Western Med 2017;27(7):54-5
- Fan Z, Li. The correlation between speckled calcium in carotid computed tomography angiography and increased risk of ischemic stroke. Stroke 2019.
- Wang S. Analysis of clinical characteristics of patients with ischemic cerebrovascular disease without vascular occlusion by digital subtraction angiography. Grass Roots Med Forum 2017;21(4):460-1
- Cheng X. Application of digital subtraction angiography in interventional examination and treatment of acute ischemic cerebrovascular disease. Chin Med Guide 2017;15(28):120-1
- Yang H, Zhang S, Yu F. The value of digital subtraction angiography in the diagnosis of ischemic cerebrovascular disease and evaluation of interventional treatment effect. J Cardiovasc Rehab Med 2017;26(6):640-4
- Zhang B, Wang J, Yang B. Effect of digital subtraction angiography in interventional treatment of cervical vascular stenosis in patients with ischemic stroke. Imaging Res Med Appl 2019;3(20):192-3
- Tang X, Liu J, Gong S. Interpretation of expert consensus on perioperative nursing of patients with acute ischemic stroke treated by intravascular intervention. Nurs Res 2022;12:36
- Jian J, Yang J, Zhu L. Application of cluster nursing in patients with acute ischemic stroke after intervention. Chin J Mod Nurs 2018;24(14):1649-52
- Huang X, Huang T, Li N. The effect of endovascular stent angioplasty on neurological function and prognosis in elderly patients with ischemic stroke. J Air Force Med 2019;35(6):499-502
- Li S, Wang L. Effect of serum globulin on inflammatory factors and neurohormones in patients with acute ischemic stroke after neurointervention. Lab Med Clinic Med 2021;18(20):2994-3001
- Siegler JE, Zha AM, Czap AL. The impact of the novel coronavirus epidemic on the treatment time of acute ischemic stroke: mUlti center cooperation of the society of vascular and interventional neurology. Stroke 2020;52(1):337-42.
- Chalos V, Ende N, Lingsma HF. National Institutes of Health Stroke Scale: Alternative main outcome indicators of acute treatment trial for ischemic stroke. Stroke 2019;51(1):282-90.
- Hofmeister J, Bernava G, Rosi A. Clone based radiology predicts successful reanalysis of mechanical thrombectomy strategies in acute ischemic stroke. Stroke 2020;51(8):2488-94.
- Ptaszkowska L, Ptaszgowski K, Halski T. Immediate effects of respiratory stimulation on ventilation parameters in survivors of ischemic stroke: A randomized intervention study. Medicine 2019;98(38):e17128.
 After intervention and
After intervention and  Before intervention
Before intervention



