- Corresponding Author:
- M. V. Kulkarni
P. G. Department of Chemistry, Karnatak University, Dharwad - 580 003, 1S. C. S. College of Pharmacy, Harapanahalli - 583 131, India
E-mail: manohar274@gmail.com
| Date of Submission | 16-Nov-2009 |
| Date of Revision | 27-Oct-2010 |
| Date of Acceptance | 19-Jan-2011 |
| Indian J Pharm Sci, 2011, 73 (1): 88-92 |
Abstract
Coumarin-4-acetic acids have been synthesized from various phenols and citric acid under Pechmann cyclisation conditions. All the compounds have been evaluated for antiinflammatory and analgesic activity in acute models. Compounds have also been evaluated for their ulcerogenic potential. Using the computer program, prediction of activity spectra for substances, prediction results and their Pharma Expert software, we have found a correlation between the observed and predicted antiinflammatory activity.
KeyWords
Antiinflammatory, analgesic, coumarin, computer-aided prediction, prediction of activity spectra for substances, ulcerogenic
Introduction
Coumarins constitute a family of naturally occurring lactones with a potential for a range of biological activities [1,2]. Substituents at C-4 position on the coumarin ring like hydroxy, aminomethyl, and arylaminomethyl have resulted in compounds with anticoagulant [3], CNS depressant [4-6], and antimicrobial [7-9] activities respectively. Parent coumarin and its metabolite 7-hydroxycoumarin (umbelliferone) were both found to inhibit the carrageenan induced edema in rats [10]. The role of hydroxyl group in arachidonic acid metabolism has been investigated which showed that they were moderate inhibitors of 5-HETE formation. This is further supported by a later observation that phenolic coumarins possess remarkable ability to scavenge peroxyl radicals [11]. During our work on 4-substituted coumarins [12,13] we have found that incorporation of biocompatible fragments like vanillin [14] and paracetamol [15] at the allylic position with respect to the biogenetically important C3-C4 double bond leads to compounds with potential antiinflammatory activity. Clinically accepted antiinflammatory drugs like indomethacin, naproxen and ibuprofen possess −CH(R)-COOH group (R= alkyl or aryl) as a pharmacophore.
In view of the above cited inflammation inhibition property associated with 4-substituted coumarins and the importance of −CH(R)-COOH group in antiinflammatory drugs, it was thought of substantial intellectual appeal to screen some coumarin-4- acetic acids for their antiinflammatory property. The prediction of activity spectra for substances (PASS) program i.e., computer-added prediction of biological activities associated with organic structures [16-22] has been applied to thiazole derivatives [23]. The present paper demonstrates the utility of the PASS program and makes a clear comparison of the predicted and the observed pharmacological properties of some coumarin-4-acetic acids.
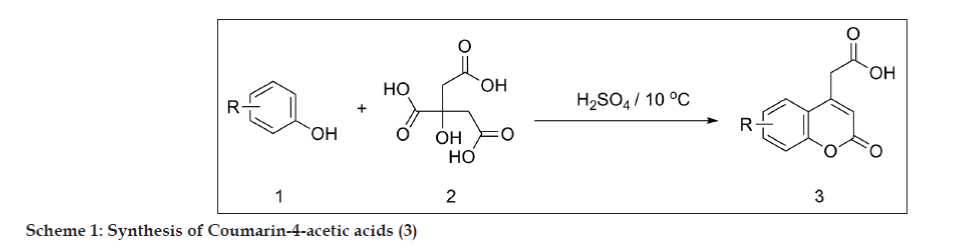
All the five substituted coumarin-4-acetic acids (3a-e) were synthesized according to the procedure reported in the literature [24]. Accordingly, a mixture of citric acid 2 (1 mol) and conc. sulphuric acid (32 ml) was stirred for 30 min, then the temperature was slowly raised during an interval of 10-15 min and as soon as the evolution of gas slackened, the flask was removed from the bath, allowed to stand for 15 min till the reaction mixture became clear and free from carbon monoxide bubbles; this was then cooled to 10°. To this solution, substituted phenol 1 (1 mol) was added at 10o, drop wise. After the addition of phenol, the reaction mixture was stirred at room temperature for 48 h. The reaction mixture was then poured onto crushed ice; the separated solid was filtered and dissolved in saturated sodium bicarbonate solution, which on acidification gave the title compounds 3 (a-e) (yield 57-73%) (Scheme 1).
Albino Wistar rats of either sex weighing 150-200 g were used. Animals were housed in groups of six per cage at a temperature of 25±1° and relative humidity of 45±5%. A 12:12 h light:dark cycle was followed during the experiments. Animals had free access to food and water, however, food was withdrawn six hours before and during the experiments. The animals were obtained from the Central Animal House of S. C. S. College of Pharmacy, Harapanahalli (India). The Institutional Animal Ethical Committee approved the protocol of the study.
The acute toxicity study was done as per the OECD guidelines (407). The compounds 3a-e were administered orally in different doses, the rats were continuously observed for 8 h for any signs of acute toxicity such as increased-decreased motor activity, ataxia, tremors, convulsions, sedation, lacrimation, etc. After 24 h the rats were sacrificed, stomach, intestine, and liver were inspected under the magnifying lenses for any ulcerhaemorrhaegic spots. The doses of the test compounds were fixed on the basis of their acute toxicity as 30 mg/kg and 100 mg/kg for evaluation (Table 1).
| Compound | R | LD50 | Screening dose |
|---|---|---|---|
| (mg/kg) | (mg/kg) | ||
| 3a | 6-CH3 | 1000 | 100 |
| 3b | 7-CH3 | 300 | 30 |
| 3c | 7,8-Benzo | 300 | 30 |
| 3d | 7-OH | 1000 | 100 |
| 3e | 7-OCH3 | 300 | 30 |
Table 1: Acute toxicity data of the compounds 3a-e
The antiinflammatory activity was studied using carrageenin-induced rat paw edema method [25]. All the test compounds 3a-e were administered in two doses 30 mg/kg and 100 mg/kg body weight based on their acute toxicity studies and the standards used for the present antiinflammatory activity testing are diclofenac sodium and indomethacin. The test compounds were administered orally to the rats suspended in 0.5% carboxymethylcellulose (CMC). The control animals received 0.5% CMC. Thirty minutes after drug administration, 0.1 ml of 1% carrageenan (Sigma) in normal saline solution was injected into subplantar region of one of the hind paws. The paw edema volume was recorded using a plethysmometer (UGO Basile, Italy) at different time intervals. The percentage inhibition of inflammation was calculated by applying Newbould formula. The results were found to be statistically significant against control at P< 0.001 by applying Scheffe′s Post Hoc method (Table 2).
| Compound | R | Dose | Mean paw volume at different | Percentage inhibition of | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg / kg) | time intervals (ml) | edema volume (ml) | ||||||||
| 0 h | 1st h | 2nd h | 3rd h | 1st h % | 2nd h % | 3rd h % | ||||
| Control | -- | -- | 1.05±0.01 | 1.33±0.02 | 1.65±0.02 | 2.06±0.03 | -- | -- | -- | |
| Diclofenac sodium | -- | 100 | 0.85±0.04 | 0.66±0.08 | 0.60±0.03 | 0.58±0.02 | (50.37) | (63.63) | (71.84) | |
| Indomethacin | -- | 5 | 0.69±0.07 | 0.79±0.03 | 0.62±0.06 | 0.52±0.03 | (40.60) | (62.42) | (74.75) | |
| 3a | 6-CH3 | 100 | 0.76±0.03 | 1.10±0.01 | 1.13±0.05 | 0.96±0.03 | (17.29) | (31.51) | (53.39) | |
| 3b | 7-CH3 | 30 | 0.77±0.04 | 1.11±0.05 | 1.20±0.04 | 1.0±0.01 | (16.54) | (27.27) | (51.45) | |
| 3c | 7,8-Benzo | 30 | 0.86±0.03 | 1.20±0.08 | 1.13±0.08 | 0.96±0.03 | (9.77) | (31.51) | (53.29) | |
| 3d | 7-OH | 100 | 0.70±0.06 | 1.23±0.03 | 1.06±0.05 | 1.03±0.02 | (7.57) | (35.75) | (50.00) | |
| 3e | 7-OCH3 | 30 | 0.96±0.05 | 1.20±0.05 | 1.16±0.04 | 0.96±0.02 | (9.77) | (29.69) | (53.39) | |
Table 2: Antiinflammatory activity data of compounds 3a-e
The analgesic activity [26] was determined in vivo by using abdominal constriction test induced by acetic acid 0.6% (0.1 ml/10 g) in mice. Albino mice of both the sexes (18-22 g) were used. Compounds were administered orally (30 mg/kg) and (100 mg/kg) as a suspension in 5% carbethoxymethylcellulose (vehicle). Diclofenac sodium (20 mg/kg) and aspirin (100 mg/kg) were used as the standard drugs under same conditions. Acetic acid solution was administered i.p. 1 h after administration of the test compounds. Ten minutes after the i.p. injection of the acetic acid solution, the number of constrictions per animal was recorded for 20 min. Control animals received on equal volume of vehicle. Analgesic activity was expressed as percentage of inhibition of constrictions when compared with the vehicle control group (Table 3).
| Compound | R | Dose (mg/kg) | Mean writhing (±SEM) | % Analgesic activity |
|---|---|---|---|---|
| Control (vehicle) | -- | -- | 29.2±2.50 | --- |
| Standard (Diclofenac sodium, 20 mg/kg, po) | -- | 20 mg/kg, po | 7.23±.20 | 75.23 |
| Standard (Aspirin, 100 mg/kg, po) | -- | 100 mg/kg, po | 7.87±1.10 | 73.28 |
| 3a | 6-CH3 | 100 | 11.25±1.31 | 61.14 |
| 3b | 7-CH3 | 30 | 17.25±1.90 | 40.92 |
| 3c | 7,8-Benzo | 30 | 7.25±1.25 | 75.17 |
| 3d | 7-OH | 100 | 2.25±0.64 | 92.29 |
| 3e | 7-OCH3 | 30 | 7.26±1.12 | 75.17 |
Table 3: Analgesic activity of compounds 3a-e
The ulcerogenic activity was measured as followed by method of DiJoseph et al. [27]. Male Wistar rats were fasted for 36 h. standard suspended in 1% CMC was given orally at a dose level of 20 mg/kg (2′10 mg/kg) body weight and the test compounds were administered twice at 2 h interval at a dose level of 400 mg/kg. Four hours later animals were sacrificed and stomach was examined for lesions. The ulcer index [27] of the test compounds showed no harmful effects on the stomach, at the dose of 400 mg/kg p.o., in fasted rats. Standard, at lower doses produced serious gastric ulcers in all animals (Table 4).
| Compound | R | Mean ulcer index (±SEM) | % Protection |
|---|---|---|---|
| Control (2% w/v gum acasia) | -- | 4.40±0.40 | -- |
| Standard (rantidine, 50 mg/kg, po) | -- | 0.20±0.12 | 95.45 |
| Standard (omeprazole, 4 mg/kg, po) | -- | 0.171±0.02 | 96.11 |
| 3c | 7,8-Benzo | 1.50±0.38 | 65.90** |
| 3d | 7-OH | 2.00±0.47 | 54.54** |
| The values are mean±SEM, N=5, **P< 0.01 Vs control |
Table 4: Effect of compounds 3c and 3d on aspirin induced ulcer model
The analysis of biological activity spectra prediction for the synthesized compounds 3 (a-e) made in this publication is a good example of study of chemical compounds before their experimental investigations using the freely available internet version of PASS and PharmaExpert: http://www.ibmc.msk.ru/PASS.
A biological activity spectrum for a substance is a list of biological activity types for which the probability to be revealed (Pa) and the probability not to be revealed (Pi) are calculated. Pa and Pi values are independent and their values vary from 0 to 1. Biological activity spectra were predicted for all five synthesized structures 3a-e with PASS 2005 version [16]. The result of prediction is valuable at planning of the experiment, but one should take into account some additional factors: Particular interest to some kinds of activity, desirable novelty of a substance, available facilities for experimental testing. Actually, each choice is always the compromise between the desirable novelty of studied substance and risk to obtain the negative result in testing. The more is Pa value, the less is the probability of false positives in the set of compounds selected for biological testing. For example, if one selects for testing only compounds for which a particular activity is predicted with Pa≥0.9, the expected probability to find inactive compounds in the selected set is very low, but about 90% of active compounds are missed. If only compounds with Pa ≥ 0.8 are chosen, the probability to find inactive compounds is also low, but about 80% of active compounds are missed etc. By default, in PASS Pa= Pi value is chosen as a threshold, therefore all compounds with Pa>Pi are suggested to be active. Another criterion for selection is the compounds′ novelty. If Pa value is high, sometimes one may find close analogues of known biologically active substances among the tested compounds. For example, if Pa>0.7 the chance to find the activity in experiment is high, but in some cases the compound may occur to be the close analogue of known pharmaceutical agents. If 0.5<Pa<0.7 the chance to find the activity in experiment is less, but the compound is not so similar to known pharmaceutical agents. If Pa<0.5 the chance to find the activity in experiment is even more less, but if it will be confirmed the compound might occur to be a new chemical entity.
The effect of coumarin-4-acetic acids on the carrageenan-induced paw edema method is mentioned in Table 2. All the compounds showed a slow onset of action during the 1st h, which was found to increase rapidly during the 2nd h and reached maximum level at the end of the 3rd h. The 7-methyl and 7-methoxy derivatives 3b and 3e were found to be active at a dose level of 30 mg/kg showing greater than 50% inhibition of inflammation. Further these compounds are able to provide significant protection against the writhing induced by acetic acid. The values are comparable with aspirin in the case of 7,8-benzo and 7-methoxy derivates 3c and 3e. The best results were observed in the case of 7-hydroxy derivative 3d (Table 3). The ulcer index of the test compounds showed no harmful effects on the stomach, at the dose of 400 mg kg-1 p.o., in fasted rats. Indomethacin, at lower doses produced serious gastric ulcers in all animals. Amongst the five compounds tested for their ulcerogenic potential the 7,8-benzo and 7-hydroxy derivatives were able to prevent the formation of aspirin-induced ulcers to a considerable degree (Table 4). Structural formulae of the presently screened coumarin-4-acetic acids and structures of the standards have been used as the input data and the PASS program has been used to predict their biological activity. The predicted activity data pertinent to the screening carried out has been presented in Table 5. It can be seen that the PASS activity indicated by the Pa values is quite high with respect to the inactivity Pi values. According to this analysis compounds 3c and 3e are expected to exhibit good inhibition of inflammation, which has been actually verified by the screening data given in Table 2. Highest analgesic activity is predicted for the 7-hydroxy derivative 3d, which has been confirmed by our preliminary results in Table 3. An interesting point observed is that the predicted toxicity Pa indices for all the compounds are less than the Pa indices for antiinflammatory activity (Table 5).
| Compound | R | Antiinflammatory | Analgesic | Ulcerogenic | Toxicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | |||||||
| 3a | 6-CH3 | 0,665 | 0,016 | 0,324 | 0,125 | 0,634 | 0,008 | 0,356 | 0,159 | |||||
| 3b | 7-CH3 | 0,667 | 0,015 | 0,353 | 0,108 | 0,609 | 0,008 | 0,371 | 0,147 | |||||
| 3c | 7,8-Benzo | 0,669 | 0,015 | Nil | 0,626 | 0,008 | 0,414 | 0,115 | ||||||
| 3d | 7-OH | 0,663 | 0,016 | 0,385 | 0,090 | 0,649 | 0,007 | 0,386 | 0,136 | |||||
| 3e | 7-OCH3 | 0,672 | 0,015 | 0,326 | 0,123 | 0,741 | 0,006 | 0,330 | 0,183 | |||||
| Diclofenac sodium | -- | 0,550 | 0,049 | 0,595 | 0,025 | 0,353 | 0,070 | 0,550 | 0,064 | |||||
| Indomethacin | -- | 0,793 | 0,010 | 0,662 | 0,018 | Nil | Nil | |||||||
| Aspirin | -- | 0,673 | 0,023 | 0,554 | 0,033 | 0,431 | 0,036 | 0,625 | 0,047 | |||||
Table 5: Pass results of compounds 3 (a-e)
In conclusion coumarin-4-acetic acids tested in this study have shown interesting antiinflammatory activities and also possess significant analgesic activity, which is sensitive to the substituents at C-7.
Acknowledgements
The authors (RNR) are grateful to the Principal Pharmacy College, Harpanahalli, and (MB and VBJ) to the Karnatak University, Dharwad for providing laboratory facilities and University Research Studentship
References
- Kulkarni MV, Kulkarni GM, Lin CH, Sun CM. Recent advances in coumarins and 1-azacoumarins as versatile biodynamic agents. Curr Med Chem 2006;13:2795-818.
- Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/ antioxidant activities. Curr Pharm Design 2004;10:3813-33.
- Schimanski CC, Burg J, Mohler M, Hohler T, Kanzler S, Otto G, et al. Phenprocoumon-induced liver disease ranges from mild acute hepatitis to (sub-) acute liver failure. J Hepatal 2004;41:67-74.
- Shieh CC, Coghlan M, Sullivan JP, Gopalkrishnan M. Potassium channels: Molecular defects, diseases, and therapeutic opportunities. Pharmacol Rev 2000;52:557-93.
- Sah P. Ca2+-activated K+ currents in neurons: Types, physiological roles and modulation. Trends Neurosci 1996;19:150-4.
- Hewawasam P, Fan W, Knipe J, Moon SL, Boissard CG, Gribkoff VK, et al. The synthesis and structure-activity relationships of 4-aryl-3-aminoquinolin-2-ones: A new class of calcium-dependent, large conductance, potassium (maxi-K) channel openers targeted for post-stroke neuroprotection. Bioorg Med ChemLett 2002;12:1779-83.
- Kulkarni MV, Patil VD. Studies on coumarins I. Arch Pharm (Weinheim) 1981;314:708-11.
- Hanamanthgad SS, Kulkarni MV, Patil VD. Synthesis of some biomimetic 4-substituted coumarins Rev Roum De Chim 1985;30:735-41.
- Adavi HH, Kusanur RA, Kulkarni MV. alpha.-Cleavage during the oxidation of benzazolyl 4-coumarinylmethyl sulfides. J Indian ChemSoc 2004;81:981-5.
- Leal LK, Matos ME, Ribeiro RA, Ferreira FV, Vianna GS. Antinociceptive and antiedematogenic effects of the hydroalcoholic extract and coumarin from Torreseacearensis. Phytomedicine 1997;4:221-7.
- Paya M, Halliwell B, Hoult JR. Interactions of a series of coumarins with reactive oxygen species: Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. BiochemPharmacol 1992;44:205-14.
- Basanagouda M, Kulkarni MV, Sharma D, Gupta VK, Pranesha, Sandhyarani P, et al. Synthesis of some new 4-aryloxymethylcoumarins and examination of their antibacterial and antifungal activities. J ChemSci 2009;121:485-95.
- Basanagouda M, Shivashankar K, Kulkarni MV, Rasal VP, Patel H, Mutha SS, et al. Synthesis and antimicrobial studies on novel sulfonamides containing 4-azidomethyl coumarin. Eur J Med Chem 2010;45:1151-7.
- Ghate MD, Kulkarni MV, Shobha R, Kattimani SY. Synthesis of vanillin ethers from 4-(bromomethyl) coumarins as anti-inflammatory agents. Eur J Med Chem 2003;38:297-302.
- Shastri LA, Ghate MD, Kulkarni MV. Dual fluorescence and biological evaluation of paracetamol ethers from 4-bromomethylcoumarins. Indian J Chem 2004;43B:2416-22.
- Available from: http://www.ibmc.msk.ru/PASS [Last accessed on 2011 Jan 04].
- Geronikaki AA, Lagunin AA, Hadjipavlou-Litina DI, Eleftheriou PT, Filimonov DA, Poroikov VV, et al. Computer-aided discovery of anti-inflammatory thiazolidinones with dual cyclooxygenase/lipoxygenase inhibition. J Med Chem 2008;51:1601-9.
- Mittal M, Goel RK, Bhargava G, Mahajan MP. PASS-assisted exploration of antidepressant activity of 1,3,4-trisubstituted-b-lactam derivatives. Bioorg Med ChemLett 2008;18:5347-9.
- Geronikaki A, Druzhilovsky D, Zakharov A, Poroikov V. Computer-aided prediction for medicinal chemistry via the internet. SAR QSAR Environ Res 2008;19:27-38.
- Poroikov VV, Filimonov DA, Ihlenfeldt WD, Gloriozova TA, Lagunin AA, Borodina YV, et al. PASS biological activity spectrum predictions in the enhanced open NCI database browser. J ChemInfComput Sci 2003;43:228-36.
- Sadym A, Lagunin A, Filimonov D, Poroikov V. Prediction of biological activity spectra via the internet. SAR QSAR Environ Res 2003;14:339-47.
- Poroikov VV, Filimonov DA. How to acquire new biological activities in old compounds by computer prediction. J Comput Aided Mol Des 2002;16:819-24.
- Geronikaki A, Lagunin A, Poroikov V, Filimonov D, Hadjipavlou-litina D, Vicini P. Computer aided prediction of biological activity spectra: Evaluating versus known and predicting of new activities for thiazole derivatives. SAR QSAR Environ Res 2002;13:457-71.
- Dey BB, Row KK. The reactivity of the methylene group in coumarin-4-acetic acids and their esters. Condensation with salicylaldehyde to 4:3'-dicoumaryls. J Indian ChemSoc 1924;1:107-22
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. ProcSocExpBiol Med 1962;111:544-8.
- Koster R, Anderson M, De Beer EJ. Acetic acid for the analgesic screening. Fed Proc 1959;18:412-6.
- DiJoseph JF, Eash JR, Mir GN. Gastric antisecretory and antiulcer effects of WHR1582A, a compound exerting alpha-2-adrenoceptor agonist activity. J Pharmacol Exp Ther 1987; 241:97-102.