- *Corresponding Author:
- T. K. Giri
NSHM Knowledge Campus, Kolkata-Group of Institutions, Behala, Kolkata, West Bengal 700053, India
E-mail: tapan_ju01@rediffmail.com
| Date of Received | 26 October 2021 |
| Date of Revision | 08 September 2022 |
| Date of Acceptance | 02 May 2023 |
| Indian J Pharm Sci 2023;85(3):581-590 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Quick removal and low therapeutic efficacy are drawbacks of ocular eye drops. In situ gels were developed using gellan gum and/or sodium carboxymethyl cellulose as an ion activated polymer to enhance the residence time of the drug on the ocular surface and improve the efficacy through the sustained release of acyclovir. The solution containing polymer converted to gel through cross-linking reaction with Ca++ ion present in simulated tear fluid. The optimized formulation (F1 GC: 0.5 % gellan gum+0.5 % carboxymethyl cellulose) showed immediate gelation (within 5 s) that remained for more than 6 h and may prevent drug elimination by tears. The developed formulations were clear and in the pH range of 6.05 to 7.84. The interaction between the carboxyl group of polymers and Ca++ ions was confirmed by Fourier transform infrared spectroscopy. All the formulations showed satisfactory drug content from 91.13-100.61 %. The gel strength of the formulations increased as the concentration of the polymers increased. The prepared in situ gels exhibited a shear thinning pseudoplastic behaviour that may help in the blinking of the eye. Optimized formulation releases the drug for over 8 h and shows the Fickian diffusion release mechanism. The developed formulation may improve the bioavailability of the drug through longer contact time and controlled drug release in the eye.
Keywords
Anionic polysaccharides, ion sensitive, ocular delivery, in situ gel, acyclovir, sustained release
Herpes eye infection is the most common and severe type caused by a virus called type 1 herpes simplex[1-2]. The infection is transferred from one person to another through skin to skin contact. The incidence of ocular herpes infection is estimated at 21 to 31 per 100 000 people per year[3-4] and worldwide up to 10 million people are thought to have a history of ocular herpes infection. The main impact on vision is through corneal scarring, thinning and neovascularization, particularly through recurrent attacks[5]. The drug of choice for the treatment of herpes infections is acyclovir[6]. Acyclovir (fig. 1) is a purine nucleoside with the molecular formula C8H11N5O3. The herpes virus encoded enzymes transform acyclovir into acyclovir triphosphate and stop the replication of viral deoxyribonucleic acid. The existing treatment modality using acyclovir for ocular herpes infections is an ointment[7]. These dosage forms require 4-5 times administration due to the short half-life (2-3 h) and the polar nature of the drug[8]. Moreover, a larger quantity of the drug was lost owing to nasolacrimal drainage. The concentration of the drug on the intraocular surface is low and exhibits low bioavailability and ineffective therapy[9,10].
Numerous research works have been carried out to enhance the ocular bioavailability of acyclovir. A nanodosage form of poly-D, L-lactic acid loaded with acyclovir was prepared for ocular delivery of acyclovir[11]. The developed dosage form exhibited controlled release with enhanced aqueous humor concentration and a favorable pharmacokinetic profile of the drug. Polyethyl-2-cyanoacrylate nanoparticles containing acyclovir were prepared and coated with polyethylene glycol[12]. Acyclovir-loaded polyethylene glycol-coated polyethyl-2-cyanoacrylate nanoparticles were compared with drug solution for aqueous humor drug concentration. The developed nanoparticles exhibited a 25-fold enhancement in drug concentration in comparison to the drug solution.
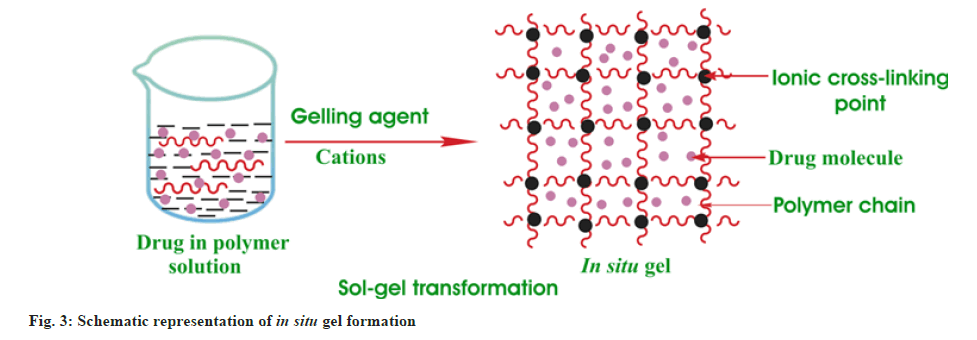
Over the past two decades, the main focus of research has been the development of ocular in situ gel for the delivery of drugs[13-15]. In situ gel offers several advantages such as ease of administration and the fact that it does not obstruct vision compared to ointments. Moreover, in situ gel resides for several hours on the ocular surface. In situ gel formulation is present in a viscous solution and administered into the eye as drops. After administration into the eye, the solution is converted into gel through different responses including temperature, ionic strength and pH on the ocular surface[16]. The formed gel extends the contact time and increases the bioavailability. It was observed by a preclinical study that the in situ gel formulations improve the treatment of ocular diseases including keratitis or conjunctivitis, glaucoma, uveitis, dry eye and cataracts[17-23]. Few in situ gel formulations are in clinical trials or available in the market for conjunctivitis and glaucoma.
The objective of the study was to develop an ion sensitive in situ gel for sustained ocular delivery of acyclovir to treat ocular herpes infection. Gellan gum and/or sodium carboxymethyl cellulose were investigated as a vehicle for the development of an ophthalmic in situ gel formulation of acyclovir. Both sodium carboxymethyl cellulose and gellan gum are anionic polysaccharides and transform into a gel in the presence of cations on the ocular surface[24-26]. A pharmaceutical evaluation of acyclovir in situ gel was conducted subsequently with regard to clarity, gelling capacity, rheological studies, pH, drug content, and in vitro drug-release behavior.
Materials and Methods
Materials:
Acyclovir was procured from East India Pharmaceuticals (Kolkata, India) as a gift sample. Gellan gum was obtained from SRL (Maharashtra, India). Carboxymethyl cellulose was purchased from Balaji drugs Pvt. Ltd. (Kolkata, India). Dialysis membrane-60 with molecular cut-off of 12-14 kDa was procured from Himedia (Mumbai, India). Analytical grade reagents were used in this study.
In situ gel preparation:
Ion sensitive in situ gel was prepared according to the reported method with medication[27]. Weighed amounts of gellan gum and/or sodium carboxy methyl cellulose were dispersed in water. The dispersion was kept at 85-90° for 20 min with stirring. After cooling to room temperature, acyclovir (1 mg/ml) was added and stirred until dissolved. Table 2 represents the composition of the ophthalmic in situ gel formulation. The developed preparations were filled in glass vials (10 ml) and sterilized with 15 psi pressure at 121° for 20 min by autoclaving. Then the preparations were stored in a refrigerator for further use. Fig. 2 represents the solution and in situ gel formulation.
Fourier Transforms Infrared (FTIR) spectroscopy study:
The FTIR study was carried out to examine the interaction between Ca++ and polymers for the formation of in situ gel. The infrared spectra of samples were obtained using FTIR spectroscopy (Alpha-T, Bruker, Germany) in the range between 4000 and 500 cm-1[28,29].
Measurement of rheological properties:
The viscosity of the solutions and in situ gel was determined by a viscometer (Brookfield DV-II) using a no. 61 spindle. Simulated tear fluids were used for the preparation of gellan gum and sodium carboxymethyl cellulose in situ gels. The simulated tear fluids were prepared using 1.4 g potassium chloride, 6.8 g sodium chloride, 2.2 g sodium bicarbonate and 0.084 g calcium chloride dihydrate in 1000 ml water[30]. All examined solutions and in situ gel samples were equilibrated at 34±0.1° prior to each measurement. The spindle was rotated at varying speeds from 10 to 100 rpm. The viscometer displays the viscosity reading.
Drug content determination:
100 µl of the formulation was taken in a volumetric flask (25 ml). Then 25 ml of 0.1(N) NaOH was added to it. The amount of acyclovir was determined using a UV-visible spectrophotometer (UV 1800, Shimadzu, Japan) at 261 nm [31].
pH determination:
The pH value of the solutions and in situ gels were measured by a pH meter (Toshcon Industries Pvt. Ltd., India)[32].
In vitro gelation study:
A gelation study was carried out to investigate the time required to convert gel and gelling capacity. The solution is converted to gel during the gelation process. The in vitro gelation study was performed by diluting polymer solution with simulated tear fluid in the ratio of 25:7 and gel formation was observed visually. The gradation and consistency of the gel are represented in Table 1[33].
| Degree of gelation | Grade |
|---|---|
| Gel not formed | (-) |
| Slow and weakly gel formed | (+) |
| Gel forms quickly and lasts for 2-3 h | (++) |
| Gel forms quickly and lasts more than 6 h | (+++) |
Table 1: Grading of In Situ Gelation
Determination of gel strength:
Gel strength was determined according to the reported method[34]. The 100 ml graduated cylinder (d=3.75 cm) was used for the study. Then 50 g of the polymer solution was added into the cylinder and maintained the temperature at 34°. The gel was formed by the addition of simulated tear fluid. A 27 g disk (d=3.5 cm, thickness=0.3 cm) was placed on the surface of the gel and the time (s) required to sink the disk 5 cm downward was determined.
In vitro release study:
The release of the drug from the developed formulation was determined according to the reported method with modification[35,36]. A formulation containing 1 mg of the drug was taken into a dialysis membrane and hung inside the beaker. Then 50 ml of simulated tear fluid was added to the beaker. The release study was carried out at 50 rpm and 34°. Periodically, 5 ml samples were taken and replace with the same volume of fresh simulated tear fluid. Then the withdrawn sample was filtered and drug content was determined by spectrophotometer at 256 nm.
Results and Discussion
In situ gels were developed using gellan gum and sodium carboxymethyl cellulose as ionic polymers for controlled delivery of acyclovir (fig. 3). Anionic polymers cross-linked with cations present in the ocular fluid and initiated the transition of solution to gel that increases the viscosity[37]. The viscosity of polymers increases with an increase in the cation concentration. An increase in tear production increases the concentration of cations and consequently enhances the viscosity of the gel resulting in improved retention time and enhanced bioavailability.
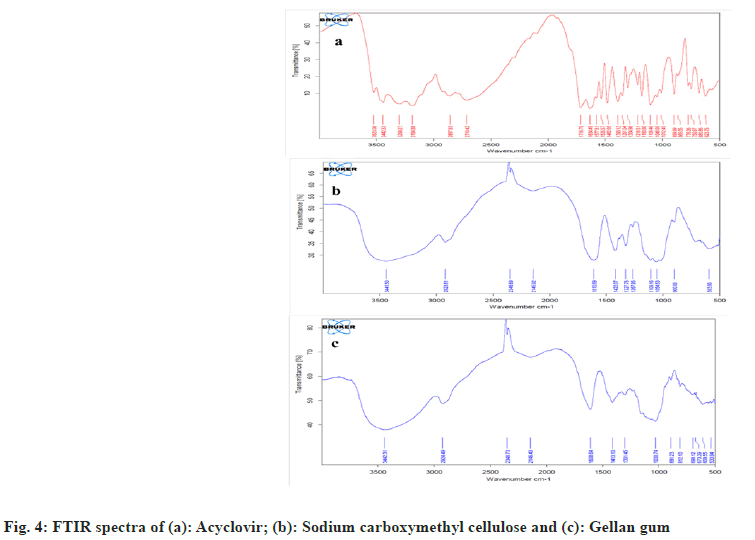
Drug and polymer interactions were characterized through FTIR spectroscopy. The FTIR spectra of acyclovir are represented in fig. 4a and exhibited a peak at 3442 cm-1 due to the stretching of the OH group. The peaks for primary amines are present at 3299 and 3184 cm-1 due to N-H stretching. The stretching vibration peak of the aliphatic C-H functional group was noted at 2857 cm-1. Furthermore, the sharp peak at 1716 cm-1 depicts C=O functional groups. The peak at 1634 cm-1 was for N-H bending. The peaks for stretching vibration of -C=N and -C-N are present at 1482 cm-1 and 1180 cm-1. Carboxymethyl cellulose exhibited a band at 3441 cm–1 owing to the OH stretching frequency (fig. 4b). The peaks for stretching asymmetric and symmetric carboxylate groups are shown at 1610 and 1422 cm−1. The stretching vibration peaks of C-H functional groups were noted at 2923 cm-1. O-H bending vibrations showed at 1327 cm-1 and CH-O-CH2 stretching vibrations showed a peak at 1054 cm-1. Gellan gum showed peaks at 1608 cm–1 for asymmetric stretching and 1413 cm–1 for symmetric stretching of the carboxylate group (fig. 4c). Peak was observed at 3442 cm–1 for the O-H functional group. Peak depicted at 2924 cm–1 owing to stretching –C–H group and at 891 cm−1 due to stretching of –C–O alkyl ether. Similar spectra were observed by other authors[38,39].
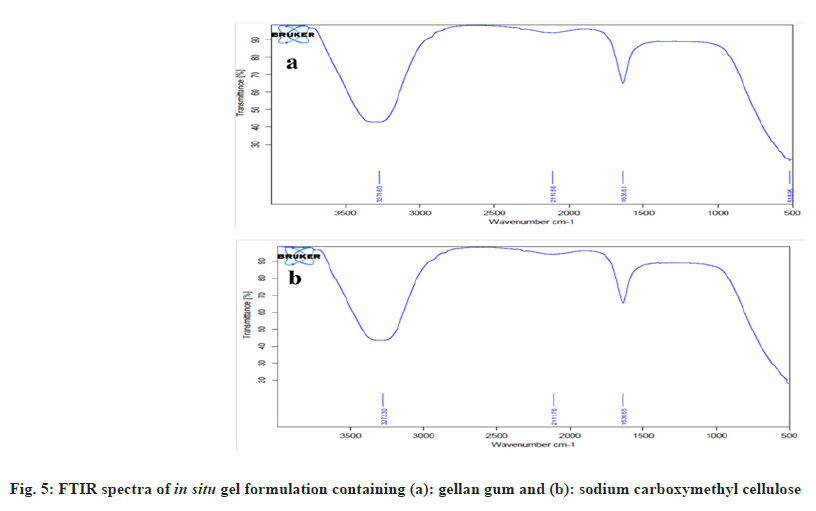
FTIR spectra for in situ gel containing gellan gum and acyclovir were represented in fig. 5a. The peaks of symmetric and asymmetric carboxylate group stretching of gellan gum at 1413 and 1608 cm-1 were shifted and fused with one band at 1636 cm-1 in the formulation of in situ gel. This may be due to the interaction between Ca++ of simulated tear fluid and carboxylic groups in gellan[40].
FTIR spectra for in situ gel containing carboxymethyl cellulose and acyclovir are represented in fig. 5b. The peaks of symmetric and asymmetric carboxylate group stretching of carboxymethyl cellulose at 1422 and 1610 cm-1 were shifted and fused with one band at 1636 cm-1 in the formulation of in situ gel. This may be due to the interaction between Ca++ in simulated tear fluid and the carboxylic groups of sodium carboxymethyl cellulose.
The in situ gel was prepared by using carboxymethyl cellulose and/or gellan gum in different concentrations (Table 2). The developed formulations were clear without any suspended particles. The solution pH was observed in the range of 6.05-7.84 (Table 2) for all the formulations. Tears pH was about 7.4 and varied in the range of 5.2-9.3 in disease conditions[41]. However, the eye can tolerate the formulations without buffering in the range of 3.5-8.5 and after administration tears adjust the physiologic pH[42]. All the formulations showed satisfactory drug content in the range of 91.13±1.51 to 100.61±0.24 % (Table 2).
| Formulation code | Gellan gum (%w/v) | Carboxymethyl cellulose (%w/v) | Appearance | pH (In situ gel solution) | Gelling capacity | Drug content (%) (Mean±SD, n=3) | Gel strength (s) (Mean±SD, n=3) |
|---|---|---|---|---|---|---|---|
| F1 G | 0.2 | - | Transparent solution | 6.05 | (+) | 91.13±1.51 | 1.40±0.08 |
| F2G | 0.3 | - | Transparent solution | 6.11 | (++) | 96.29±3.79 | 2.30±0.02 |
| F3 G | 0.4 | - | Transparent solution | 6.14 | (++) | 97.82±3.99 | 4.25±0.02 |
| F4 G | 0.5 | - | Transparent solution | 6.67 | (+++) | 98.10±0.64 | 12.83±0.40 |
| F5 G | 0.6 | - | Transparent solution | 6.96 | (+++) | 100.05±0.48 | 44.90±1.51 |
| F6 G | 1 | - | Transparent solution | 7.15 | Very stiff gel | 100.61±0.24 | More than 12 h |
| F1 C | - | 0.2 | Transparent solution | 7.22 | (+) | 96.01±0.52 | 1.42±0.07 |
| F2 C | - | 0.3 | Transparent solution | 7.42 | (+) | 87.09±0.20 | 1.92±0.02 |
| F3 C | - | 0.4 | Transparent solution | 7.56 | (+) | 97.54±0.86 | 2.08±0.15 |
| F4 C | - | 0.5 | Transparent solution | 7.83 | (++) | 98.44±0.31 | 2.30±0.14 |
| F5 C | - | 0.6 | Transparent solution | 7.34 | (++) | 99.73±0.42 | 2.33±0.21 |
| F6 C | - | 1 | Transparent solution | 7.84 | (+++) | 100.47±0.19 | 4.27±0.14 |
| F1GC | 0.5 | 0.5 | Transparent solution | 6.86 | (+++) | 98.52±0.19 | 144.64±0.44 |
Table 2: Physicochemical Properties of In Situ Gel
The viscosity and gel forming ability are the two important parameters for in situ gel formulation. Different concentrations of gellan gum and sodium carboxymethyl cellulose were prepared and determined the gel forming ability and viscosity. The solution to be administered requires optimal viscosity for ease of administration. The solution should be converted into gel quickly in contact with tear fluid and reside for a long period of time on the ocular surface. The shear rate during inter-blinking is 0.03 s−1 and blinking time is 4250-28500 s−1[43]. Therefore, in situ gel formulations with pseudoplastic behavior are preferred for ophthalmic drug delivery. Polymer solutions with pseudoplastic behavior show a decreasing viscosity trend with increasing shear rates[23].
Gel formation time of in situ gel formulation is critical to prevent drug loss after administration. Quick gelation of solutions in the ocular surface could prevent dilution and removal of drug by tears. It was observed that the quick gelation of the solution permitted more drug entrapment in the gel. Batch F1G (0.2 % gellan gum), F1C (0.2 % carboxymethyl cellulose), F2 C (0.3 % carboxymethyl cellulose), and F3C (0.4 % carboxymethyl cellulose) showed slow weak gelation (Table 2). Batch F2G (0.3 % gellan gum), F3G (0.4 % gellan gum), F4C (0.5 % carboxymethyl cellulose), and F5C (0.6 % carboxymethyl cellulose) showed immediate gelation which remained for 2-3 h. Batch F4G (0.5 % gellan gum), F5G (0.6 % gellan gum), F6C (1 % carboxymethyl cellulose), and F1GC (0.5 % gellan gum+0.5 % carboxymethyl cellulose) showed immediate gelation (within 5 s) and remained for more than 6 h. Among all the formulations, F6G (1 % gellan gum) showed a very stiff gel. 1 % gellan gum solution was very viscous and not suitable for eye application.
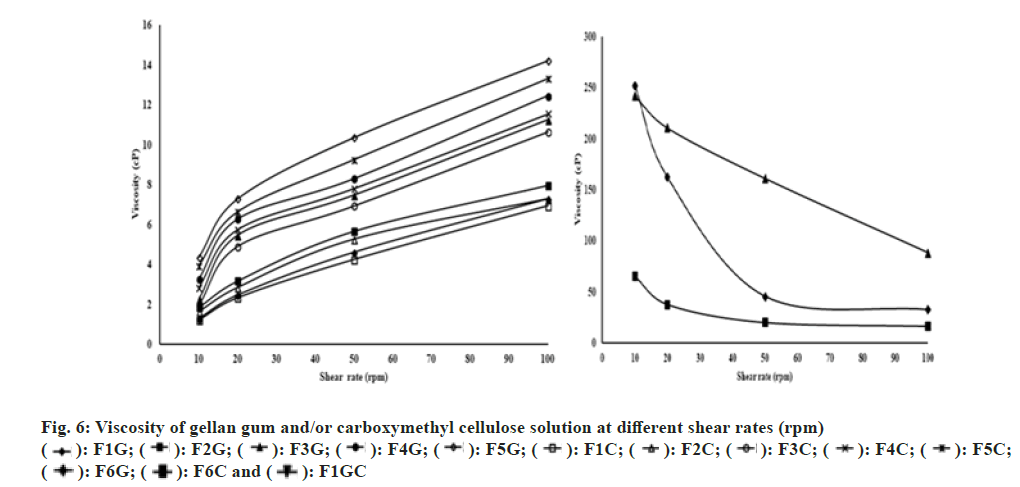
The viscosities of gellan gum and/or carboxymethyl cellulose solutions are represented in fig. 6. The viscosity of the polymer solution increased with increasing concentration. Both gellan gum and carboxymethyl cellulose solution show Newtonian flow behavior (viscosity increased with increasing shear rate) at concentrations of 0.2-0.6% (w/v) (fig. 6). However, gellan gum (1 % w/v), carboxymethyl cellulose (1 % w/v) and combinations of gellan gum and carboxymethyl cellulose (0.5 % w/v+0.5 % w/v) solutions show non-Newtonian flow behavior (fig. 6). At the higher shear rate the non-Newtonian flow shows lesser resistance. At rest, the polymer has a long entangled chain and exhibited high viscosity (high internal resistance). With an increase in shear rate, the molecular chain disentangles and exhibits shear thinning behavior (low internal resistance). Moreover, increasing the shear rate decreases the interaction between the molecules and consequently reduced viscosity.
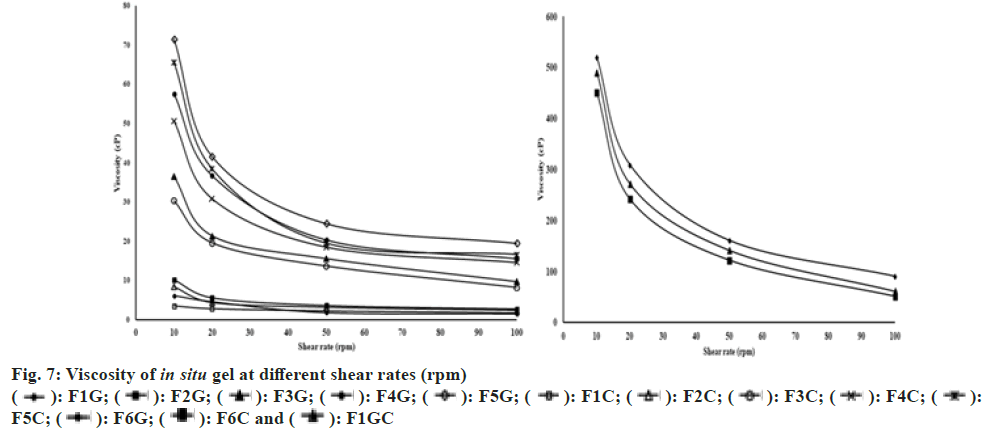
The addition of simulated tear fluid to polymer solutions resulted in the enhancement of viscosity due to gelation. The effect of shear rate on viscosity was represented in fig. 7. It was observed that all the in situ gel formulations showed shear thinning behavior. After gelation in the simulated tear fluid a reduced viscosity was observed with increasing shear rate. Such in situ gels will not show difficulty in blinking during the conversion of gel in the eye.
Gel strength is very essential for the development of in situ gel. The formulation must be instilled as a solution without difficulty and have enough strength to prevent formulation drainage from the eye. The gel strength of the prepared formulations is represented in Table 2. The gel strength of all the formulations was increased with an increase in the concentration of polymer. The gel strength of the in situ gel formulation was associated with gel viscosity. The gel strength increased with increasing polymer concentration. The same observation was observed by other authors[44].
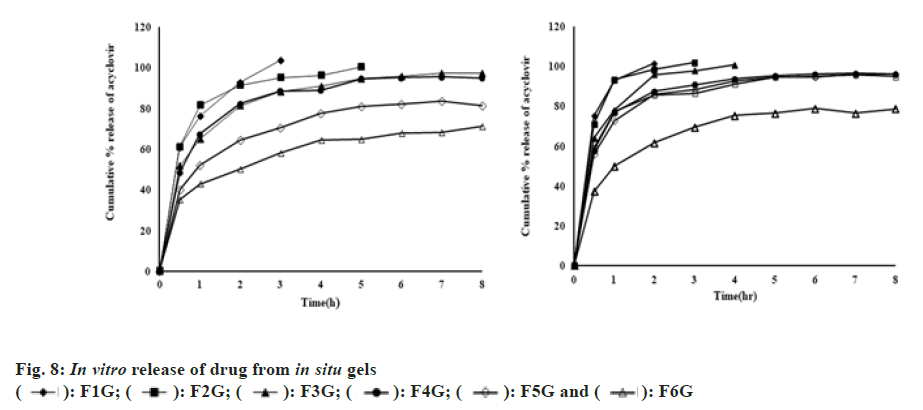
The effect of gellan gum and/or carboxymethyl cellulose on the release of the drug was studied (fig. 8). The release of the drug after 2 and 8 h was determined and represented in Table 3. The initial quick release and then the sustained release of the drug were observed from all the in situ gels. The burst release helps to attain the therapeutic drug concentration in a minimum amount of time and later releases the drug in a sustained manner to achieve the drug concentration for an extended period of time. The initial release of the drug may be due to the surface migration of the drug in the matrix. The release of the drug was retarded by increasing the concentration of the polymer. With an increase in the concentration of polymer, the viscosity and cross-linking density was increased resulting in a retardation of drug release. Among all formulations, F5G (0.6 % gellan gum), F6G (1 % gellan gum) and F1GC (0.5 % gellan gum+0.5 % carboxymethyl cellulose) show the better sustained release profile. 64.2 %, 50.26 % and 61.58 % drug was released from F5G, F6G and F1GC formulations in 2 h. 81.3 %, 71.09 % and 78.52 % drug was released from F5G, F6G and F1GC formulations in 8 h. Formulation F6G which contains 1 % gellan gum shows more sustained release of the drug. However, this solution is too viscous and produces a stiff in situ gel that is unsuitable for eye instillation. So, formulation F1GC containing 0.5 % gellan and 0.5 % carboxymethyl cellulose is an optimized formulation that sustains the release of the drug.
The release of drugs from in situ gels was treated with the Korsmeyer-Peppas model[45,46].
Mt/M∞=ktn (1)
Where Mt/M∞=Fraction of acyclovir release at time t; k=constant that determines release velocity; n=diffusional exponent that describes the mechanism of drug release. The n and k values can be measured from the slopes and intercepts of log Mt/M∞ and log t. The value of n was calculated and represented in Table 3. Release of drug from in situ gel formulations in simulated tear fluid was Fickian diffusion. The release of the drug is controlled by drug diffusion driven by a chemical potential in tear fluid (Type I transport). The relaxation time of polymer is much greater compared to solvent diffusion time in Fickian diffusion.
| Formulation code | % released after 2 h (Mean±SD, n=3) | % released after 8 h (Mean±SD, n=3) | n value (Mean±SD, n=3) | R2 |
|---|---|---|---|---|
| F1 G | 92.72±1.05 | 103.59±0.43 | 0.296±0.015 | 1 |
| F2G | 91.15±1.26 | 100.29±0.84 | 0.243±0.008 | 0.92 |
| F3 G | 81.17±1.50 | 97.02±1.01 | 0.227±0.008 | 0.95 |
| F4 G | 82.26±0.12 | 94.82±1.27 | 0.229±0.005 | 0.9 |
| F5 G | 64.20±0.17 | 81.30±1.51 | 0.265±0.007 | 0.96 |
| F6 G | 50.26±0.32 | 71.09±1.11 | 0.257±0.018 | 0.99 |
| F1 C | 101.64±0.62 | 101.64±0.62 | 0.218±0.011 | 0.94 |
| F2 C | 98.35±1.29 | 102.04±0.64 | 0.235±0.014 | 0.86 |
| F3 C | 95.83±1.25 | 100.90±0.31 | 0.224±0.005 | 0.95 |
| F4 C | 87.55±0.93 | 96.16±1.18 | 0.168±0.000 | 0.87 |
| F5 C | 86±1.61 | 95.97±0.88 | 0.182±0.006 | 0.9 |
| F6 C | 85.75±1.68 | 94.76±1.35 | 0.157±0.011 | 0.89 |
| F1 GC | 61.58±1.04 | 78.52±1.83 | 0.268±0.008 | 0.94 |
Table 3: Mechanism and Percentage Drug Release Through Hydrogel Matrix
Acyclovir loaded ion sensitive in situ gels forming ophthalmic solutions was developed using gellan gum and/or sodium carboxymethyl cellulose as gelling agents. A reduction in viscosity of in situ gel formulation was observed in a physiologic environment with an increase in shear rate and this will help in the blinking process. The optimized formulation showed gelation within 5 s and remained for more than 6 h. Moreover, the optimized formulation provides sustained drug release for more than 8 h. The developed formulation may improve bioavailability through longer contact times and controlled drug release in the eye.
Conflict of interest:
The authors declared no conflict of interests.
References
- Bagga B, Kate A, Joseph J, Dave VP. Herpes simplex infection of the eye: An introduction. Community Eye Health 2020;33(108):68.
- Shukla SD, Valyi-Nagy T. Host molecules that promote pathophysiology of ocular Herpes. Front Microbiol 2022;13:818658.
[Crossref] [Google Scholar] [PubMed]
- Labetoulle M, Auquier P, Conrad H, Crochard A, Daniloski M, Bouée S, et al. Incidence of herpes simplex virus keratitis in France. Ophthalmology 2005;112(5):888-95.
[Crossref] [Google Scholar] [PubMed]
- Liesegang TJ, Melton LJ, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex: Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol 1989;107(8):1155-9.
[Crossref] [Google Scholar] [PubMed]
- Wilhelmus KR, Beck RW, Moke PS, Dawson CR, Barron BA, Jones DB, et al. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. New Engl J Med 1998;339(5):300-6.
- Birkmann A, Bonsmann S, Kropeit D, Pfaff T, Rangaraju M, Sumner M, et al. Discovery, chemistry, and preclinical development of pritelivir, a novel treatment option for acyclovir-resistant herpes simplex virus infections. J Med Chem 2022;65(20):13614-28.
[Crossref] [Google Scholar] [PubMed]
- Chodosh J, Ung L. Adoption of innovation in herpes simplex virus keratitis. Cornea 2020;39(1):S7.
[Crossref] [Google Scholar] [PubMed]
- Khan S, Ali A, Singhavi D, Yeole P. Controlled ocular delivery of acyclovir through rate controlling ocular insert of eudragit: A technical note. AAPS Pharm Sci Tech 2008;9(1):169-73.
[Crossref] [Google Scholar] [PubMed]
- Gide PS, Gidwani SK, Kothule KU. Enhancement of transdermal penetration and bioavailability of poorly soluble acyclovir using solid lipid nanoparticles incorporated in gel cream. Indian J Pharm Sci 2013;75(2):138.
[Google Scholar] [PubMed]
- Robinson JR. Ocular drug delivery mechanism (s) of corneal drug transport and mucoadhesive delivery systems. STP Pharma 1989;5(12):839-46.
- Giannavola C, Bucolo C, Maltese A, Paolino D, Vandelli MA, Puglisi G, et al. Influence of preparation conditions on acyclovir-loaded poly-d, l-lactic acid nanospheres and effect of PEG coating on ocular drug bioavailability. Pharm Res 2003;20(4):584-90.
[Crossref] [Google Scholar] [PubMed]
- Fresta M, Fontana G, Bucolo C, Cavallaro G, Giammona G, Puglisi G. Ocular tolerability and in vivo bioavailability of poly (ethylene glycol) (PEG)?coated polyethyl?2?cyanoacrylate nanosphere?encapsulated acyclovir. J Pharm Sci 2001;90(3):288-97.
[Crossref] [Google Scholar] [PubMed]
- Ranch KM, Maulvi FA, Naik MJ, Koli AR, Parikh RK, Shah DO. Optimization of a novel in situ gel for sustained ocular drug delivery using Box-Behnken design: In vitro, ex vivo, in vivo and human studies. Int J Pharm 2019;554:264-75.
[Crossref] [Google Scholar] [PubMed]
- Bhalerao H, Koteshwara KB, Chandran S. Levofloxacin hemihydrate in situ gelling ophthalmic solution: Formulation optimization and in vitro and in vivo evaluation. AAPS Pharm Sci Tech 2019;20(7):272.
[Crossref] [Google Scholar] [PubMed]
- Chowhan A, Giri TK. Polysaccharide as renewable responsive biopolymer for in situ gel in the delivery of drug through ocular route. Int J Biol Macromol 2020;150:559-72.
[Crossref] [Google Scholar] [PubMed]
- Thrimawithana TR, Rupenthal ID, Young SA, Alany RG. Environment-sensitive polymers for ophthalmic drug delivery. J Drug Deliv Sci Technol 2012;22(2):117-24.
- Geethalakshmi A, Karki R, Kumar Jha S, P Venkatesh D, Nikunj B. Sustained ocular delivery of brimonidine tartrate using ion activated in situ gelling system. Curr Drug Deliv 2012;9(2):197-204.
[Crossref] [Google Scholar] [PubMed]
- Lai JY. Biodegradable in situ gelling delivery systems containing pilocarpine as new antiglaucoma formulations: Effect of a mercaptoacetic acid/N-isopropylacrylamide molar ratio. Drug Des Devel Ther 2013:1273-85.
[Crossref] [Google Scholar] [PubMed]
- Wu H, Liu Z, Peng J, Li L, Li N, Li J, Pan H. Design and evaluation of baicalin-containing in situ pH-triggered gelling system for sustained ophthalmic drug delivery. Int J Pharm 2011;410(1-2):31-40.
[Crossref] [Google Scholar] [PubMed]
- Oechsner M, Keipert S. Polyacrylic acid/polyvinylpyrrolidone bipolymeric systems. I. Rheological and mucoadhesive properties of formulations potentially useful for the treatment of dry-eye-syndrome. Eur J Pharm Biopharm 1999;47(2):113-8.
[Crossref] [Google Scholar] [PubMed]
- Song J, Bi H, Xie X, Guo J, Wang X, Liu D. Preparation and evaluation of sinomenine hydrochloride in situ gel for uveitis treatment. Int Immunopharmacol 2013;17(1):99-107.
[Crossref] [Google Scholar] [PubMed]
- Abraham S, Furtado S, Bharath S, Basavaraj BV, Deveswaran R, Madhavan V. Sustained ophthalmic delivery of ofloxacin from an ion-activated in situ gelling system. Pak J Pharm Sci 2009;22(2):175-9.
[Google Scholar] [PubMed]
- Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm 2006;315(1-2):12-7.
[Crossref] [Google Scholar] [PubMed]
- Rupenthal ID, Green CR, Alany RG. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: Physicochemical characterisation and in vitro release. Int J Pharm 2011;411(1-2):69-77.
[Crossref] [Google Scholar] [PubMed]
- Cao SL, Ren XW, Zhang QZ, Chen E, Xu F, Chen J, et al. In situ gel based on gellan gum as new carrier for nasal administration of mometasone furoate. Int J Pharm 2009;365(1-2):109-15.
[Crossref] [Google Scholar] [PubMed]
- Rolando M, Zierhut M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol 2001;45:S203-10.
[Crossref] [Google Scholar] [PubMed]
- Vijaya C, Goud KS. Ion-activated in situ gelling ophthalmic delivery systems of azithromycin. Indian J Pharm Sci 2011;73(6):615-20.
[Crossref] [Google Scholar] [PubMed]
- Giri TK, Thakur A, Tripathi KD. Biodegradable hydrogel bead of casein and modified xanthan gum for controlled delivery of theophylline. Curr Drug Ther 2016;11(2):150-62.
- Giri TK, Verma P, Tripathi DK. Grafting of vinyl monomer onto gellan gum using microwave: Synthesis and characterization of grafted copolymer. Adv Compos Mater 2015;24(6):531-43.
- Qi H, Chen W, Huang C, Li L, Chen C, Li W, et al. Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int J Pharm 2007;337(1-2):178-87.
[Crossref] [Google Scholar] [PubMed]
- Datta S, Bhowmik R, Nath R, Chakraborty R, Chakraborty A. Formulation and evaluation of a nanoparticle laden in situ gel system for enhancing the ocular delivery of ciprofloxacin. Int J Pharm Sci Rev Res 2021;70(2):156-63.
- Nisha GS, Maithil P, Charyulu RN. Formulation and development of nasal in situ gels of triptans for anti-migraine activity. Int J Res Pharm Biomed Sci 2012;3(2):862-70.
- Carlfors J, Edsman K, Petersson R, Jörnving K. Rheological evaluation of Gelrite in situ gels for ophthalmic use. Eur J Pharm Sci 1998;6(2):113-9.
[Crossref] [Google Scholar] [PubMed]
- Mahajan HS, Gattani S. In situ gels of metoclopramide hydrochloride for intranasal delivery: In vitro evaluation and in vivo pharmacokinetic study in rabbits. Drug Deliv 2010;17(1):19-27.
[Crossref] [Google Scholar] [PubMed]
- Sun J, Zhou Z. A novel ocular delivery of brinzolamide based on gellan gum: In vitro and in vivo evaluation. Drug Des Dev Ther 2018;12:383-9.
[Crossref] [Google Scholar] [PubMed]
- Giri TK, Dey B, Maity S. Preparation and characterization of nanoemulsome entrapped in enteric coated hydrogel beads for the controlled delivery of capsaicin to the colon. Curr Drug Ther 2018;13(1):98-105.
- Balasubramaniam J, Pandit JK. Ion-activated in situ gelling systems for sustained ophthalmic delivery of ciprofloxacin hydrochloride. Drug Deliv 2003;10(3):185-91.
[Crossref] [Google Scholar] [PubMed]
- Kirchmajer DM. Robust biopolymer based ionic–covalent entanglement hydrogels with reversible mechanical behaviour. J Mater Chem B 2014;2(29):4694-702.
- Dey M, Ghosh B, Giri TK. Enhanced intestinal stability and pH sensitive release of quercetin in GIT through gellan gum hydrogels. Colloids Surf B 2020;196:111341.
- Singh BN, Kim KH. Characterization and relevance of physicochemical interactions among components of a novel multiparticulate formulation for colonic delivery. Int J Pharm 2007;341(1-2):143-51.
[Crossref] [Google Scholar] [PubMed]
- Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev 2005;57(11):1595-639.
[Crossref] [Google Scholar] [PubMed]
- Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS Pharm Sci Tech 2009;10:808-19.
[Crossref] [Google Scholar] [PubMed]
- Srividya BJ, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J Control Release 2001;73(2-3):205-11.
[Crossref] [Google Scholar] [PubMed]
- Morsi N, Ibrahim M, Refai H, El Sorogy H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur J Pharm Sci 2017;104:302-14.
[Crossref] [Google Scholar] [PubMed]
- Kassem MA, El Shaboury KM, Mohamed AI. Application of central composite design for the development and evaluation of chitosan-based colon targeted microspheres and in vitro characterization. Indian J Pharm Sci 2019;81(2):354-64.
- Giri TK, Choudhary C, Alexander A, Badwaik H, Tripathy M, Tripathi DK. Sustained release of diltiazem hydrochloride from cross-linked biodegradable IPN hydrogel beads of pectin and modified xanthan gum. Indian J Pharm Sci 2013;75(6):619.
[Google Scholar] [PubMed]