- *Corresponding Author:
- Narges shokri
Department of Pharmaceutics, School of Pharmacy, Ardabil University of Medical Sciences, Iran
E-mail: n.shokri@arums.com
| Date of Submission | 17 July 2014 |
| Date of Revision | 19 January 2015 |
| Date of Acceptance | 14 November 2015 |
| Indian J Pharm Sci 2015; 77(6): 694-704 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Notice of Retraction: After careful and considered review of the content of this paper by a duly constituted expert committee, this paper has been found to be in violation of IJPS Publication Principles. We hereby retract the content of this paper. Reasonable effort should be made to remove references to this paper.
Evidence
It has come to our attention that there are substantial overlaps between the text of the above article and the following papers, especially in the introduction, methods, and discussion.
It has been pointed out that the above study appeared to contain a high level of similarity with four articles by the same authors published as below:
Shokri N, Akbari Javar H. Comparison of calcium phosphate and zinc oxide nanoparticles as dermal penetration enhancers for albumin. Indian J Pharm Sci. 2015 Nov-Dec; 77(6): 694?704
Shokri N, Akbari Javar H. Using a Synergistic Combination of Two Enhancers for Dermal Delivery of Collagen in Pharmaceutical and Cosmetic Products. Journal of Pharmaceutical Research. 2015 Jan-Mar, 14(1); 1-6
Shokri N, Akbari Javar H. Zinc Oxide Nanoparticles as Skin Permeation Enhancer for Solvents and Surfactants. Journal of Pharmaceutical Research. 2014;13(3):85-91. -Shokri N, Goodarzi MT, Akbari Javar H, Soltani Y. Zinc Oxide and Zinc Oxide Nanoparticles as Enhancers in Topical Pharmaceutical and Cosmetic Products. Journal of Pharmaceutical Research. 2014;13(2): 40-44.
Shokri N. Different Inorganic Nanoparticles as Enhancers for Dermal Delivery of Proteins in Pharmaceutical and Cosmetic Products. Journal of Pharmaceutical Research. 2014 Oct; 13(4):116-121.
The published paper in IJPS and the paper in the Journal of Pharmaceutical Research. 2014 Oct; 13(4): 116-121, had overlap in the introduction, aim, methods, results, discussion, and conclusion indicate that these papers are an example of redundancy. Following an internal investigation, it was established that the studies were conducted along broadly similar lines. Furthermore, submitting multiple articles derived from the same data set with the overlap without cross-referencing is not legitimated based on the ethical principles of Scopus. Hence the article was retracted from the Indian Journal of Pharmaceutical Sciences.
Keywords
Albumin, calcium phosphate nanoparticles, zinc oxide nanoparticles, skin permeation, skin distribution, enhancer
Dermal administration of drugs is usually preferred by patients because of its several advantages including noninvasive and painless administration, controlled delivery and termination, bypassing first-pass drug metabolism, loss of GI absorption steps and threats like pH variations, enzymes and transit time[1].
The most important barrier for dermal and transdermal drug delivery is the skin’s horny layer or stratum corneum (SC). This layer must be altered for penetration of drugs to the skin[2]. Extensive research on chemical penetration enhancers (CPEs) has been performed, which form the main strategy of formulation-design approaches for dermal and transdermal drug delivery[3]. It is now well known that CPEs improve dermal penetration or transdermal absorption of drugs[4]. This is dependent to physicochemical characteristics of the CPE and the drug. Therefore, skin permeation of drugs are different in the presence of various CPEs[5,6]. Thus each pair of drug-CPE should be examined separately. More than two hundred CPEs have been shown to enhance skin permeation of drugs[3] mainly including aliphatic acids, fatty acids, esters, alcohols, oils and terpens. The mechanism of many CPEs is constructing a new skin microstructure[1]. One group of such CPEs are hydrophobic nanoparticles (NPs) made from lipids or hydrophobic polymers. The drug is trapped inside these NPs. Such NPs involve some problems including that lipid NPs should be evaluated in terms of size, stability, safety and efficient drug loading and release, and polymeric NPs should be evaluated in terms of size, surface charge, safety, biocompatibility and especially degradation kinetics and byproducts. Therefore, they should be accurately designed to become suitable for such uses[7-9].
Another group is inorganic NPs. Among them, titanium dioxide NPs have been introduced as dermal enhancer and its physicochemical optimization as an enhancer was investigated[10]. In this study inorganic NPs including calcium phosphate nanoparticles (CaP-NPs) and zinc oxide nanoparticles (ZnO-NPs) were used as enhancers. CaP with molecular weight (MW) of 310.176 g/mol is a safe and inexpensive natural chemical containing calcium and phosphate, which are essential nutrients[11]. CaP-NPs has shown significantly less toxicity than other metal NPs[12]. It has excellent biocompatibility and biodegradability in comparison with similar NPs, because of its chemical similarity to human hard tissues[11,13-15]. It was shown that CaP-NPs have some cytoprotective effects and high compatibility with peptides and proteins[13,16]. CaP was used in nanoparticulate dispersed form and size of 100 nm, as a carrier in biological systems, e.g. to transfer nucleic acids or other drugs[11,14,15] and beside that, CaP-NPs have been successfully used for transcutaneous vaccine delivery as carriers[15], which is the only study on the enhancing effect of CaP-NPs. Topical use of CaP-NPs is safe because they are slowly dissolved and thus eliminated from the skin as ions. It means that the resulted ions have enough time to exit the skin. Therefore they do not remain in the skin layers for a long time[11]. Zinc is also a relatively inexpensive, biocompatible and non-toxic vital nutrient with almost no topical side effects[17]. Zinc has shown no interactions with most pharmaceutically active molecules[15]. It is proved that zinc has antioxidant and cytoprotective effects on skin keratinocytes in cell (HaCaT) culture[18,19]. In addition, ZnO with MW of 81.408 g/mol has been applied topically to heal wounds and to cure other skin disorders[18]. In this regard, ZnO has been more effective than ZnSO4 [6]. In topical application, zinc inhibits the adverse effect of sunlight UV on the skin proteins especially in form of ZnO-NPs[19,20]. Also zinc desirably inhibits the degradation of proteins[19]. It has been said that metals like zinc involve the metaloenzymes of the skin and thus protect the skin proteins[21]. The skin distribution of zinc has shown a peak in the epidermal layer decreasing toward the SC, but increased in the SC[22-24]. ZnO-NPs are fluorescent NPs and very biocompatible and safer than other similar metal NPs[25]. In similar to zinc, it has been proved that ZnO-NPs can not pass through the skin thus they have no systemic toxicity[26,27]. On the other hand, ZnO-NPs slowly solve in aqueous medium as ions[28], which leave the skin slowly into the circulation followed by their elimination via liver, kidneys and intestine[29]. This process desirably cause elimination of the NPs from the skin. We have proved the enhancer effect of ZnONPs on skin penetration of ibuprofen and also on skin penetration of liquid CPEs in our previous works for the first time[30,31]. These findings highlight the lack of research on the enhancing effects of the CaP-NPs and/ or ZnO-NPs in dermal drug delivery. Such NPs do not involve the problems mentioned for polymeric or lipid NPs. In our method, drug was not confined within the inorganic NPs and the NPs were not used as drug carriers. Indeed, drug did not attach to or loaded into the NPs. Instead, the NPs were used simultaneously with the drug but separated from the drug in each formulation. In this regard, the NPs positioned through out the skin layers and therefore, could alter the skin microstructure especially because of their crystalline state[25]. This helps the drug to pass the skin. Such a method of using NPs as enhancer (simultaneous use with drug and without loading the drug), was successfully employed in our previous works[32,33]. Also in this investigation, the NPs have been used as penetration enhancers and not as carriers.
Fortunately, the inorganic NPs used usually do not show toxicity irrespective of zinc[6] nor CaP[14,15]. Moreover, they can not practically and easily pass the skin[18,19,23,27]. As a result, they have not the potential to cause toxicity. On the other hand, because the target organ is the skin bearing a permanent turnover, all materials accumulated in the skin (especially outer skin) will release out along with the skin within a few days.
Since recently proteins have found extensive applications as therapeutic agents and many proteinbased active ingredients have been developed and used in topical (dermal) pharmaceutical and cosmetic products for various purposes[32], in this study, albumin (MW of 67 KDa) was selected as the active ingredient and as a model for other therapeutic proteins. It has been reported that albumin which is non permeable through skin was able to cross the skin when administered CaP-NPs[13]. We have previously proved that CPEs were effective for enhancing the skin permeation of albumin and other proteins[33,34]. In this work, we studied effects, process and differences of CaP-NPs and ZnO-NPs in enhancing the skin permeation of albumin.
Materials and Methods
ZnO-NPs (nominal average particle size of 100 nm), CaP-NPs (nominal average particle size of 100 nm), human serum albumin, TES buffer, and other used chemicals were purchased from Sigma-Aldrich company, USA. Fluorescein-5-isothiocyanate cadaverine (FITC-cad) was from Ana-Spect, Inc. (San Jose, CA, USA).
Preparation of albumin-FITC
The process of albumin binding to FITC was performed according to Wu et al. FITC-cad was chosen for this purpose. Briefly, 30 μl albumin was added to TES buffer in each well of microplate at 4º. Then 30 μl reagent-A was added to each, following by incubation at 37º. After 10 min, 60 μl reagent-B was added. The free FITC-cad was removed by charcoal[35].
Preparation of formulations
Formulations numbered 1-8 were prepared. Their constituents are listed in Table 1. Formulations 1 and 2 were prepared by mixing 300 mg NPs (ZnO NPs and CaP NPs, respectively) into 3 ml deionized water (DW) using magnetic stirrer (Heidolph, MR Hei-Tec, Germany) to obtain a paste. Formulation 3 and 6 contained only 500 mg albumin and albumin-FITC, respectively, mixed evenly into 3 ml deionized water. Formulations 4 and 5 were prepared similar to the formulation 3 but 300 mg ZnO-NPs and CaP-NPs were added to them, respectively. Formulations 7 and 8 were prepared similar to the ormulation 6 but 300 mg ZnO-NPs and CaP-NPs were added to them, respectively. Albumin was the active ingredient and the NPs were used as enhancers for skin permeation of albumin. Such a dose for NPs (300 mg) was their optimum concentration as topical enhancer, obtained in our previous work[32].
| Formulation number | Active ingredient(permeant) (500 mg) | Solvent(3 ml) | Permeation enhancer (300 mg) |
|---|---|---|---|
| 1 | - | DW | ZnO-NPs |
| 2 | - | DW | CaP-NPs |
| 3 | Albumin | DW | - |
| 4 | Albumin | DW | ZnO-NPs |
| 5 | Albumin | DW | CaP-NPs |
| 6 | Albumin-FITC | DW | - |
| 7 | Albumin-FITC | DW | ZnO-NPs |
| 8 | Albumin-FITC | DW | CaP-NPs |
Table 1: Formulations 1-8 and their Constituents
Permeation test
Permeation in this study could be referred to the release of albumin from the skin slice. It was determined by an ex vivo method using a two chamber (donor and receiver) diffusion cell. The cell had an effective diffusion area of 9.6 cm2 between the two chambers. This method is supported by the fact that SC, the main site of enhancer action, shows similar behavior in vivo and in vitro (ex vivo)[36]. Thirty millilitres of phosphate buffered saline (PBS), pH 7.4., was poured into the receiver chamber as the medium. A piece of mouse full skin was cut from mouse back. The skin was placed and fixed between the two chambers. The whole amount of every formulation listed in the Table 1, was placed and spread on the skin by a clean swab. Then the cap was placed and fixed. After that, the cell was placed in a shaker-incubator (Heidolph incubator 1000, Heidolph co., Germany) with a temperature of 32º for 2 h[3,37,38]. Choosing an exposure time of 2 h was because the topical products usually do not remain on the skin for a long time. During this period, the formulation was rubbed by a swab to spread and help the albumin permeation.
After 0, 0.5, 1, 1.5 and 2 h, 3 ml samples of PBS was taken out from the medium of the diffusion cells containing the formulations 3, 4, 5 and 6, for evaluation of albumin permeation or release through the skin. The samples were analyzed for the concentration of albumin using a UV/Vis spectrophotometer (Perkin-Elmer-Lambda 25, USA) at 278 nm[39]. Three milliliter of fresh PBS was replaced every time. The total amount of albumin (in 30 ml PBS) was calculated for every sample and reported as the dermal permeated albumin (mg). The cumulative permeated amount of albumin was plotted versus time in figs. 1 to 4 for formulations 3 to 6, respectively.
After finishing the above permeation test, skin slices of the cells containing formulations 1, 2, 6, 7 and 8, were removed from the cells for preparation for microscopic imaging. Skin slices related to the formulations 1 and 2 were prepared for light microscopy and skin slices related to the formulations 1, 6, 7 and 8 were prepared for fluorescence microscopy. It should be noted that the permeation experiment was performed 2 times for the formulation 1 to use the related skin slices for both light and fluorescence microscopy.
Microscopic imaging
Skin slices obtained from the permeation experiment, were used for obtaining skin sections for microscopic imaging. To prepare skin sections, the remained formulation was whipped carefully from the skin by a clean cotton swab. The skin was immersed in liquid nitrogen and snap frozen and kept at -80º until imaging. Then the skin was embedded in Tissue Tec ?? following by cross-sectioning with thickness of 10 μm[40].
Light microscopic imaging process was performed for untreated skin and skins treated by formulations 1 and 2. Fluorescence microscopic imaging was performed for untreated skin and skins treated by formulations 1, 6, 7 and 8. Before light microscopy, skin slices were washed with saline and formalin[41]. Fluorescence images of skin slices were obtained by a fluorescent microscope (IX81, Olympus, Tokyo, Japan). The excitation wavelength range and emission wavelength for ZnO-NPs were 330-380 and 420 nm, respectively. The excitation and emission wavelengths for albumin-FITC-cad were 485 and 535 nm, respectively[35].
Statistical analysis
The permeation experiments were performed 3 times for each formulation and the reported data as cumulative amounts of permeated albumin, are mean±SD (n=3). One-way analysis of variance (ANOVA) was used for comparing the differences. SPSS for Windows (release 11.5.0) was employed for statistical analysis. The P-value <0.05 was considered to be significant.
Results
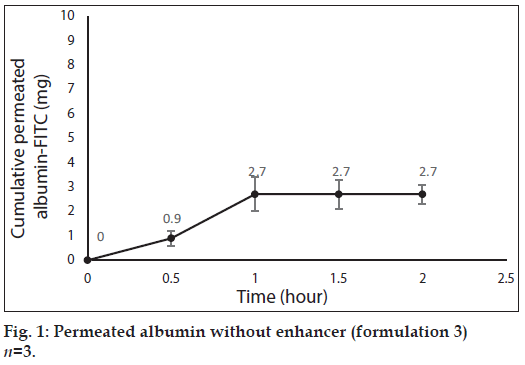
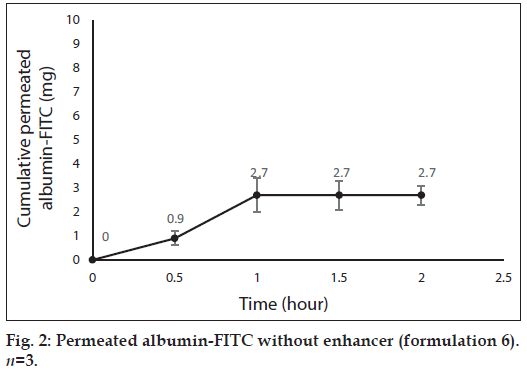
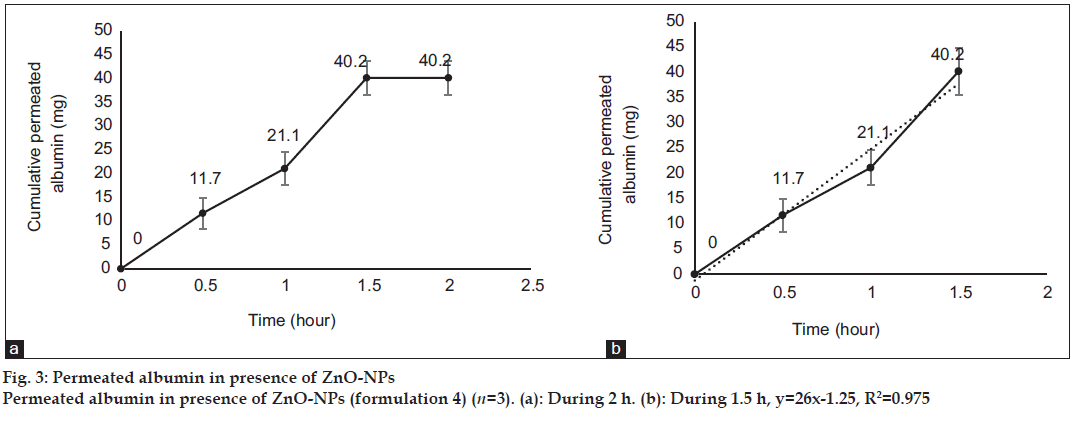
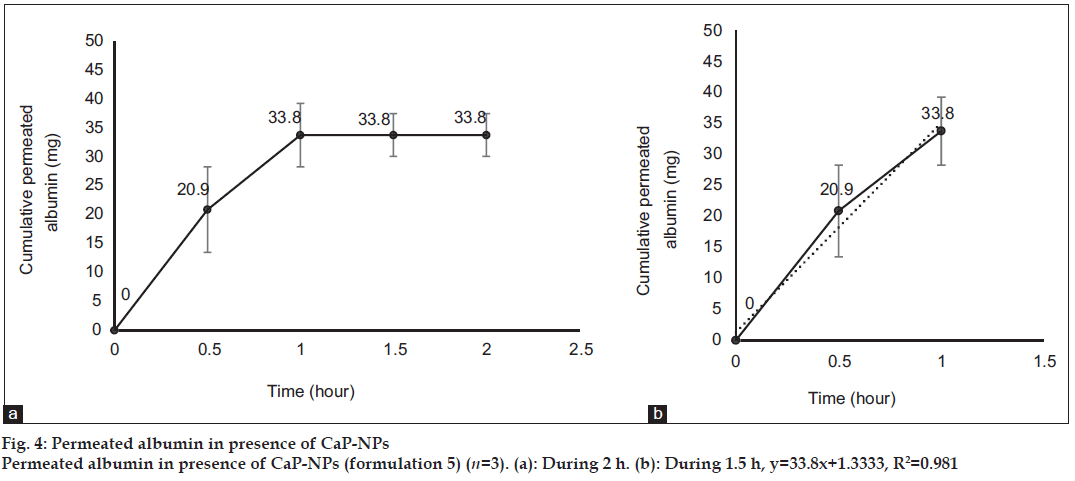
Fig. 1 shows profile of permeation of albumin (formulation 3) through the skin during 2 h. Indeed it shows permeated albumin in presence of just DW (without any enhancer). The cumulative amounts of skin permeated albumin at 0, 0.5, 1, 1.5 and 2 h after application of the formulation, were 0±0, 11.2±0.2, 5±0.5, 5±0.5 and 5±0.5 mg, respectively. Fig. 2 shows a similar profile of permeation of albumin-FITC (formulation 6). It shows the cumulative amounts of permeated albumin-FITC in presence of just DW, which were 0±0, 0.9±0.3, 2.7±0.7, 2.7±0.6 and 2.7±0.4 mg, respectively for the mentioned sampling times. Fig. 3a presents a similar profile of permeation of albumin in presence of ZnO-NPs (formulation 4) where ZnO-NPs were used as enhancer for permeation of albumin. The related cumulative amounts of permeated albumin were 0±0, 11.7±3.3, 21.1±3.5, 40.2±3.6 and 40.2±3.6 mg, respectively. Fig. 3b is the same data as fig. 3a but it is limited to the time of 1.5 h, which indicates a linear relationship between permeated albumin and time where R2=0.97. Fig. 4a presents similar data in presence of CaP-NPs as enhancer (formulation 5), which were 0±0, 20.9±7.4, 33.8±5.5, 33.8±3.7 and 33.8±3.7 mg, respectively. Fig. 4b is the same data as fig. 4a but it is limited to the time of 1 h, which indicates a linear relationship permeated albumin and time where R2=0.98.
Fig. 5 offers images of skin cross-sections obtained by light microscopy. Fig. 5a presents images of untreated skin as control. Fig. 5b presents images of skin treated by ZnO-NPs (formulation 1) at 0.5, 1 and 1.5 h after application of the formulation. Fig. 5c presents images of skin treated by CaP-NPs (formulation 2) after 0.5, 1 and 1.5 h. Actually the images offer skin distribution patterns of the two kinds of NPs in the absence of albumin. As expected, in fig. 5a no particles were observed while in fig. 5b and c the NPs were observed in the skin. In case of fig. 5b and c, the NPs were distributed into different layers of the skin. Fig. 5b showed that application of ZnO-NPs alone on the skin was followed by a partial penetration of the NPs into the skin where most of them positioned in upper layers of the skin (SC and epidermis). In this process, the NPs did not penetrate the skin after 0.5 h but most of them penetrated after 1 h. The maximum amount of the NPs in the skin layers was observed after 1.5 h where most of them accumulated in upper layers and their accumulation in deeper layers was very low or almost negligible. Fig. 5c shows that CaP-NPs did not penetrated into the skin after 0.5 h. After 1 h part of the NPs entered the upper skin layers and even deeper layers. The maximum concentration of the NPs in the skin was observed after 1.5 h, which was observed all over the skin depth.
Fig. 6 offers images of skin cross-sections obtained by fluorescence microscopy. Fig. 6a is related to untreated skin as control where as expected, no fluorescence signal was observed. Fig. 6b-e offer fluorescence images at 0.5, 1 and 1.5 h after application of the formulations. Since ZnO-NPs possess intrinsic fluorescence, fluorescence images were taken of the skin treated by ZnO-NPs beside their light microscopy images. Therefore, fig. 6b shows the fluorescence images of the skin treated by ZnO-NPs (formulation 1). After 0.5 h, almost all the NPs was observed at the top of the skin and small amount of them penetrated into the skin. After 1 h, considerable amount penetrated and distributed into the upper layers of the skin. After 1.5 h, most of the NPs entered the skin and positioned just in the upper layers of the skin. It means that after 1.5 h, negligible amount of the NPs penetrated the deeper skin layers. Also after 1.5 h, the concentration of the NPs on the top of the skin was decreased indicating the penetration of most of the NPs into the skin.
Figure 6: Fluorescence microscopy of mice skin.
(a) Control (untreated skin), (b) treated by Zno-NPs (formulation 1), (c) treated by albumin-FITC (formulation 6), (d) treated by ZnO-NPs and albumin-FITC (formulation 7), (e) treated by CaP-NPs and albumin-FITC (formulation 8), obtained by fluorescence microscopy after 0.5, 1 and 1.5 h.
Fig. 6c shows the fluorescence images of skin treated by albumin-FITC (formulation 6), where no signal was observed throughout the skin layers but just at the top of the skin. It means that following by application of albumin-FITC alone, almost all the amount of albumin was remained on the skin and not penetrating into the skin at any of the times of 0.5, 1 and 1.5 h. Fig. 6d and e present images of skin treated by ZnO-NPs and albumin-FITC (formulation 7), and CaP-NPs and albumin-FITC (formulation 8), respectively. In fig. 6d and e, penetration of albumin-FITC into the skin was obvious, in contrast to the fig. 6c for albumin-FITC alone. Indeed they showed penetration and distribution of albumin into the skin in presence of the NPs. It means that according to the images, after 0.5 h, the penetration of albumin into the skin was almost negligible although it was somewhat more in presence of CaP-NPs than ZnO-NPs. After 1 h albumin partially penetrated into the skin in presence of ZnO-NPs and distributed into all over the skin layers in presence of CaP-NPs. After 1.5 h, albumin distributed into all over the skin in presence of ZnO-NPs while the concentration of NPs was totally decreased in presence of CaP-NPs.
Discussion
Since penetration of albumin into skin is very poor, it needs enhancers to permeate the skin efficiently. Because albumin is a hydrophilic high MW molecule[12,34] hindering it to pass the skin. The profile of albumin skin permeation showed its maximum after 1 h, which was 5±0.5 mg. The permeated albumin slowly increased during this 1 h and remained constant after that. The profile of permeation of albumin-FITC was similar but its maximum was 2.7±0.7 mg (after 1 h), which was less than that for albumin (P<0.05). This difference can be attributed to that the MW of albumin is lower than albumin-FITC, which led to the more permeation of albumin. Especially that FITC is not a very small molecule and its MW is 389.38 g/mol. The main obstacles for penetration of albumin are its large size and the lipids of SC, which limit the permeation of hydrophilic molecules.
Simultaneous use of ZnO-NPs with albumin, dramatically increased the albumin permeation (P<0.05) as well as the simultaneous use of CaP-NPs with albumin (P<0.05). Using ZnO-NPs or CaP-NPs, led to maximum albumin permeations of 40.2±3.6 and 33.8±5.5 mg, respectively. This not only confirmed the permeation enhancer effect of the both NPs, but also revealed that ZnO-NPs were more powerful enhancer (P<0.05) than CaP-NPs. On the other hand, the amount of permeated albumin after 0.5 h in case of CaP-NPs (20.9±7.4 mg) was more than that in case of ZnO-NPs (11.7±3.3 mg, P<0.05) as well as the amount of permeated albumin after 1 h in case of CaP-NPs (33.8±5.5 mg), which was more than that in case of ZnO-NPs (21.1±3.5 mg, P<0.05). This shows although the maximum and final permeated amount in presence of ZnO-NPs was more than that in presence of CaP-NPs, but the speed of permeation in presence of CaP-NPs was more than that in presence of ZnO-NPs. In this regard, the maximum cumulative permeated albumin in presence of ZnO-NPs was observed after 1.5 h and the maximum cumulative permeated albumin in presence of CaP-NPs was observed after 1 h. Knowing such delivery periods are useful for designing related formulations (containing albumin or other similar proteins) for treatment of different diseases.
The enhancer effect of the both NPs can be referred to their penetration and deposition into the skin layers following by their retention in the skin layers due to their nanometer size and insolubility. This phenomenon disorders the skin microstructure and damage the consistency of skin components, which helps the penetration and permeation of albumin[23]. On the other hand, it has been proved that ZnO-NPs interact with the components of the skin layers[22-24]. Such interactions with skin components may lead to a more altered skin structure and therefore a more albumin permeation in comparison with that for CaP-NPs.
In addition, albumin permeation was not instant and it took place in 1.5 h in the presence of ZnO-NPs and 1 h in the presence of CaP-NPs. Formerly, it has been shown that albumin binds the NPs. In addition, we showed that albumin did not penetrate the skin without presence of the NPs. Therefore, beside the large size and hydrophilicity of albumin, the binding of albumin to the NPs can be responsible for the delayed permeation of albumin through the skin, especially in case of ZnO-NPs. As is observed in the curves, such delay when albumin was used alone and when albumin was used with CaP-NPs was 1 h, but when albumin was used with ZnO-NPs was 1.5 h. On the other hand, the solubility of CaP-NPs is more and quicker than ZnO-NPs. Thus ZnO-NPs remain in the skin for a more period of time. This can be the reason for the greater delay observed for albumin permeation in presence of ZnO-NPs.
In addition, albumin binds low MW cations (e.g. Ca2+, Na+ and K+) with a relatively high affinity[42]. Therefore, albumin binds to the CaP-NPs with high affinity. This can be the reason for the lower total permeation of albumin in the presence of CaP-NPs in comparison with that in the presence of ZnO-NPs.
Trendlines have been plotted according to the ascending part of the curves. The R2 of the line related to the albumin permeation in the presence of ZnO-NPs and CaP-NPs, were 0.97 and 0.98, respectively, which shows that in the presence of the NPs, the rate of albumin permeation over time was almost constant. Such a process makes the permeation of albumin predictable, which is desired for designing related formulations (containing albumin or other similar proteins) and doses for treatment of different diseases. The very close values of the two R2 of the curves, indicated a general similarity between the kinetic and mechanism of the two kinds of NPs.
All the characteristics of the two kinds of NPs mentioned above, caused the differences between their related profiles of albumin permeation. Such different characteristics of the two kinds of NPs, also caused different skin permeation pathways for albumin. The pathways are observed in the microscopic images of skin slices. The images gave more information about the kinetic and deposition of the NPs in the skin and therefore their mechanism as enhancer.
According to the light microscopy images, positioning of the NPs in the different layers of skin, was different, confirming the previous findings by other scientists[23]. This not only confirmed that the ZnO-NPs and CaP-NPs penetrated the skin, but also revealed that there were differences between the distribution patterns of the two kinds of NPs. In addition, there were differences between the distribution patterns of every kind of the NPs after different time intervals. Actually greater amount of the NPs penetrated the skin over time and more amount of the NPs reached the deeper skin layers over time. It means that both NPs delayed to penetrate the skin and delayed to reach the deeper layers of skin. These patterns were employed to describe the enhancer action of the NPs for skin penetration of albumin. Images almost confirmed the previously found data as ZnO-NPs deposit in the SC, connective tissue of the dermis and hair roots[43]. But, we proved a considerable concentration of the NPs in the epidermis beside the SC. Such a deposition of the ZnO-NPs in the skin layers, confirmed the above hypothesis as the NPs altered the microstructure of the skin and therefore led the permeation of albumin. It also conform the finding of other researchers as every change or alteration of the skin structure, especially upper skin, can dramatically change the skin morphology affecting the skin permeability[44].
In contrast to the ZnO-NPs, according to images, using CaP-NPs alone was followed by their distribution into all layers (SC, epidermis and dermis). In case of both NPs, after 0.5 h, almost no NPs penetrated the skin. After 1 h, both NPs to some extent penetrated the skin while ZnO-NPs penetrated the skin more than CaP-NPs. After 1.5 h, the total penetrated NPs again increased and seemed to be equal for both NPs. As mentioned, at this time, ZnONPs were observed mostly in the upper layers while CaP-NPs were observed in all over the skin dept. Therefore accumulation of ZnO-NPs in the SC was more than CaP-NPs and thus ZnO-NPs could alter the SC more than CaP-NPs. Since SC is the main barrier for permeation of molecules, the more accumulation of the ZnO-NPs in SC can be the reason for the more albumin permeation in presence of ZnO-NPs in comparison with that in presence of CaP-NPs. Thus with the exception that ZnO-NPs mostly positioned in the upper layers, the distribution kinetics of the both kinds of NPs were similar, especially in terms of length of time. Therefore, generally the light microscopy images proved the ability of the both NPs to enter and distribute into the skin. This confirms the hypothesis mentioned above that such deposition of the NPs occurred and led to the altering of skin structure and helping the albumin permeation.
The kinetics of skin distribution of the NPs observed in the images, strongly correlated with the albumin permeation profile in presence of the related NPs especially in terms of time and the permeation delay was observed for both cases, penetration of NPs alone and profiles of albumin in presence of the NPs. This phenomenon again demonstrated the enhancer effect of ZnO-NPs on albumin permeation. Besides, this phenomenon indicated that the delay in albumin permeation, would be referred to the delayed distribution kinetics observed for the NPs.
Although permeation of albumin-FITC was shown to be less than albumin, but it was necessary to use albumin-FITC for tracking the albumin in the skin via fluorescence imaging. Fluorescence images of ZnO-NPs depicted a skin distribution pattern similar to their related light microscopy images. They showed little permeated amount after 0.5 h, increased permeated amount after 1 h, and even more increased permeated amount after 1.5 h. During this period of time, the ZnO-NPs accumulated in the upper layers, confirming the previous images.
The intensity of the fluorescence signals of albumin-FITC was more than that of ZnO-NPs at every time of 0.5, 1 or 1.5 h. It can be due to the intrinsic stronger fluorescence of FITC than ZnO-NPs.
Fluorescence images confirmed the above explained patterns of skin distribution of the NPs obtained by light microscopy and the permeation profiles. Images showed that albumin-FITC remained at the top of the skin after 0.5, 1 and even 1.5 h, indicating lack of penetration. Such distribution patterns of albumin-FITC correlated with the albumin or albumin-FITC permeation profiles, revealing negligible amount of permeated albumin after 0.5, 1 and even 1.5 h.
Distribution patterns of albumin-FITC in presence of the NPs, closely correlated with the light microscopy images of the distribution of the NPs without albumin. The patterns also conformed the albumin permeation profiles in presence of the NPs. Distribution of albumin-FITC in presence of the NPs showed that although slight amount of albumin permeated after 0.5 h in presence of each NPs, but great amounts permeated after 1 h. In fact, in presence of ZnO-NPs a considerable amount of albumin permeated after 1 h while in presence of CaP-NPs a huge amount of albumin permeated after 1 h. At this time, albumin was mostly observed in the upper layers in presence of ZnO-NPs while it was observed all over the skin depth in presence of CaP-NPs. However, after 1.5 h, albumin was observed in all the layers in presence of ZnO-NPs and signals of deeper skin increased, indicating the process of albumin permeation. It means that the albumin was still passing through the skin. At this time, the accumulation of the albumin at the top of the skin decreased compared to the time of 1 h, indicating the entrance of considerable amount of albumin into the skin. After 1.5 h, total concentration of albumin in the skin layers in presence of CaP-NPs was decreased, indicating the permeation and exit of part of the amount of albumin from the skin. It means that most of the albumin was passed through the skin at this time.
Although the both ZnO-NPs and CaP-NPs slowly solve in the skin intercellular aqueous medium, but the rate of this dissolution is lower for ZnONPs[11,28] leading to their greater resident time in the skin, which can be responsible for the more total permeation of albumin in the presence of ZnO-NPs. It can also be responsible for the slower permeation of albumin in the presence of ZnO-NPs in comparison with the presence of CaP-NPs. In addition, the quicker dissolution of the CaP-NPs can increase the osmotic pressure of inside of the skin[12], which itself can stimulate the skin penetration and permeation of albumin. Such increase in the osmotic pressure, itself can be the reason of the quicker permeation of albumin in presence of CaP-NPs than in presence of ZnO-NPs.
It can be observed in the images that the fluorescence intensity of the skin images treated by albumin-FITC and ZnO-NPs was slightly more than that of treated by albumin-FITC and CaP-NPs, especially for the image of 0.5 h. It can be attributed to the fact that in contrast to the CaP-NPs, each of the albumin-FITC and ZnO-NPs possess fluorescence emission.
In addition to all the content discussed above, it should be considered that albumin interact with NPs, namely they adsorb on the surface of the NPs like ZnO-NPs[43]. Many studies have proved such adsorption of albumin on metal NPs like Ag, Fe2O3, ZnO and CaP in aqueous medium and neutral pH. This adsorption improves stability of albumin and inhibits aggregation of the NPs[45-47]. Both of these effects are desirable for the present work although ZnO-NPs intrinsically do not highly tend to aggregate[46]. Such interaction between albumin and metal NPs are reversible[28,46] and do not change the structure of albumin as well as its characteristics[48,49]. Such interactions improve stability and function of albumin and are widely used in drug delivery[36]. Besides, such adsorptions have increased translocation of albumin-NPs via cellular barriers[49]. Beside the above reported data, a controlled, delayed or sustained release of adsorbed albumin from CaP-NPs[47] or from other NPs[50] and other similar systems (protein-NPs)[48] have been reported. These reports indicate that if albumin is just adsorbed and is not incorporated in the NPs during preparation, the release of albumin from NPs is delayed but does not take a long time especially about the ZnO-NPs[50]. As a result, the relatively delayed release or permeation of albumin from the skin found in the present study, can be attributed to such adsorption of albumin onto the both kinds of NPs following by a slow release of albumin from the NPs into the skin and then the receiver medium. Such a process was reinforced by the similarity of the images of skins treated by the NPs and treated by albumin and NPs. It means that the distribution patterns of the both kinds of NPs over time, was similar to those of albumin and NPs, which not only revealed the role of the NPs in the permeation of albumin, but also proved such interactions between albumin and the NPs. Finally, the similarities between albumin permeation in presence of ZnO-NPs and CaP-NPs were referred to the similar characteristics of the two kinds of NPs such as size, neutral surface charge and reversible adsorption of albumin. These findings state that metal NPs such as ZnO-NPs and CaP-NPs act as enhancers for skin permeation of albumin in a similar mechanism. Moreover, the differences between albumin permeation in presence of ZnONPs and CaP-NPs were referred to the different deposition and solubility of the two kinds of NPs.
ZnO-NPs and CaP-NPs acted as enhancers for skin permeation of albumin. Their enhancer effect was attributed to their positioning and remaining in the skin layers. After 0.5 h little amount of albumin was permeated in presence of every kind of the NPs. After 0.5 or 1 h the permeated albumin in presence of CaP-NPs was more than that in presence of ZnO-NPs and after 1.5 h the permeated albumin in presence of ZnO-NPs was more than that in presence of CaP-NPs. Indeed the total permeated albumin in presence of CaP-NPs (after 1 h) was 33.8±5.5 mg and the total permeated albumin in presence of ZnO-NPs (after 1.5 h) was 40.2±3.6 mg. Therefore, the enhancer effect of ZnO-NPs was stronger while the enhancer effect of CaP-NPs was quicker. Such differences were attributed mainly to the different skin distribution and solubility of the NPs. The albumin permeation over time in presence of every kind of the NPs was relatively delayed and the both related profiles were linear with different slopes. The similarity of skin distribution of each of the NPs alone and skin distribution of albumin in the presence of the NPs, proved the accompaniment of albumin with the NPs (adsorption), which not only demonstrated the enhancer effect of the NPs, but also was responsible for the delayed release or permeation of albumin from skin. Such findings about the mechanism, dose, duration, linearity and etc. of the ZnO-NPs and CaP-NPs as skin permeation enhancers for albumin, can be used for designing and preparation of such topical formulations containing albumin or other similar proteins as active ingredients.
Acknowledgements
The research was supported by Ardabil University of Medical Sciences.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Barry BW. Breaching the skin?s barrier to drugs. Nat Biotechnol2004;22:165-7.
- Prausnitz MR, Mitragotri S, Langer R. Current status and futurepotential of transdermal drug delivery. Nat Rev Drug Discov2004;3:115-24.
- Smith EW, Maibach HI. Penetration enhancer classification. In: BarryBW, editor. Percutaneous Penetration Enhancers. London: Taylor andFrancis; 2006. p. 4-14.
- Karadzovska D, Brooks JD, Monteiro-Riviere NA, Riviere JE.Predicting skin permeability from complex vehicles. Adv Drug DelivRev 2013;65:265-77.
- Lee CK, Uchida T, Noguchi E, Kim NS, Goto S. Skin permeationenhancement of tegafur by ethanol/panasate 800 or ethanol/water binaryvehicle and combined effect of fatty acids and fatty alcohols. J PharmSci 1993;82:1155-9.
- Bae YS, Hill ND, Bibi Y, Dreiher J, Cohen AD. Innovative uses forzinc in dermatology. DermatolClin 2010;28:587-97.
- Shokri N, AkbariJavar H, FouladdelSh, Khalaj A, Khoshayand M,Dinarvand R, et al. Preparation and evaluation of poly(caprolactonefumarate) nanoparticles containing doxorubicin HCI. Daru2011;19:12-22.
- Shokri N, AkbariJavar H, Fouladdel SH, Khalaj A, Dinarvand R,Azizi E. Preparation and characterization of crosslinked andnon-crosslinkedpolycaprolactonefumarate (PCLF) NPs as carriers fordoxorubicin HCl. Afr J Pharm Pharmacol 2011;5:797-805.
- Shokri N, Azizi E, AkbariJavar H, Fouladdel SH, Khalaj A,Dinarvand R. In vitro and in vivo evaluation of poly (caprolactonefumarate) nanoparticles. Indian J Pharm Sci Res 2012;3:3106-15.
- Peira E, Turci F, Corazzari I, ChirioD, Battaglia L, Fubini B, et al. The influence of surface charge and photo-reactivity on skin-permeationenhancer property of nano-TiO2 in ex vivo pig skin model under indoorlight. Int J Pharm 2014;467:90-9.
- Sun L, Chow LC, Frukhtbeyn SA, Bonevich JE. Preparation andproperties of nanoparticles of calcium phosphates with various Ca/Pratios. J Res NatlInst Stand Technol 2010;115:243-55.
- Li J, Chen YC, Tseng YC, Mozumdar S, Huang L. Biodegradablecalcium phosphate nanoparticle with lipid coating for systemic siRNAdelivery. J Control Release 2010;142:416-21.
- Sokolova V. Dissertation 2006. Synthesis, Characterization andApplication of Calcium Phosphate Nanoparticles for the Transfectionof Cells. University of Duisburg-Essen; 2006.
- Epple M, Ganesan K, Heumann R, Klesing J, Kovtun A, Neumann S,et al. Application of calcium phosphate nanoparticles in biomedicine. JMater Chem 2010;20:18-23.
- Sahdev P, Podaralla S, Kaushik RS, Perumal O. Calcium phosphatenanoparticles for transcutaneous vaccine delivery. J BiomedNanotechnol 2013;9:132-41.
- Paul W, Sharma C. Synthesis and characterization of alginate coatedzinc calcium phosphate nanoparticles for intestinal delivery of insulin.Process Biochem 2012;47:882-6.
- Aksoy B, Atakan N, Aksoy HM, Tezel GG, Renda N, Ozkara HA,et al. Effectiveness of topical zinc oxide application on hypertrophicscar development in rabbits. Burns 2010;36:1027-35.
- Forslind B. The skin barrier: Analysis of physiologically importantelements and trace elements. ActaDermVenereolSuppl (Stockh)2000;208:46-52.
- Parat MO, Richard MJ, Pollet S, Hadjur C, Favier A, Béani JC. Zincand DNA fragmentation in keratinocyte apoptosis: Its inhibitory effectin UVB irradiated cells. J PhotochemPhotobiol B 1997;37:101-6.
- Lima J, Martins R, Neri C, Serra O. ZnO:CeO2-based nanopowderswith low catalytic activity as UV absorbers. Appl Surf Sci2009;255:9006-9.
- Parkinson TF, Millikan LE, Anderson DF. Manganese, copper andzinc concentrations in human skin lesions. Int J ApplRadiatIsot1979;30:411-5.
- Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, et al. Selectivechemical labeling reveals the genome-wide distribution of5-hydroxymethylcytosine. Nat Biotechnol 2011;29:68-72.
- Desouza ED, Atiya IA, Al-Ebraheem A, Wainman BC, Fleming DE,McNeill FE, et al. Characterization of the depth distribution of Ca, Feand Zn in skin samples, using synchrotron micro-x-ray fluorescence(SµXRF) to help quantify in vivo measurements of elements in theskin. ApplRadiatIsot 2013;77:68-75.
- Trevor A, Katzung B, Masters S, Knuidering-Hall M, editors. Drugswith important actions on blood, inflammation, and gout. Katzung andTrevor?s Pharmacology Examination and Board Review. USA: Appletonand Lange; 2013. p. 317-25.
- Kathiravan A, Paramagurn G, Renganathan R. Study on the binding ofcolloidal zinc oxide nanoparticles with bovine serum albumin. J MolStruct 2009;934:129-37.
- Vandebriel RJ, De Jong WH. A review of mammalian toxicity of ZnOnanoparticles. NanotechnolSciAppl 2012;5:61-71.
- Labouta HI, Schneider M. Interaction of inorganic nanoparticles withthe skin barrier: Current status and critical review. Nanomedicine2013;9:39-54.
- Agren MS, Chvapil M, Franzén L. Enhancement of re-epithelializationwith topical zinc oxide in porcine partial-thickness wounds. J Surg Res1991;50:101-5.
- Choi SJ, Choy JH. Biokinetics of zinc oxide nanoparticles:Toxicokinetics, biological fates, and protein interaction. Int JNanomedicine 2014;9:261-9.
- Shokri N, Goodarzi MT, AkbariJavar H, Soltani Y. Zinc oxide and zincoxide nanoparticles as enhancers in topical pharmaceutical and cosmeticproducts. J Pharm Res 2014;13:40-4.
- Shokri N, AkbariJavar H. Zinc oxide nanoparticles as skin permeationenhancer for solvents and surfactants. J Pharm Res 2014;13:85-91.
- Fujiwara S, Amisaki T. Fatty acid binding to serum albumin: Molecularsimulation approaches. BiochimBiophysActa 2013;1830:5427-34.
- Shokri N, AkbariJavar H, Khalaj A. Effect of hydrophilic solventson enhancing transdermal delivery of albumin. Int J Pharm Sci Res2014;5:1000-7.
- Shokri N, AkbariJavar H, Ghadermazi R. Effects of skin penetrationenhancers in topical antiaging products containing a-Hydroxy acids andhyaluronic acid. Avicenna J Med Biochem 2014;2:e18611.
- Wu YW, Chen SF, Yang CB, Tsai YH. Screening, purification, andidentification of a copper-dependent FITC-binding protein in humanplasma: Albumin. J ChromatogrB AnalytTechnol Biomed Life Sci2008;863:187-91.
- Warner KS, Li SK, He N, Suhonen TM, Chantasart D, Bolikal D, et al.Structure-activity relationship for chemical skin permeation enhancers:Probing the chemical microenvironment of the site of action. J PharmSci 2003;92:1305-22.
- Warner RR, Bush RD, Ruebusch NA. Corneocytes undergo systematicchanges in element concentrations across the human inner stratumcorneum. J Invest Dermatol 1995;104:530-6.
- Kim MH, Park DH, Yang JH, Choy YB, Choy JH.Drug-inorganic-polymer nanohybrid for transdermal delivery. Int JPharm 2013;444:120-7.
- Jasi-Ska A, Ferguson A, Mohamed W,Szreder T. The study of interactions between ibuprofen and bovine serum albumin. Food ChemBiotechnol 2009;73:15-24.
- De Bruijn HS, Meijers C, van der Ploeg-van den Heuvel A,Sterenborg HJ, Robinson DJ. Microscopic localisation ofprotoporphyrin IX in normal mouse skin after topical applicationof 5-aminolevulinic acid or methyl 5-aminolevulinate. J PhotochemPhotobiol B 2008;92:91-7.
- Zhai Y, Yang X, Zhao L, Wang Z, Zhai G. Lipid nanocapsules fortransdermal delivery of ropivacaine: In vitro and in vivo evaluation. IntJ Pharm 2014;471:103-11.
- Burtis C, Ashwood E, Bruns D. Tietz Textbook of Clinical Chemistryand Molecular Diagnostics. St. Louis: Elsevier; 2006.
- Chang Y, Zhang M, Xia L, Zhang J, Xing G. The toxic effects andmechanisms of CuO and ZnO nanoparticles. Materials 2012;5:2850-71.
- Hansen S, Lehr CM, Schaefer UF. Modeling the human skin barrier ?Towards a better understanding of dermal absorption. Adv Drug DelivRev 2013;65:149-51.
- Ravindran A, Singh A, Raichur AM, Chandrasekaran N, Mukherjee A.Studies on interaction of colloidal Ag nanoparticles with Bovine SerumAlbumin (BSA). Colloids Surf B Biointerfaces 2010;76:32-7.
- Wells MA, Abid A, Kennedy IM, Barakat AI. Serum proteinsprevent aggregation of Fe2O3 and ZnO nanoparticles. Nanotoxicology2012;6:837-46.
- Tarafder S, Banerjee S, Bandyopadhyay A, Bose S. Electricallypolarized biphasic calcium phosphates: Adsorption and release ofbovine serum albumin. Langmuir 2010;26:16625-9.
- Bi Y. Study on the Interactions between Nanomaterials and Proteins.University of Western Ontario, Electronic Thesis and DissertationRepository, Paper 946; 2012.
- Saptarshi SR, Duschl A, Lopata AL. Interaction of nanoparticleswith proteins: Relation to bio-reactivity of the nanoparticle. JNanobiotechnology 2013;11:26.
- Dasgupta S, Banerjee SS, Bandyopadhyay A, Bose S. Zn-and Mg-dopedhydroxyapatite nanoparticles for controlled release of protein. Langmuir2010;26:4958-64.
Notice of Retraction: After careful and considered review of the content of this paper by a duly constituted expert committee, this paper has been found to be in violation of IJPS Publication Principles. We hereby retract the content of this paper. Reasonable effort should be made to remove references to this paper.
Evidence
It has come to our attention that there are substantial overlaps between the text of the above article and the following papers, especially in the introduction, methods, and discussion. It has been pointed out that the above study appeared to contain a high level of similarity with four articles by the same authors published as below:
-Shokri N, Akbari Javar H. Comparison of calcium phosphate and zinc oxide nanoparticles as dermal penetration enhancers for albumin. Indian J Pharm Sci. 2015 Nov-Dec; 77(6): 694–704
-Shokri N, Akbari Javar H. Using a Synergistic Combination of Two Enhancers for Dermal Delivery of Collagen in Pharmaceutical and Cosmetic Products. Journal of Pharmaceutical Research. 2015 Jan-Mar, 14(1); 1-6
-Shokri N, Akbari Javar H. Zinc Oxide Nanoparticles as Skin Permeation Enhancer for Solvents and Surfactants. Journal of Pharmaceutical Research. 2014;13(3):85-91.
-Shokri N, Goodarzi MT, Akbari Javar H, Soltani Y. Zinc Oxide and Zinc Oxide Nanoparticles as Enhancers in Topical Pharmaceutical and Cosmetic Products. Journal of Pharmaceutical Research. 2014;13(2):40-44.
-Shokri N. Different Inorganic Nanoparticles as Enhancers for Dermal Delivery of Proteins in Pharmaceutical and Cosmetic Products. Journal of Pharmaceutical Research. 2014 Oct; 13(4):116-121
The published paper in IJPS and the paper in the Journal of Pharmaceutical Research. 2014 Oct; 13(4):116-121, had overlap in the introduction, aim, methods, results, discussion, and conclusion indicate that these papers are an example of redundancy.
Following an internal investigation, it was established that the studies were conducted along broadly similar lines. Furthermore, submitting multiple articles derived from the same data set with the overlap without cross-referencing is not legitimated based on the ethical principles of Scopus. Hence the article was retracted.