- *Corresponding Author:
- Manjoosha Srivastava
Phytochemistry Division,
Council of Scientific and Industrial Research-National Botanical Research Institute,
Lucknow,
Uttar Pradesh 226001
E-mail: m.srivastava@nbri.res.in
| Date of Received | 24 December 2020 |
| Date of Revision | 26 October 2021 |
| Date of Acceptance | 05 May 2022 |
| Indian J Pharm Sci 2022;84(3):552-559 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Acacia nilotica, indigenously known as ‘Babool’ of family Leguminosae has many traditional and ethnobotanical uses. All the parts of tree are important and seeds are most abundantly available which are rich in different phytochemicals. Thus, the aim of this study was qualitative and quantitative analysis of phytochemicals and evaluation of the antioxidant activity in Acacia nilotica seed along with their parts viz. cotyledon and endosperm as a potential source in nutritional and medicinal applications. Phytochemicals as carbohydrate, protein, alkaloid, flavonoids, phenolics, saponin and tannin were present in seed, cotyledon and endosperm. Cotyledon was rich in sugar, carbohydrate, protein and oil, i.e. 4.73 %, 17.83 %, 30.45 % and 11.78 % respectively whereas endosperm was rich in phenolics and flavonoids i.e. 2.66 mg/g gallic acid equivalent and 7.66 mg/g quercetin equivalent respectively. Antioxidant activity studies showed that endosperm exhibited maximum percentage inhibition i.e. 74.07 % compared to the standard butylated hydroxyanisole having 82.88 % inhibition of 2,2-diphenyl-1-picrylhydrazyl radicals. The study revealed that the specific utilization of Acacia nilotica seed have scope in nutritional supplements and as useful bioactive extracts for sustainable health benefits.
Keywords
Acacia nilotica, 2,2-diphenyl-1-picrylhydrazyl, gallic acid equivalent, quercetin equivalent, nutritional, phytochemicalsuse
Plants and their parts are good source of effective medicines and nutritional supplements for human health and well-being. Nearly three quarter population of the world depends upon the plants and their products for healthcare management and about 30 % plant species are in medicinal use. In Indian medicine system such as Ayurveda and Unani, drugs of herbal origin have been used traditionally and ethnbotanically since ancient times [1].
Acacia nilotica (A. nilotica), indigenously known as ‘Babool’, belonging to subfamily Mimosoideae [2,3] and family Leguminosae [4,5], is an important and evergreen tree of Indian dried regions. Tree is generally 10 m in height, bark is longitudinally fissured with dark brown or black color and young plant exhibit terete, pubescent and slender branchlets [6]. A. nilotica recognized as a pioneer species for high bioactive compounds and economically important source of gum, tannins, fodder, fuel and timber from long time [7,8]. The species are widespread throughout Asia, Africa and America [9], and naturally found in Botswana, Kenya, Egypt, Zimbabwe, India, Saudi Arabia, Burma, Tanzania, Nepal, Pakistan, Nigeria, Sri Lanka, Indonesia, Sudan, Iraq, Iran, Namibia, Yemen and Ethiopia [10]. The plant A. nilotica has been used in traditional medicines and their all parts viz. gum, seeds, pods, roots, leaves, flowers and bark are medicinally important [11-13]. The plant is rich in different phytochemicals viz. carbohydrates, alkaloids, proteins, fatty acids, phenolics, flavonoids, tannins, saponins, cyanogenic glycosides, diterpenes, phytosterols, triterpenegenins, amino acids viz. cystine, methionine, threonine, lysine, tryptophan and macro elements such as potassium, phosphorus, magnesium, iron and manganese [14,15]. A. nilotica possesses antioxidant, antimicrobial, antifungal, antibacterial, antipyretic, antiinflammatory, antidiarrhoeal, antiulcer, antihelmintic, wound healing, diuretic, antispasmodial, antihypertensive, antitumor and anticancer properties [16-19] and effective in coughs, cold, congestion, nerve stimulation, leucorrhea, dysentery, ophthalmia, hemorrhages and sclerosis relief [20]. Antioxidant property plays a vital role for the treatment of inflammation and cancer which are caused by free radicals [21] and presence of tannin which is beneficial for activation of glucose transfer and prevents lipolysis [22]. Phenolic compounds in A. nilotica makes the species potential for antioxidant property due to free radical scavenger activity [23,24].
A. nilotica seed exhibit edible characteristics and has nutritive and medicinal values due to various phytochemicals. Although, some studies are available on seed [25], but part specific study of seed on phytochemicals and bioactivity has not been reported yet. Thus, A. nilotica seed was exploited for phytochemical investigation and antioxidant property.
Materials and Methods
Plant material:
Seeds were collected from Kalli Pashchim region of Lucknow district (Uttar Pradesh), India. The material was authenticated (LWG No.102994) and specimen was deposited in the herbarium at Council of Scientific and Industrial Research (CSIR)-National Botanical Research Institute (Uttar Pradesh), India. Different parts of the seed viz. cotyledon and endosperm (seed gum) were separated mechanically using grinder (Philips grinder) and sieved by 40 mesh sieve to obtain 40 mesh powder of each part for study.
Chemicals viz. Gallic acid, Quercetin, 2,2-Diphenyl-1- Picrylhydrazyl (DPPH) and Butylated Hydroxyanisole (BHA) of Merck, solvents viz. n-hexane, chloroform, acetone, ethanol and methanol of Qualigens and glasswares made of Borosil were used.
Preliminary study:
Powdered materials were examined for organoleptic characteristics viz. colour, odour, taste and texture. The solubility was studied in solvents viz. hexane, chloroform, acetone, ethanol, distilled water and boiling water [26].
Extraction and phytochemical screening:
Materials were extracted under cold and hot conditions using different polarity gradient solvents viz. n-hexane, chloroform, acetone, alcohol and water. Cold extraction was carried out using percolation method by exhausting 50 g powder materials each in 250 ml solvents for 24 h at room temperature (25°-30°). Extracts were then filtered using Whatman filter paper (No. 42). Hot extraction was carried out through successive soxhlet extraction method by taking 50 g powder materials and 250 ml different solvents in soxhlet apparatus on water bath. The extracts were concentrated through Rotary Evaporator (IKA-RV 10 digital) at 40°-55° and dried at low temperature (-80°) and pressure (0.200 millibar (mBar)) using lyophilizer (Labconco- FreeZone Plus 4.5). Dried extracts were used in qualitative tests for the identification of various phytochemicals viz. carbohydrates, sugar, starch, glycosides, alkaloids, phenolics, flavonoids, saponins, tannins and sterols which were estimated quantitatively [27,28].
Phytochemical estimation:
Phytochemicals identified in different polarity gradient solvent extracts, viz. n-hexane, chloroform, acetone, ethanol and water were estimated quantitatively according to standard estimation methods.
Total sugar:
0.5 g powdered material was homogenized with 5 ml 80 % ethanol and centrifuged using centrifuge (Eltek TC 4100 F) for 15 min at 2000 rpm. The supernatant volume obtained was adjusted to 10 ml with 80 % ethanol. 0.1 ml sample was taken, mixed 0.1 ml 80 % phenol and 5 ml concentrated sulphuric acid respectively and made up the volume to 10 ml using 80 % ethanol. Thereafter, prepared samples were kept in an ice bath for 30 min. The D-glucose stock solution of 0.1 mg/ml concentration was prepared as standard and 1, 2, 3, 4 and 5 ml of stock solutions were taken for dilutions. The calculation of total sugar was carried out by measuring the absorbance at 490 nm double beam Ultraviolet-Visible (UV) spectrophotometer (Thermo Scientific-Evolution 201 UV spectrophotometer) [29].
Total carbohydrate:
100 mg material was taken into different boiling tubes, added 5.0 ml of 2.5 N Hydrochloric Acid (HCl) in each tube and boiled for 3 h on water bath. Samples were cooled at room temperature, neutralized by adding sodium carbonate and centrifuged. Dilutions were prepared by mixing 0.2 ml of sample, 1 ml 5 % phenol and 5 ml of 96 % sulphuric acid. Samples kept for 10 min at room temperature, then heated on water bath for 15 min and cooled. Stock solution of D-glucose as standard was prepared in 0.1 mg/ml concentration and dilutions were prepared by taking 0.2, 0.4, 0.6, 0.8 and 1 ml from stock solution. Total carbohydrate was calculated by measuring the absorbance at 630 nm [30] through UV spectrophotometer.
Total protein:
500 mg powdered materials was homogenized with 5 ml of phosphate buffer of pH 7.4 and centrifuged for 20 min at 4000 rpm. The supernatant was filtered and volume was maintained to 10 ml with phosphate buffer. 0.1 ml of sample was taken and made up to 1 ml with distilled water. Then 5 ml of Reagent-C was added which was prepared by adding 50 ml Reagent-A, i.e. 2 % sodium carbonate in 0.1 N sodium hydroxide and 1 ml Reagent-B, i.e. 0.5 % copper sulphate in 1 % potassium sodium tartrate. Mixed the solutions and let stand for 10 min. Thereafter, 0.5 ml 1 N Folin-Ciocalteu Reagent (FCR) was added, mixed the samples and kept in the dark for 30 min. The stock solution of Bovine Serum Albumin (BSA) as standard of 1 mg/ml concentration was prepared. 0.2, 0.4, 0.6, 0.8 and 1 ml of BSA stock solution taken for dilutions. Total protein was calculated by measuring the absorbance at 660 nm [31] through UV spectrophotometer.
Oil content:
Seed, cotyledon and endosperm powder was extracted with 250 ml n-hexane through soxhlet apparatus for 8 h on water bath. The excess solvent from the extract was evaporated through rotary evaporator at 40°, dried in vacuum and oil content was gravimetrically quantified [32].
Total starch:
0.5 g powdered material was homogenized with 5 ml of 80 % ethanol and centrifuged for 15 min at 2000 rpm. The centrifugate was separated and added 4 ml distilled water, heated for 15 min on water bath and macerated using a glass rod. 3 ml of 52 % perchloric acid was then added in the macerated sample, homogenized and centrifuged for 15 min at 2000 rpm. Thus, the supernatant obtained was made up to the volume with distilled water. In 0.1 ml of sample, mixed 0.1 ml of 80 % phenol, 5 ml of concentrated sulphuric acid, make up the volume up to 10 ml with distilled water and kept in an ice bath for 30 min. The dilutions of standard were prepared by taking 1, 2, 3, 4 and 5 ml of D-glucose stock solution of 0.1 mg/ml concentration. Total starch was calculated by measuring the absorbance at 490 nm [29] through UV spectrophotometer.
Total tannin:
1 g powdered material was extracted with 50 ml distilled water by boiling on the water bath for 6-8 h, filtered the extracts and volume was made up to 50 ml in volumetric flask. Then mixed 0.5 ml of FCR, 1 ml saturated sodium carbonate solution in 0.1 ml of sample and made the volume to 10 ml with distilled water. The stock solution of tannic acid at 0.1 mg/ml concentration was used as standard and different dilutions were prepared by taking 0.2, 0.4, 0.6, 0.8 and 1 ml of stock solution. Total tannin was calculated through tannic acid standard curve with the help of absorbance using UV spectrophotometer at 760 nm [28].
Total phenolics:
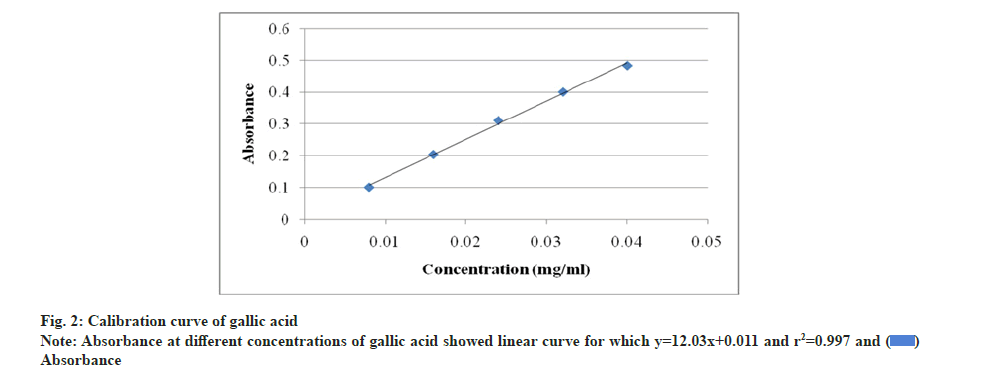
Total phenolic content was estimated by extracting 50 g of powdered materials with 250 ml methanol. Extracts were filtered after 24 h, concentrated by rotary evaporator and dried in vacuum. Stock solutions of 1 mg/ml concentration were prepared by dissolving the extracts in methanol. 0.5 ml of stock solutions was taken in 25 ml volumetric flask, added 10 ml of distilled water, 1.5 ml of FCR and allowed to stand for 5 min. 4 ml of 20 % sodium carbonate solution was then added and made up the volume to 25 ml with distilled water. The prepared mixtures were kept in dark for 30 min. The stock solution of gallic acid of 0.1 mg/ml concentration was prepared as standard. Total phenolic content was calculated by using gallic acid standard curve with the measurement of absorbance at 765 nm through UV spectrophotometer. The equation: y=115.9x+0.113, correlation coefficient (r2)=0.999 was used according to calibration curve (y=Absorbance, x=Gallic Acid Equivalent (GAE)) [33].
Total flavonoids:
Total flavonoid content was estimated by extracting 50 g of powdered materials with 250 ml methanol. The stock solutions of methanolic extracts at 1 mg/ml concentration were prepared. In 1 ml sample, added 1 ml of 2 % methanolic aluminium chloride solution, made the volume to 10 ml using methanol and allowed to stand the mixtures for 1 h. Similar process was repeated for preparation of quercetin stock solution of 0.1 mg/ ml concentration as standard and prepared the dilutions using 0.2, 0.4, 0.6, 0.8 and 1 of stock solution. Total flavonoid content was calculated through quercetin standard curve with the help of absorbance recorded at 420 nm using UV spectrophotometer. The equation 74.61x+0.058, r2=0.998 was used for the calculation of total flavonoid content (y=Absorbance, x=Quercetin Equivalent (QE) in mg/ml) [34].
Antioxidant activity:
The samples were prepared by extracting the materials in methanol. The stock solutions of methanolic extract of samples and 0.135 mM methanolic solution of DPPH were prepared in 1 mg/ml concentration. Different dilutions were prepared by taking 0.02, 0.04, 0.06, 0.08 and 0.1 ml methanolic stock solution of samples, added 1 ml DPPH solution and made the volume up to 2 ml with methanol. The mixtures were allowed to stand for 30 min in the dark. The similar process was repeated to prepare the dilutions of BHA of 1 mg/ml concentration as antioxidant standard. The absorbance was measured at 517 nm through UV spectrophotometer [35].
Statistical analysis:
The statistical analysis of data was carried out by performing the experiments in triplicate. The data was represented by mean±Standard Deviation (SD). The linear correlation coefficient was observed using MS Office Excel 2007. Regression equation was used for phenolics, flavonoids concentrations and Half-Maximal Inhibitory Concentration (IC50) values in DPPH radical scavenging assay calculation. The p values less than 0.05 were considered significant.
Results and Discussion
The organoleptic properties and solubility of seed, cotyledon and endosperm powders were determined for the quality and acceptability of powdered materials (Table 1 and Table 2). The qualitative study showed that phytochemicals were majorly found in alcohol and water extracts of the seed, cotyledon and endosperm (Table 3).
| Sample | Color | Odour | Taste | Texture |
|---|---|---|---|---|
| Seed | Creamish brown | Characteristic | Neutral | Amorphous |
| Cotyledon | Cream | Characteristic | Nutty with slight bitterness | Amorphous |
| Endosperm | Brown | Characteristic | Nutty with slight bitterness | Amorphous |
Table 1: Organoleptic Properties of A. nilotica Seed and their Parts.
| Sample | Hexane | Chloroform | Acetone | Ethanol | Water | Boiling water |
|---|---|---|---|---|---|---|
| Seed | - | - | - | - | + | + |
| Cotyledon | - | - | - | - | + | + |
| Endosperm | - | - | - | - | + | + |
Note: (+): Soluble and (-): Insoluble
Table 2: Solubility of A. nilotica Seed and their Parts.
| Phytochemical groups | Seed | Cotyledon | Endosperm |
|---|---|---|---|
| H Chl Ac Al W | H Chl Ac Al W | H Chl Ac Al W | |
| Carbohydrate | - - - + + | - - - + + | - - - + + |
| Protein | - - - + + | - - - + + | - - - + + |
| Alkaloid | - + + + + | - + - + + | - - - + - |
| Phenolic | - - - + - | - - - + - | - - - + - |
| Flavonoid | - - - + - | - - - + - | - - - + - |
| Tannin | - - - + + | - - - + + | - - - + + |
| Saponin | - - - - + | - - - - + | - - - - + |
| Glycoside | - - - - - | - - - - - | - - - - - |
| Tri-terpenoid | - - - - - | - - - - - | - - - - - |
| Sterols | - - - - - | - - - - - | - - - - - |
Note: H: n-hexane; Chl: Chloroform; Ac: Acetone; Al: Alcohol; W: Water; (+): Present and (-): Absent
Table 3: Phytochemical Screening of A. nilotica Seed and their Parts.
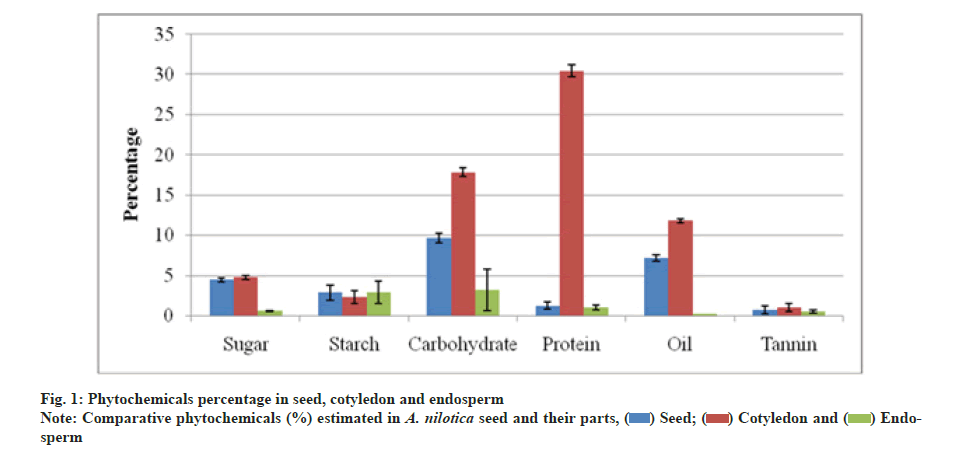
Quantitative estimation revealed that total sugar was 4.43 % in seed, 4.73 % in cotyledon and 0.56 % in endosperm. Cotyledon part was found to have 17.83 % carbohydrate which was higher than in the seed i.e. 9.66 % and in endosperm i.e. 3.16 %. Total protein was estimated up to 30.45 % in the cotyledon part which was significantly higher than in seed i.e. 1.23 % and in endosperm i.e. 0.99 %. The cotyledon was also rich in oil i.e. 11.78 % while seed had 7.16 % and endosperm part had only 0.18 %. Starch was estimated in similar ranges i.e. 2.86 % in the seed and endosperm part and 2.30 % in the cotyledon. Total tannin varied as 1 % in cotyledon, 0.7 % in seed and 0.46 % in the endosperm respectively (fig. 1).
The quantification of phenolic and flavonoid content were determined through calibration curves of gallic acid and quercetin (fig. 2 and fig. 3). The phenolic content was 2.66 mg/g GAE in endosperm part which was significantly higher than 1.80 mg/g and 1.26 mg/g GAE in seed and cotyledon respectively. Endosperm part was also rich in flavonoid content with 7.66 mg/g QE which was noteworthy and more than in seed 4.0 mg/g QE and cotyledon 1.33 mg/g QE (Table 4).
| Sample | Total phenolic content [GAE (mg/g)]±SD (n=3) | Total flavonoid content [QE (mg/g)]±SD (n=3) |
|---|---|---|
| Seed | 1.80±0.40 | 4.0±1.0 |
| Cotyledon | 1.26±0.11 | 1.33±0.57 |
| Endosperm | 2.66±0.18 | 7.66±1.52 |
Note: GAE: Gallic Acid Equivalent; QE: Quercetin Equivalent; ±SD: Standard Deviation in values, n=3. Values were performed in triplicates and taken as mean which are significant (p<0.05)
Table 4: Total Phenolic and Flavonoid Content in A. nilotica Seed and their Parts.
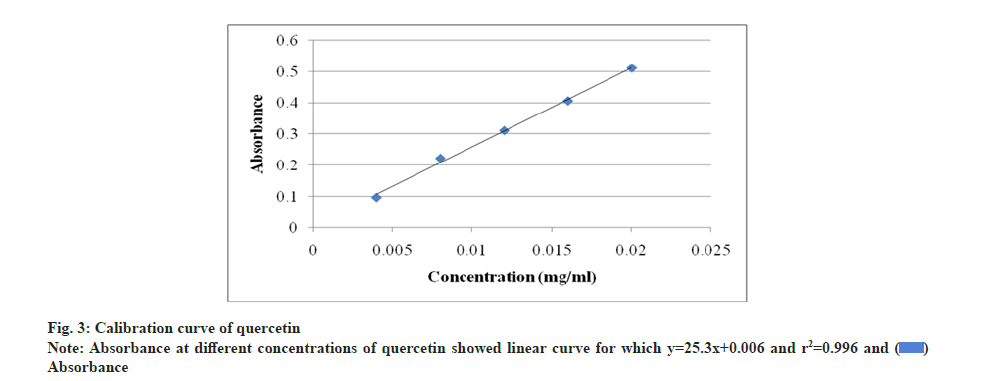
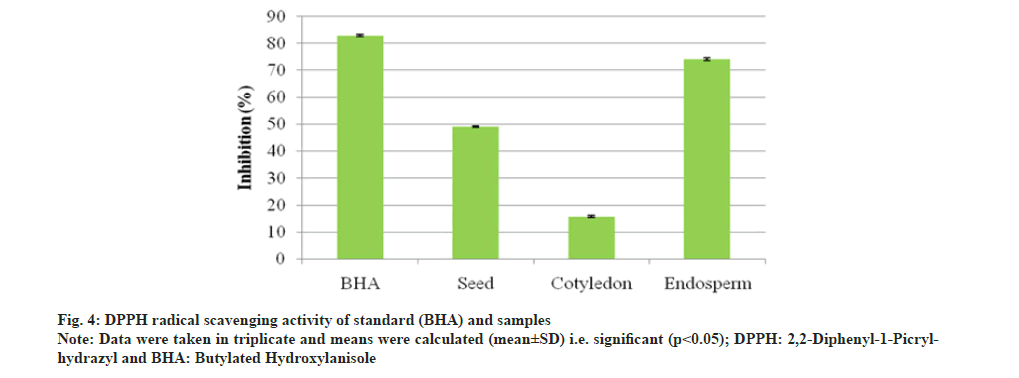
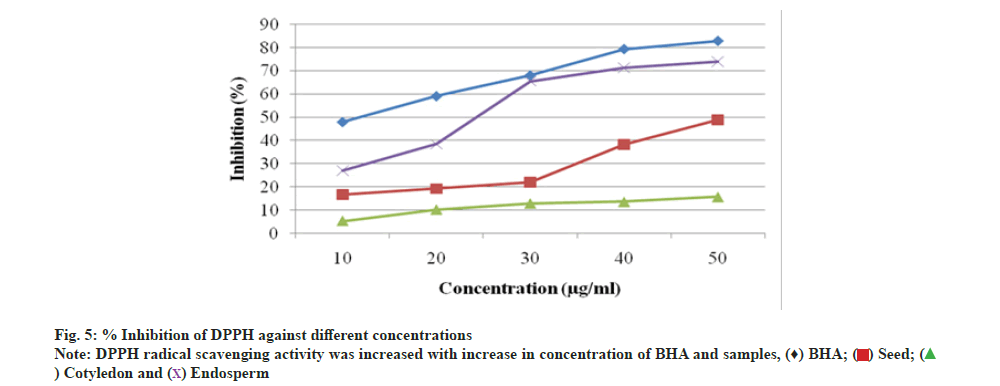
The DPPH radical scavenging assay of A. nilotica seed, cotyledon and endosperm in comparison to standard BHA showed that percentage (%) inhibition of free radicals in BHA was 82.88 % and in seed, cotyledon and endosperm was found to be 49.02 %, 15.69 % and 74.07 % respectively (fig. 4). A. nilotica endosperm was comparable to standard and exhibit more potentiality for free radical reducing ability than the seed and cotyledon. The inhibitory concentration (IC50) value and % inhibition for antioxidant activity are inversely proportional to each other; % inhibition increases and IC50 value decreases. % inhibition of BHA was higher with lower IC50 value, i.e. 10.61 μg/ml. Endosperm showed most significant antioxidant activity with IC50 value, i.e. 25.84 μg/ml, lower than that of seed and cotyledon, i.e. 55 and 191.10 μg/ml respectively as compared to BHA (Table 5). Thus, endosperm was identified as most potential inhibitor of DPPH radicals. Further, the graph (fig. 5) of reducing power capacity of seed, cotyledon and endosperm in methanol extracts as compared to standard BHA showed that as the concentration of the extracts increased, inhibition of DPPH radicals also increased.
| Sample | IC50 value (µg/ml)±SD (N=3) |
|---|---|
| BHA | 10.61±0.63 |
| Seed | 55±0.78 |
| Cotyledon | 191.10±18.79 |
| Endosperm | 25.84±0.21 |
Note: Values were represented in triplicate (mean±SD). Mean values were statistically significant (p<0.05) and BHA: Butylated Hydroxylanisole
Table 5: Ic50 Values in DPPH Radical Scavenging Model in A. nilotica Seed and their Parts.
The results revealed that the A. nilotica seed contained nutritionally and medicinally important phytochemicals. The part specific study of seed explored the utilization of seed parts for specific purpose. The cotyledon part was rich in sugar, carbohydrate, protein and oil for nutritional applications, while the endosperm part was useful for antioxidant property due to phenolics and flavonoids. The antioxidants reduce the level of Reactive Oxygen Species (ROS). ROS is a major cause of oxidative stress diseases such as pain, inflammation, tissue damage or injury, neurodegenerative disorder, cardiovascular problem and cancer. Thus, the study prospect for plant based better source materials to develop safe and eco-friendly healthcare industrial products.
Acknowledgements:
Authors are thankful to the Director, CSIR-National Botanical Research Institute, Lucknow, for providing facilities and support. Geetendra Kumar is also thankful to University Grant Commission for financial support.
Conflict of interests:
The authors declared no conflict of interest.
References
- Joy PP, Thomas J, Mathew S, Skaria PB. Medicinal plants, Kerala agricultural university. India: Aromatic and Medicinal Plants Research Station; 1998. p. 4-6.
- Prajapati ND, Purohit SS. Agro’s colour atlas of medicinal plants. India: Agrobios; 2004. p. 3.
- Rastogi RP, Mehrotra BN. Compendium of Indian medicinal plants. Lucknow/New Delhi: Central Drug Research Institute/National Institute of Science Communication; 2001. p. 4-7.
- Nadkarni KM. The Indian plants and drugs. New Delhi: Shrishti Book Distributors; 2005. p. 4-5.
- Dymock W, Warden CJH, Hooper D. Pharmacographica Indica: A history of the principal drugs of vegetable origin. New Delhi: Shrishti Book Distributors; 2005. p. 556.
- Kritikar KR, Basu BD. Indian Medicinal plants with illustrations. 2nd ed. Uttaranchal: Oriental Enterprises; 2001. p. 1289-92.
- Gupta RK. Resource survey of gummiferous Acacias in western Rajasthan. Trop Ecol 1970;11(2):148-61.
- Mahgoub S. On the subspecies of Acacia nilotica in the Sudan. Khartoum, Sudan: Sudan Silva 1979;4:57-62.
- Raj A, Haokip V, Chandrawanshi S. Acacia nilotica: A multipurpose tree and source of Indian gum Arabic. South Indian J Biol Sci 2015;1(2):66-9.
- Orwa C, Mutua A, Kindt R, Jamnadass R, Simons A. Agroforestree Database: A tree reference and selection guide. Agroforestry Database: 4.0; 2009.
- El-Hadiyah TM, Abdulhadi NH, Badico EE, Mohammed EY. Toxic potential of ethanolic extract of Acacia nilotica (Garad) in rats. Sud J Med Sci 2011;6(1):1-5.
- Said HM. Hamdard Pharmacopoeia of Eastern Medicine. 2nd ed. New Delhi: Sri Satguru Publications; 1997. p. 353.
- Ghani MN. Khazainul Advia. New Delhi: Idarae Kitabul Shifa, YNM; 2011. p. 254.
- Seigler DS. Phytochemistry of Acacia sensu lato. Biochem Syst Ecol 2003;31(8):845-73.
- Singh R, Singh B, Singh S, Kumar N, Kumar S, Arora S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. Toxicol In Vitro 2008;22(8):1965-70.
[Crossref] [Google Scholar] [PubMed]
- Singh BN, Singh BR, Sarma BK, Singh HB. Potential chemoprevention of N-nitrosodiethylamine-induced hepatocarcinogenesis by polyphenolics from Acacia nilotica bark. Chem Biol Interact 2009;181(1):20-8.
[Crossref] [Google Scholar] [PubMed]
- Sultana B, Anwar F, Przybylski R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica and Eugenia jambolana Lam. trees. Food Chem 2007;104(3):1106-14.
- Baravkar AA, Kale RN, Patil RN, Sawant SD. Pharmaceutical and biological evaluation of formulated cream of methanolic extract of Acacia nilotica leaves. Res J Pharm Technol 2008;1(4):480-3.
- Singh R, Arora S. In vitro evaluation of peroxyl radical scavenging capacity of water extract/fractions of Acacia nilotica (L.) Willd. Ex Del. Afr J Biotechnol 2009;8(7):1270-2.
- Saini ML, Saini R, Roy S, Kumar A. Comparative pharmacognostical and antimicrobial studies of Acacia species (Mimosaceae). J Med Plant Res 2008;2(12):378-86.
- Pareek P, Choudhry M. Management of type 2 diabetics by Indian gum arabic (Acacianilotica) pods powder. Int J Food Nutr Sci 2013;2(2):77-83.
- Liu X, Kim JK, Li Y, Li J, Liu F, Chen X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells. J Nutr 2005;135(2):165-71.
[Crossref] [Google Scholar] [PubMed]
- Kalaivani T, Mathew L. Free radical scavenging activity from leaves of Acacia nilotica (L.) Wild. ex Delile, an Indian medicinal tree. Food Chem Toxicol 2010;48(1):298-305.
[Crossref] [Google Scholar] [PubMed]
- Vadivel V, Biesalski HK. Total phenolic content, in vitro antioxidant activity and type II diabetes relevant enzyme inhibition properties of methanolic extract of traditionally processed underutilized food legume, Acacia nilotica (L.) Willd ex. Delile. Int Food Res J 2012;19(2):593-601.
- Srivastava M, Misra A, Srivastava S, Malhotra S. Traditional medicine and globalization: The future of ancient system of medicine. In: Mukherjee PK editor. Kolkata: Maven Publishers; 2012.
- Siddiqui A, Hakim MA. Format for the pharmacopoeial analytical standards of compound formulation, workshop on standardization of Unani drugs (appendix). New Delhi: Central council for research in Unani medicine; 1995. p. 25.
- Helrich K. Anonymous: Official methods of analysis (AOAC). 14th ed. Virginia: Association of Official Analytical Chemists Inc; 1984.
- Srivastava M, Kumar G, Mohan R, Malhotra S. Phytochemical studies and antimicrobial activity of babool seeds. J Sci Ind Res 2014; 73(11):724-728.
- Montgomery R. Determination of glycogen. Arch Biochem 1957;67:378-86.
[Crossref] [Google Scholar] [PubMed]
- Robert R, Elias R. Determination of carbohydrate using sulphuric acid method. 4th ed. In: Nielson S editors. Food analysis. Springer; 2011.
- Lowry OI, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem 1951;193(1):265-75.
[Crossref] [Google Scholar] [PubMed]
- Straccia MC, Siano F, Coppola R, La Cara F, Volpe MG. Extraction and characterization of vegetable oils from cherry seed by different extraction processes. Chem Eng Trans 2012;27:391-6.
- Bray HG, Thorpe WV. Analysis of phenolic compounds of interest in metabolism. Methods Biochem Anal 1954;1:27-52.
[Crossref] [Google Scholar] [PubMed]
- Ordonez AA, Gomez JD, Vattuone MA. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem 2006;97(3):452-8.
- Liyana-Pathirana CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agric Food Chem 2005;53(7):2433-40.
[Crossref] [Google Scholar] [PubMed]
 .
.

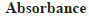 .
.

 .
.



