- Corresponding Author:
- Radha Natarajan
Post Graduate and Research Department of Chemistry, Seethalakshmi Ramaswami College, Tiruchirappalli-620 002
E-mail: radha.chem1955src@gmail.com
| Date of Submission | 12 July 2013 |
| Date of Revision | 20 June 2014 |
| Date of Acceptance | 27 June 2014 |
| Indian J Pharm Sci 2014; 76(4): 370-374 |
Abstract
Novel bicyclo[3.3.1]nonane derivatives were synthesized by an efficient methodology from acetoacetanilide, 2-methoxy and 4-methoxyacetoacetanilides, 1,3,5-trinitrobenzene and triethylamine. The structures of the compounds were characterized by UV/Visible, FTIR, 1 H NMR and 2D-correlation spectroscopy analysis. The in vitro cytotoxic studies were performed using Ehrlich Ascites Carcinoma cell line by Trypan blue dye exclusion assay and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell proliferation assay. The IC 50 values of the 8-(4'-/2'-methoxy/unsubstituted phenylcarbamoyl)bicyclo[3.3.1]nonanes were found to be 110.65 µg/ml, 148.23 µg/ml and 151.71 µg/ml, respectively. Thus (4-methoxyphenylcarbomyl)bicyclo[3.3.1]nonane was more potent compared to other two bicyclic adducts.
Keywords
Bicyclo [3.3.1] nonanes, Ehrlich Ascites Carcinoma cell line, trypan blue, MTT assay, methoxy group activity
Synthetic drugs are important in cancer research and chemotherapy [1] because of their purity and specificity. Bicyclic compounds [2-8] are versatile lead molecules for designing biologically active molecules which possess broad spectrum of activities. Bicyclo [3.3.1] nonane derivatives [9-12] possess remarkable anticancer activity. The cytotoxic activities of several compounds are greatly influenced by the presence of methoxy substituents [13,14]. It has been reported that methoxy substituted pyrimido [4,5-c]quinolin- 1(2H)-ones [15] and triazole-5-ones [16] possess enhanced anticancer activity compared to other derivatives. Since the methoxy group is known for increasing the potency of anticancer compounds, we thought it worthwhile to screen and compare the cytotoxicity of bicyclo [3.3.1] nonanes possessing 2-methoxyphenylcarbomyl and 4-methoxyphenylcarbomyl substituents with the unsubstituted bicyclic adduct. Comparison of anticancer activities of the synthesized compounds was performed by in vitro cytotoxicity study on EAC cell line by trypan blue dye exclusion assay and MTT cell profileration assay.
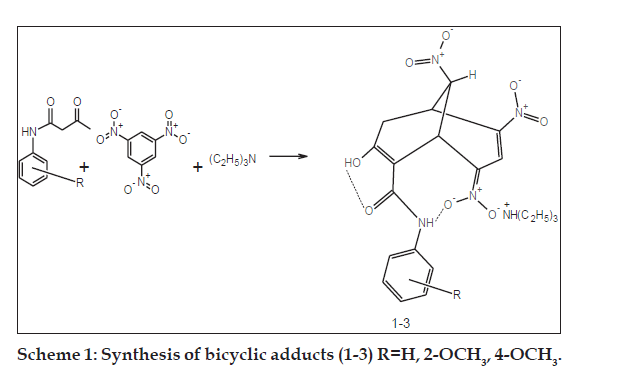
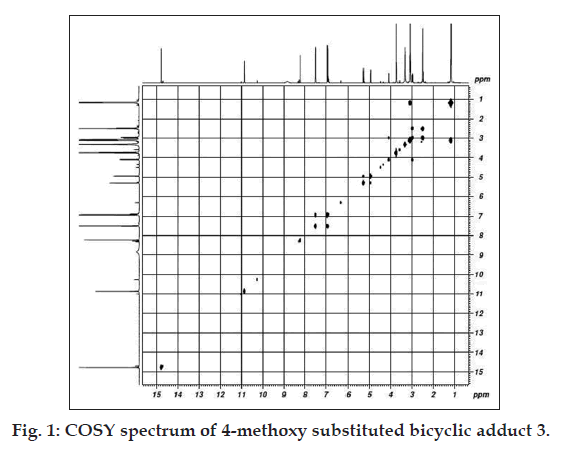
Synthetic and analytical studies were carried out using laboratory and analytical grade reagents. Melting points were determined using open-ended capillary tubes and were uncorrected. UV/Vis spectra were recorded on a Lambda-25 UV/Vis spectrophotometer in ethanol. The FTIR spectra were recorded as KBr pellets using Perkin-Elmer RXI Infrared spectrometer. The 1H NMR and 2D-correlation spectroscopy analysis (COSY) spectra were obtained from Bruker RX-300MHz and AV III-500 MHz FT-NMR spectrometers with DMSO-d6 as solvent and tetramethylsilane (TMS) as internal reference. The physical parameters and spectral results are presented in Tables 1 and 2. The synthetic route for the bicyclic adducts (1-3) is depicted in Scheme 1.
| Bicyclic adduct | Molecular formula | Yield (%) | M.P. (°) | λmax (nm) |
|---|---|---|---|---|
| H | C22H29N5O8 | 93 | 177 | 480 |
| 2-OCH3 | C23H31N5O9 | 90 | 146 | 485 |
| 4-OCH3 | C23H31N5O9 | 91 | 186 | 480 |
Table 1: Physical Parameters of Bicyclic Adducts (1-3)
| Bicyclic | d values in ppm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| adduct | Triethylammonium | Bicyclic oxidanide anion | ||||||||
| Cation | ||||||||||
| CH3 | CH2 | Bridge head | Bridging | C6H5 | Propenide | N-H | O-H | OCH3 | ||
| (t, 9H) | (q, 6H) | (m, 1H), (m, 1H) | (m, 1H) | (s, 1H) | (s, 1H) | (s, 1H) | (S,3H) | |||
| H | 1.25 | 3.11 | 4.31, 4.79 | 5.1 | 7.0-7.7 (m, 5H) | 8.40 | 10.96 | 14.9 | - | |
| 2-OCH3 | 1.17 | 3.10 | 4.18, 5.03 | 5.4 | 6.8-7.8 (m, 4H) | 8.32 | 10.42 | 14.3 | 3.88 | |
| 4-OCH3 | 1.19 | 3.12 | 4.17, 5.05 | 5.2 | 6.93, 7.57 (dd, 4H) | 8.30 | 10.56 | 14.2 | 3.76 | |
Table 2: 1H Nmr Data of Bicyclic Adducts (1-3)
Acetoacetanilides were synthesized using transacetoacetylation method as given below. Aniline, 2-methoxyaniline or 4-methoxyaniline (0.1 mol) was refluxed with t-butylacetoacetate (0.15 mol) in xylene (25 ml) for 15-30 min. The reaction mixture was distilled to remove excess xylene. The concentrated coloured solution was cooled in an ice bath with continuous stirring. Ether (20 ml) was added and the resulting amorphous solid was filtered. This method of using t-butylacetoacetate [17] resulted in high yield within a short time. Yield ~80- 85%, mp H-85°, 2-OCH3- 87°, 4-OCH3-114°. FT-IR (KBr) cm-1 of acetoacetanilide: 3338-3360 (N-H), 2912-2926 (C-H), 1720 (C=O), 1683 (C=O of amide), 1620-1625 (C=C), 1490-1495 (C-C) 1100- 1120 (C-O).
The general procedure for the synthesis of bicyclic adducts triethylammonium { [7-hydroxy-4,9-dinitro- 8-(4'-/2'-methoxy/unsubstitutedphenylcarbamoyl) bicyclo [3.3.1]nona-3,7-dien-2-ylidene](oxido)-?5? azanyl}oxidanides were elaborated below. The bicyclic adducts (1-3) were synthesized [9,18] from 1,3,5-trinitrobenzene and acetoacetanilide, 2-methoxy and 4-methoxy acetoacetanilides. A mixture of acetoacetanilide (0.01 mol) and 1,3,5-trinitrobenzene (0.01 mol) was warmed in absolute alcohol to dissolve the compounds. After cooling, a two to three fold excess of triethylamine was added and then mechanically stirred for 2-4 h. The intensely coloured residual liquid was kept overnight at 30-40° till bright orange solid or oily liquid separated out. The product was filtered or triturated with ether and the mother liquor was further treated with copious amounts of anhydrous ether and stirred in an ultrasonic bath for 15 min. The separated solid was combined with the filtered crystals, powdered well, washed with ethanol-ether solution and recrystallised from absolute alcohol. FT-IR (KBr) cm-1 of 1: 3310-3320 (N-H), 2915-2920 (C-H), 1656-1648 (C=O), 1620-1623 (C=C), 1493-1496 (C-C), 1545- 1549 (NO2 asymm), 1400-1405 (NO2 symm), 1085- 1055 (C-O).
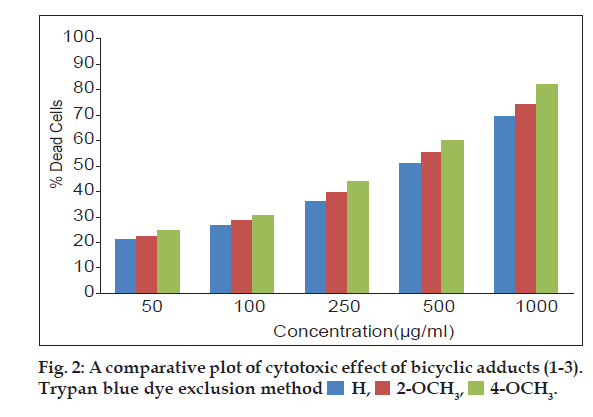
The three bicyclic adducts (1-3) were screened for in vitro cytotoxic studies against EAC cell line using Trypan blue dye exclusion assay and MTT assay. EAC cell lines obtained from a recognized centre was maintained and used for experiments.Trypan blue dye exclusion assay [19] was performed by incubating EAC cells (1×106) in 1 ml phosphate buffer saline at 37º for 3 h in CO2 atmosphere with varying concentrations of the adducts (1-3) in DMSO. Then 25 µl of trypan blue solution was added to differentiate dead (stained) and viable (unstained) cells using haemocytometer and the results are expressed as % dead cells.
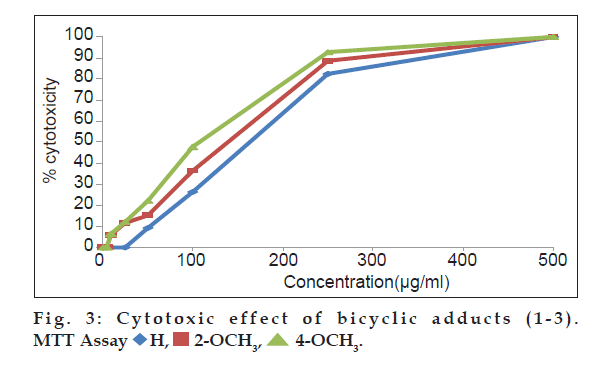
In MTT assay, cell suspension (100 µl of 1×106 cells per ml) was cultured in 96 well flat bottom micro plate with growth medium RPMI 1640 and 10% (v/v) fetal calf serum (FCS). Increasing concentrations of the adducts (1-3) were added separately to the cells and incubated at 37º for 14 h in co2 incubator with 5% co2. A fresh growth medium along with 20 µl of, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to each well and incubated for 4 h at 37° repeatedly. The purple precipitate of MTT-formazan clearly visible under the inverted microscope was dissolved in 200 ml of 0.1% acidic isopropyl alcohol (0.1N) after the removal of growth medium. Then the covered plates were kept in the dark at 18-24° for 24 h and the optical density was measured at 570 nm. The experiments were repeated thrice and mean (s±0.03) of the three results were compared with the control test samples. The percentage growth inhibition was calculated using theformula [20] percent growth inhibition=(control ODtreated OD/control OD)×100.
The formation of adducts (Scheme 1) from acetoacetanilide, 2- or 4-methoxy acetoacetanilides, 1,3,5-trinitrobenzene and triethylamine is evidenced by the absorbance (?max ~480 nm, ethanol) of nitropropene nitronate moiety in the UV/Vis spectra (Table 1). In the carbonyl region of the FTIR spectra there is no peak at 1720 cm-1, although in acetoacetanilides sharp peaks are observed. This can be explained by the enolisation of the diketo moiety during adduct formation. The absorptions in the region 1500-1445 cm-1 are due to anionic NO2 asymmetric and symmetric stretching modes [18,21]. The presence of bridgehead and bridging HCNO2 are confirmed by the 1H NMR spectral results (Table 2) in which two multiplets occur in the regiond 4-5 ppm. Multiplets around 7.0-7.7 ppm, 6.8-7.8 ppm and double doublets at 6.93, 7.57 ppm are due to phenyl, 2-methoxy or 4-methoxy phenyl rings, respectively. The 2- and 4-methoxy group in the two bicyclic adducts appear at 3.88 and 3.76 ppm, respectively. The propenide protons in the bicyclic adducts (1-3) at 8.40, 8.32 and 8.30 ppm indicate the presence of nitropropene nitronate moiety [9,18,21-24] in all the compounds. The highly deshielded amide N-H protons are found at 10.96, 10.42 and 10.56 ppm. The signals around 14 ppm are due to enolic hydrogens [18]. It is interesting to find that methoxy substituents (Table 2) exhibit shielding effects on both NH (2-OCH3, 0.54 ppm; 4-OCH3, 0.4 ppm) and OH (2-OCH3, 0.6 ppm; 4-OCH3, 0.7 ppm) signals [25]. Inorder to determine whether methoxy protons interact with the protons present in the bicyclic ring, a COSY spectrum (fig. 1) is recorded. The signal at 3.76 ppm due to 4-methoxyprotons do not exhibit coupling with NH and OH protons in the region 10-14 ppm. Hence, it can be inferred that the adduct possess a free methoxy group. The twin effect of electron donation and the isolated methoxy group devoid of interactions within the bicyclic ring may be the reason for enhanced cytotoxicty.
A comparative assessment on the cytotoxic activity of the three adducts revealed that, 4-methoxy substituted adduct exhibited significant increment in dead cells (fig. 2) in trypan blue cell viability assay [26] at all test concentrations. Cytotoxic activity of all the three adducts are also compared through MTT assay (fig. 3). The mitochondrial dehydrogenase enzymes present in the metabolic active cells reduce the yellow tetrazolium salt to purple MTT-formazan which could be followed spectrophotometrically since there is a large change in the colour of the solution during reduction [27]. Hence, this assay is used widely to examine cell proliferation. In this apoptosis pathway, the test drug under study causes death of the cells due to the loss of mitochondria.The drug concentrations (Table 3) required to cause 50% cytotoxicity for unsubstituted and methoxy substituted phenylcarbomyl bicyclo [3.3.1] nonanes (1-3) are H (151.71 µg/ml), 2-methoxy (148.23 µg/ml) and 4-methoxy (110.65 µg/ml). It has been reported by earlier workers that methoxy substitutents in pyrimido quinolinones [15] and triazol- 5-ones [16] caused enhanced cytotoxicity. Similarly in this research work, it is also observed that the electron donating 2- and 4-methoxy substituents at the phenyl functionality of the bicyclic adducts cause increased cytotoxic activities. The electron donating ability of the 4-methoxy substituent is greater than the 2-methoxy substituent. This may be the reason for the increased cytotoxicity of the 4-methoxy substituted bicyclo [3.3.1] nonane compared to 2-methoxy and unsubstituted bicyclo [3.3.1] nonanes.
| Concentration (µg/ml) | % cytotoxicity | |||
|---|---|---|---|---|
| H | 2-OCH3 | 4-OCH3 | ||
| Control | 0a | 0 | 0 | |
| 1 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | |
| 10 | 0 | 5.97 | 6.35 | |
| 25 | 0.11 | 11.48 | 12.16 | |
| 50 | 9.26 | 15.31 | 22.23 | |
| 100 | 26.30 | 36.48 | 47.65 | |
| 250 | 82.39 | 88.56 | 92.66 | |
| 500 | 100.00 | 100.00 | 100.00 | |
| IC50 b (µg/ml) | 151.71 | 148.23 | 110.65 | |
aZero indicates that no cells have died at that concentration. bDosage required to cause 50% cytotoxicity
Table 3: Cytotoxicity of Methoxy and Unsubstituted Bicyclic Adducts (1-3) Mtt Assay
The present paper highlights the following salient features. The methodology adopted for transacetoacetylation in the synthesis of acetoacetanilides resulted in high yield at a very short duration. The synthesis of bicyclic adducts occur at ambient conditions. Among the three test drugs, the adduct bearing 4-methoxy substituent in the phenylcarbamoyl moiety possesses higher cytotoxic potency compared to 2-methoxy and unsubstituted phenylcarbamoyl bicyclo [3.3.1]nonane derivatives.
Acknowledgements
The authors are thankful to SAIF IIT Madras and Department of Chemistry, Madurai Kamaraj University for NMR spectral studies. The authors are also grateful to the Management, Seethalakshmi Ramaswami College for the infrastructure, Srimad Andavan College and SASTRA for in vitro studies.
References
- Patel R, Vijayan P, Suresh B. In-vitro cytotoxic activity of chalcones bearing quinoline nucleus and N-phenyl-3-(phenylsubstituted) propenamide. Int J Pharmacol Bio Sci 2007;3:87-92.
- Renolds RC, Johnson CA, Piper JR, Sirotnak FM. Synthesis and antifolate evaluation of the aminopterin analogue with a bicyclo. Eur J Med Chem 2001;36:237-42.
- Smirnova NO, Plotnikov OP, Vinogradova NA, Sorokin VV, Kriven?ko AP. Synthesis and biological activity of substituted7-aza-8-aza(oxa)-bicyclo[4.3.0]-6,9 nonadienes. Pharm Chem J 1995;29:49-50.
- Miller JA, Ullah GM, Welsh GM, Hall AC. 8-Aminobicyclo[3.2.1] octanes: Synthesis and antiviral activity. Tetrahedron Lett 2001;42:7503-7.
- Seebacher W, Schlapper C, Brun R, Kaiser M, Saf R, Weis R. Antiprotozoal activity of new bicyclo[2.2.2]octane-2-imines and esters of bicyclo[2.2.2]octane-2-ols. Eur J Pharm Sci 2005;24:281-9.
- Razdan BK, Sharma AK, Kumari K, Bodla RB, Gupta BL, Patnaik GK. Studies on azabicyclo systems: Synthesis and spasmolytic acitivity of analogues of 9-Methyl-3,9-diazabicyclo[4.2.1]nonane and 10-Methyl- 3,10-diazabicyclo[4.3.1]decane. Eur J Med Chem 1987;22:573-7.
- Berger H, Weis R, Kaiser M, Brun R, Saf R, Seebacher W. Novel azabicyclo[3.2.2]nonane derivatives and their activities against Plasmodium falciparum K1 and Trypanosome brucei rhodesiense. BioorgMed Chem 2008;16:6371-8.
- Sharma CS, Nema RK, Sharma VK. Synthesis and screening for analgesic and anti-inflammatory activity of some novel amino acid containing bicyclo compounds. J Young Pharm 2009;1:170-4.
- Dhivya C, Vandarkuzhali SA, Sridharan G, Radha N, Brindha P. Synthesis and In vitro cytotoxic screening of triethylammonium {[7-hydroxy-4,9-dinitro-8-(phenylcarbamoyl)bicyclo[3.3.1]nona-3,7-dien-2-ylidene](oxido)-?5-azanyl}oxidanide against EAC cell lines. Pharmacol Online 2012;3:113-7.
- Geirrsson JK, Jonsson S, Valgeirsson J. Synthesis and antitumor activity of bicyclo[3.3.1] nonenol derivatives. Bioorg Med Chem 2004;12:5563-9.
- Blechart S, Jasnsen R, Velder J. Synthesis of new taxoids. Tetrahedron 1994;50:9649-56.
- Nurieva EV, Zefirova ON, Nuriev VN, Zyk NV, Weiss DG, Kuznetsov SV, et al. Synthesis of compounds with potential antitumor activity. (2R,3S)-benzoylphenylisoserine esters with susbstituted bicyclo[3.3.3] nonanes. Moscow Univ Chem Bull 2009;64:219.
- Cushman M, Nagarathnam D, Gopal D, He HM, Lin CM, Hamel E. Synthesis and evaluation of analogs of (z)-1-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)ethene as potential cytotoxic and antimitotic agents. J Med Chem 1992;35:2293-306.
- Burnham BS, Gupton JT, Krumpe K, Webb T, Shuford J, Bowers B, et al. Cytotoxicity of substituted alkyl-3,4-bis (4-methoxyphenyl) pyrrole-2-carboxylates in L1210 lymphoid leukemia cells. Arch Pharm (Weinheim) 1998;331:337-41.
- Metwally K, Khalil A, Sallam A, Pratsinis H, Kletsas D, Sayed KE. Strucuture?activity relationship: Investigation of methoxy substitution on anticancer pyrimido[4,5-C]quinolin-1(2H)-ones. Med Chem Res 2013;22:4481-91.
- Bekiran O, Kucuk M, Kahveci B, Bektas H. Synthesis and anticancer evaluation of some new 4-amino-3-(p-methoxybenzyl)4,5-dihydro-1,2,4-triazole-5-one derivatives. Z Naturforsch 2008;63b:1305-14.
- Witzeman JS, Nottingham WD. Transacetoacetylation with tert-Butyl acetoacetate: Synthetic Applications. J Org Chem 1991;56:1713-8.
- Gnanadoss LM, Radha N. Condensation-cyclization of acetoacetanilides with 1,3,5-trinitrobenzene. formation and structure of some stable delocalised anions containing the bicyclo[3.3.1] nonane skeleton. J Org Chem 1983;48:570-4.
- Sheeja KR, Kuttan G, Kuttan R. Cytotoxic and antitumour activity of berberin. Amala Res Bull 1997;17:73-6.
- Scudiero DA, Shoemaker RH, Paul KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of soluble tetrazolium formazan assay for cell growth and drug sensitivity in clusters using human and other tumor cell lines. Cancer Res 1988;48:4827-33.
- Kalaivani D, Malarvizhi R, Subbalakshmi R. Synthesis of a novel barbiturate from 1-chloro-2,4-dinitrobenzene as a anticonvulsant agent. Med Chem Res 2008;17:369-73.
- Strauss MJ, Jensen TC, Schran H, O?Conner H. Condensation? cyclization of diketones with ketoesters with electron-deficient aromatics-I. Formation and structure of some stable delocalised anions containing the bicyclo[3.3.1] nonane skeleton. J Org Chem 1970;35383 8.
- Gnanadoss LM, Kalaivani D. Condensation and cyclization of carbanions with bicyclo[3.3.1]nonane skeleton. J Org Chem 1985;50:1174-7.
- Radha N, Kamala V. Cyclic condensation of alkylacetoacetates with 1,3,5-trinitrobenzene and base. Indian J Chem 1995;34B:399-403.
- Gnanadoss LM, Radha N. Correlation of proton chemical shifts with substitiuent constant: A study in anionic sigma complexes based on the bicyclo[3.3.1]nonane skeleton. Spectrochim Acta A 1985;41A:1343-7.
- Haldar PK, Kar B, Bala B, Bhattacharya B, Mazumder UK. Antitumor activity of Sansevieria roxburghianarhizome against ehrlichascites carcinoma in mice. Pharm Biol 2010;48:1337-43.
- Potten CS. The significance of spontaneous and induced apoptosis in gastrointestinal tract of mice. Cancer Metast Rev 1992;11:179-95.
 H,
H,  2-OCH3,
2-OCH3,
 H,
H,  4-OCH3.
4-OCH3.



