- *Corresponding Author:
- Sudhakar Bhalerao
Obesity-Diabetes lab,
Centre for Innovation in Nutrition Health Disease,
Interactive Research School for Health Affairs,
Bharati Vidyapeeth,
Pune,
Maharashtra 411043,
India
E-mail: supriya.bhalerao@gmail.com
| Date of Received | 05 October 2020 |
| Date of Revision | 29 September 2021 |
| Date of Acceptance | 20 April 2022 |
| Indian J Pharm Sci 2022;84(2):513-518 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In view of the wide usage of Triphala for large number of therapeutic indications, ethno medicinal practice of extract preparation viz. extracting the three plants together (sample A) and mixing the extracts of three plants prepared separately (sample B), were compared. Both the test samples were analyzed with respect to their physicochemical, phytochemical and pharmacological profiles. In case of physicochemical properties, ash values and moisture content of both samples were comparable. Sample A showed less acidic nature and high water soluble extractive (96.15±1.7) value which was statistically significant (p<0.05). Phytochemical estimation of gallic acid and ellagic acid by high-performance thin-layer chromatography showed higher content in sample B (-97.7 ng/μg) and sample A (-49.2 ng/μg) respectively. Pharmacological activity of both the samples was comparable for total phenol content, total antioxidant and free radical scavenging activity. Although both samples inhibited the activities of acetylcholine esterase and lipase in a concentration dependent manner; sample B showed better activity. Usually, multi-plant extracts were proven more superior over single plant extracts. In our study extract prepared by mixing the extracts of 3 plants prepared separately showed better pharmacological activity profile than the extract prepared by extracting the three plants togethers.
Keywords
Gallic acid, ellagic acid, acetyl choline esterase, lipase
Medicinal plants and their active phytoconstituents are important in drug discovery. Triphala, a combination of 3 fruits viz. Terminalia chebula (T. chebula) Retz. Phyllanthus emblica Linn. and Terminalia bellerica (T. bellerica) Roxb., is one of the most extensively used and the most researched Ayurvedic formulation. It is available in the market individually or as an ingredient of various polyherbal formulations[1,2]. It has myriad of therapeutic activities and is therefore indicated in more than 74 disease indications[3,4,5]. There are 2 different methods practiced to prepare Triphala extract viz. extracting the three plants together or mixing the extracts of 3 plants prepared separately. Mixing of separately prepared extracts is a common practice in Industry. These rules out the need to prepare Triphala extract instead the separately prepared extracts can be mixed when needed. Another advantage of this method is that the separately prepared extracts can be used for other purposes as well. Considering the wide use and importance of the formulation, it is important to know whether these two preparation methods are comparable in terms of their chemistry as well as activities. The present study was thus carried out to compare the differences in Triphala extracts prepared by the above-mentioned 2 methods using a battery of tests. The Ayurvedic Pharmacopeia of India standards were used for physicochemical tests. From more than 190 phytoconstituents reported in Triphala, the phytochemical analysis was restricted to quantification of 2 major active components namely Gallic Acid (GA) and Ellagic Acid (EA)[6-8]. The pharmacological activities included comparison of anti-oxidant activity and enzymes (acetyl cholinesterase and pancreatic lipase) inhibitory activity considering our intended use of the formulation for obesity associated cognitive impairment.
The chemicals and reagents used were of analytical grade. The samples of GA and EA were procured from Sigma Aldrich, USA. A standardized aqueous extract of Triphala prepared using 2 methods; extracting the three plants together (sample A) and mixing the extracts of 3 plants prepared separately (sample B) was procured from Pharmanza Herbals Pvt. Ltd. Gujrat, India. In brief 1 kg part of each constituent plant was taken in the equal part, its powder was then processed in 4 l of water at 70°. Three extraction cycles were performed. The filtered, semisolid material was dried in the form of powder through spray dryer. The raw materials used for the preparation of the extract were authenticated at Botanical Survey of India, Jodhpur and the voucher specimens were preserved (PHPL/HB/024.A: T. bellerica Gaertn, PHPL/HB/007.A: Embelica officinalis L., and PHPL/HB/008.A: T. chebula Retz.). The stock solution of both the samples was prepared in 10 mg/ml of concentration in water.
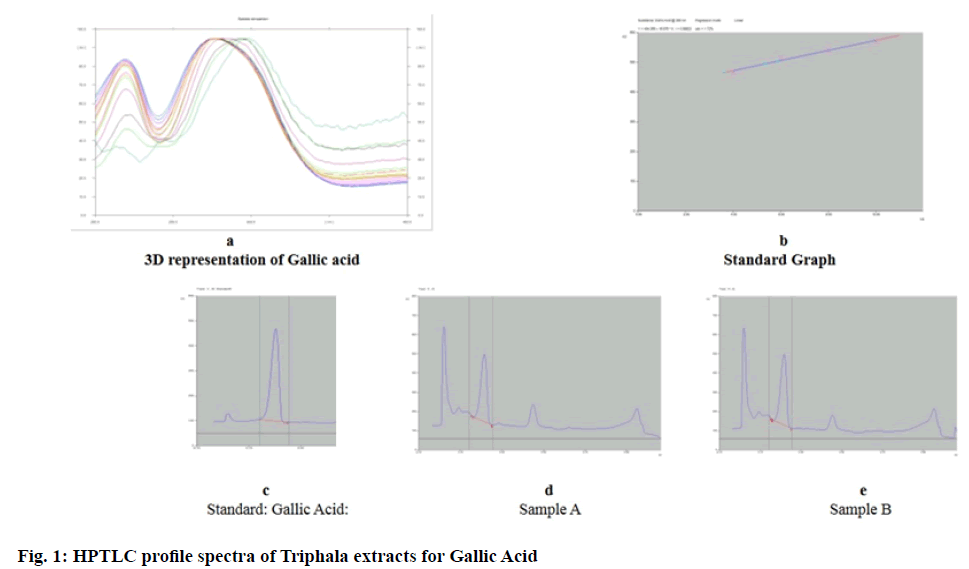
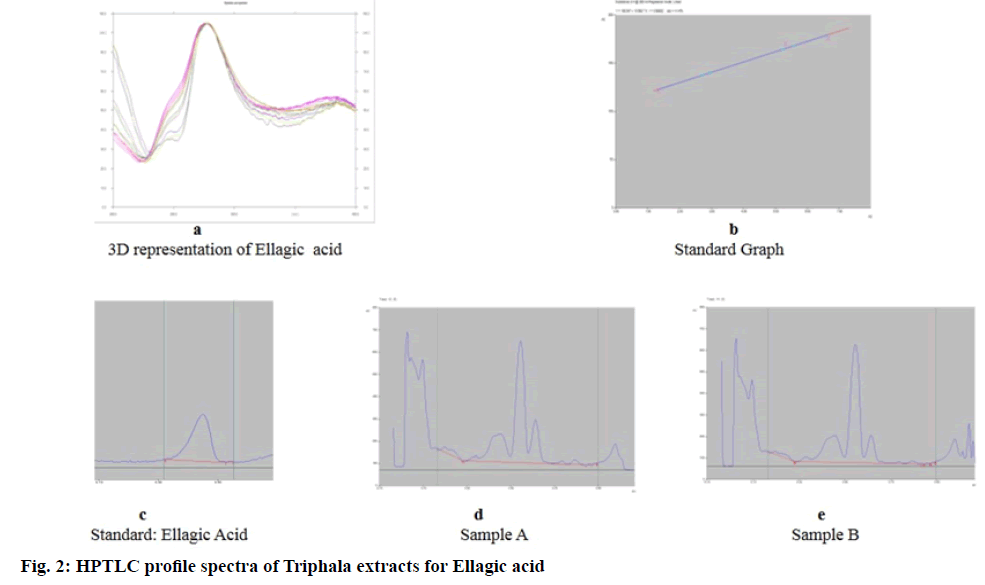
For Physicochemical tests[9] the samples were compared for percent ash content, moisture content, pH and water extractive value. Using High Performance Thin Layer Chromatography (HPTLC), quantification of GA and EA was carried out. CAMAG linomat applicator and scanner equipped with Win Computer Aided Transcription System (CATS) Software (version 1.4.3.6336) were used for the purpose. Working stock was made of 1 mg/ml extract. Test samples (50 µl each) and marker compound (1-10 µl) were applied on pre coated silica gel 60 F254 plate of uniform thickness of 0.2 mm. 10 cm×10 cm, Merck, Germany. The plates developed in the solvent system (Toluene: Ethyl acetate: Formic acid: Methanol (6:2.5:0.2:0.8)[10] and (3:3:0.8:0.2)[11] respectively for GA and EA were examined under ultraviolet light at 280 nm.
Regarding the anti-oxidant activity, the free radical scavenging activities were measured in relation to the hydrogen donating or radical scavenging capacity using the stable (2,2-diphenyl-1- picrylhydrazyl) (DPPH) radicals and other assay employed were Total phenolic content, Total antioxidant activity by Ferric Reducing Antioxidant Potential (FRAP) assay (Table 1).
| Assay | Unit of measurement | Method |
|---|---|---|
| Total phenolic content | GA equivalents | Pourmorad et al.[12] |
| Ferric Reducing Anti-oxidant Potential (FRAP) | µm Fe (II)/g dry mass | Modified method of Benzie and Strain[13] |
| DPPH free radical scavenging activity | Percentage inhibition | Brand Williams et al. method[14] |
Table 1: Anti-Oxidant Activity Assay
Total phenolic content[12] was measured as gallic acid equivalents. Briefly 5 ml of Folin ciocalteau reagent added to 1 ml sample, followed by addition of 4 ml of 1 m sodium carbonate and reaction mixture was incubation in dark at room temperature for 30 min. Absorbance was measured at 765 nm. A gallic acid standard curve (R2=0.99) was used to measure the phenolic content and was expressed as mg/g of dry mass of gallic acid equivalents.
Total antioxidant activity by FRAP assay was carried out by using modified method of Benzie et al.[13]. The FRAP reagent was prepared freshly by mixing 25 ml acetate buffer (300 mm acetate buffer), 2.5 ml 10 mm 2,4,6-tri(2-pyridyl)-1,3,5-Triazine (TPTZ) and 2.5 ml of 20 mm Ferric Chloride Hexahydrate (FeCl3·6H2O) solution. After attaining the temperature of the solution to 37°, 150 µl of plant extract was allowed to react with 2850 µl of FRAP reagent for 30 min in dark. The absorbance of coloured product was measured at 593 nm. Results are expressed in µm Fe (II)/g dry mass.
It was determined as per Brand Williams et al. method[14]. 1 ml of various concentrations (10 to 100 µg/ml) of samples was made and 5 ml of methanolic DPPH (33 mg/l) was added, the reaction mixture was incubated at 37° for 30 min. Methanolic DPPH was kept as control. The radical scavenging activity of the test samples was expressed as percentage inhibition. Percent scavenging=(A0-A1)/A0×100, where, A0 is the absorbance of the control and A1 is the absorbance of the sample. IC50 values were calculated from the slope of the standard graph using ‘y=mx+c’ formula for every cases.
Acetylcholine Esterase (AChE) inhibitory activity[15] of extracts was examined by Ellmans method with slight modifications. Galanthamine was kept as positive control. The absorbance of the mixture was measured at 412 nm. Percent inhibition of enzyme activity was calculated using the formula, percentage inhibition=A0− A1/A0×100, where A0 was the absorbance of the control and A1, the absorbance of the sample.
For Lipase enzyme inhibitory activity[16], porcine pancreatic lipase enzyme inhibitory assay was adapted from Bustanji et al.[16] with some modifications. Orlistat was used as a positive control. The 0.10 ml of pancreatic lipase solution was pre incubated with different concentrations of the extract and the selected compounds for 5 min at 37°, then the P-Nitrophenyl Butyrate (PNPB) substrate (10 mm in acetonitrile) was added. The volume was diluted to 1 ml using the Tris- Hydrochloride (Tris-HCl) buffer before measuring the solution absorbance spectrophotometrically at 410 nm.
Percentage (%) inhibition=A0−A1/A0×100, where A0 was the absorbance of the control and A1 the absorbance of the sample.
All the assays were done in triplicate (n=3) and the mean±Standard Deviation (SD) of the data has been provided in the manuscript. Further data has been analyzed using unpaired t test. p<0.05 was considered as statistically significant. Percent ash content and moisture content in both the samples was comparable i.e. sample A (5.11±0.9 and 3.82±0.12) and sample B (5.53±0.16 and 3.96±0.13). The pH of both samples was acidic in nature[17] though sample A (4.08±0.02) had significantly higher value than sample B (3.97±0.01) (p<0.01). Both the samples showed solubility in water (90.00 %) and partial solubility in alcohol (52.56 %). The water soluble extractive value (%) was significantly (p<0.05) higher in sample A (96.15±1.7).
Using HPTLC method, GA (Rf 0.21)[10] and EA (Rf 0.45) (fig. 1 and fig. 2)[11] were quantified. The content of GA and EA in the dry weight of the extracts was calculated. GA was found higher in sample B -97.7 ng/ μg as compared to sample A -89.0 ng/μg, while EA was slightly higher in sample A -49.2 ng/μg than sample B -45.1 ng/μg. Anti-oxidant activity-All 3 studied activities were comparable in both samples (Table 2). The activity of both samples was much lower than the control ascorbic acid.
| S No. | Test Assay | Sample A | Sample B |
|---|---|---|---|
| 1 | Total phenol content | 305.47±1.083 | 321.73±11.975 |
| [mg of Gallic Acid Equivalent (GAE)/gm of extract] | |||
| 2 | Total antioxidant activity by FRAP | 3.498±0.024 | 3.513±0.023 |
| [µm/ml FeII at 100µg/ml concentration] | |||
| 3 | DPPH free radical scavenging activity | 80.821±0.609 | 75.49±0.399 |
Table 2: Comparison of Antioxidant Activity
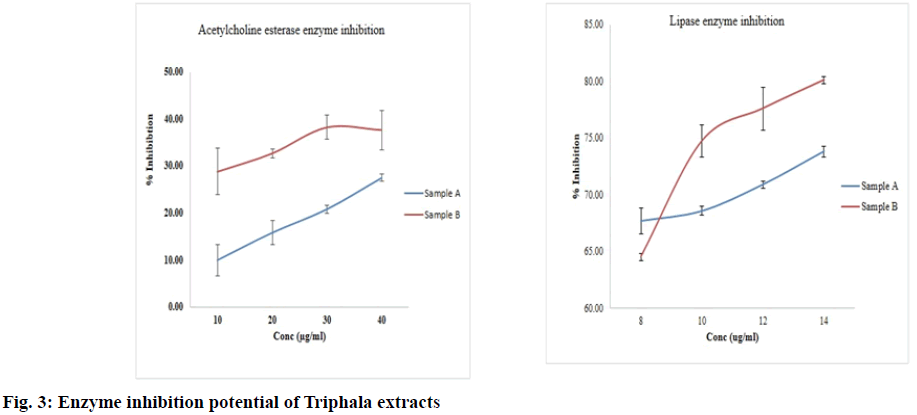
Acetylcholine esterase enzyme inhibitory activity-Both samples inhibited the enzyme activity in a concentration dependent manner, (fig. 3). Which was higher in sample B (32.81 %) as compared to sample A (10.00 %) p<0.01 at 10 μg/ml concentration. IC50 value of galanthamine was found to be 0.531 μg/ml (-1.44 μm/ml), which is in agreement with previous findings[18,19]. Lipase enzyme inhibition assay-Both samples inhibited the lipase enzyme activity in a concentration dependent manner (fig. 3). Orlistat had an IC50 value of 12.38±2.3 μg/ml. The activity of both extracts was found comparable at 2 μg/ml concentration. However, with the increasing concentration, sample B (showed significantly (p<0.001) higher inhibition as compared to sample A.
The present study was planned to evaluate difference in Triphala extract prepared by 2 different methods. It was found that when the extract was prepared by extracting the three plants together showed more watersoluble extractive value, less acidic nature and more amount of EA. While the extract prepared by mixing the extracts of 3 plants prepared separately revealed more ash content, moisture content, acidic nature of extract, more GA content, higher acetylcholine esterase and lipase inhibitory potential. Some of the parameters viz. total phenol content, total antioxidant activity and DPPH free radical scavenging activity were comparable in both the samples. The findings of our study indicate that mixing the extracts of 3 plants prepared separately has better activity profile. The higher value of gallic acid estimated in this sample may be responsible for the difference in the activities[20].
Triphala is a well-known Ayurvedic formulation with wide range of activities and therapeutic indications. Our study first time documents that addition of 3 plant extracts separately can prove more effective for managing various disease conditions rather than extracting the 3 plants together.
There are previous reports that the different extracts of the same plant from the different origins, harvest seasons, solvents and plant parts exhibit differences in terms of activities[21-23]. In a recent study, similarities/ dissimilarities of T. chebula (one of the ingredient of Triphala) prepared using 3 different methods viz. traditionally prepared extract (History of Safe Use (HOSU) Extract), HOSU Extract prepared commercially using water as solvent and methanolic extract. A significant difference was observed in the physicochemical analysis and quantitative testing for few marker compounds of these extracts[24].
Pathompak et al.[25] in have reported no significant changes with respect to inhibition of cholesterol esterase as per the proportion of ingredient plants of Triphala but higher total phenol content with higher proportion of T. bellirica[25]. On the similar lines, the present study has focused on 2 different methods of processing the extract. It is expected that when the extract was prepared by extracting the three plants together would involve chemical reaction among the different phytoconstituents of the three plants, while the extract prepared by mixing the extracts of 3 plants prepared separately would provide a little window for such interaction. Triphala, though a combination of only 3 plants, is a complex in nature as it contains more than 174 bio-actives[5]. The nature of the interaction among these phytoconstituents while extracting Triphala is not much studied. The preliminary findings from our study can prove helpful further in elucidating these interactions. The most important observation of our study is the better activity profile when the plants are extracted separately and then mixed. This finding can change the usage pattern of Triphala in clinical settings.
Triphala extract prepared using 2 methods showed considerable variation in physicochemical, phytochemical and pharmacological profiles. The extract prepared by mixing the extracts of 3 plants prepared separately was found better than the extract prepared by extracting the three plants together. This finding can be validated further using high end technology for further application by herbal industries.
Acknowledgement:
The authors acknowledge financial assistance from Sakal India Foundation and Pharmanza Herbal Pvt. Ltd, Gujarat for providing the extract samples.
Conflict of interests:
The authors have no conflict of interest to report.
References
- Acharya Jadavji Trikamji. Sushruta Samhita. Commentary of Dalhana. Sutrasthana. 38 verse 56-57, 1st Ed. Chaukhambha Sanskrit Sansthan; Varanasi: Reprint 2011;168.
- Peterson CT, Denniston K, Chopra D. Therapeutic uses of triphala in ayurvedic medicine. J Altern Complement Med 2017;23(8):607-14.
[Crossref] [Google Scholar] [Pub Med]
- Baliga MS, Meera S, Mathai B, Rai MP, Pawar V, Palatty PL. Scientific validation of the ethnomedicinal properties of the Ayurvedic drug Triphala: A review. Chin J Integr Med 2012;18(12):946-54.
[Crossref] [Google Scholar] [Pub Med]
- Naik GH, Priyadarsini KI, Mohan H. Free radical scavenging reactions and phytochemical analysis of triphala, an ayurvedic formulation. Curr Sci 2006;90:1100-5.
- Chandran U, Mehendale N, Tillu G, Patwardhan B. Network pharmacology of ayurveda formulation Triphala with special reference to anti-cancer property. Comb Chem High Throughput Screen 2015;18(9):846-54.
[Crossref] [Google Scholar] [Pub Med]
- Sarkaki A, Fathimoghaddam H, Mansouri SM, Shahram Korram M, Saki G, Farbood Y. Gallic acid improves cognitive, hippocampal long-term potentiation deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Pak J Biol Sci 2014;17(8):978-90.
[Crossref] [Google Scholar] [Pub Med]
- Shivakumar A, Paramashivaiah S, Anjaneya RS, Hussain J, Ramachandran S. Pharmacognostic evaluation of triphala herbs and establishment of chemical stability of triphala caplets. Int J Pharm Sci Res 2016;7(1):244-51.
- Mukherjee PK, Rai S, Bhattachar S, Kumar DP, Biswas TK, Jana U, et al. Clinical study of ‘Triphala’-A well-known phytomedicine from india. Iran J Pharmacol Ther 2006;5(1):51-4.
- Anonymous. The Ayurveda Pharmacopeia of India. Part.1, Vol. 1. 1st ed. Appendix II (2.2).2010. New Delhi: Government of India, Ministry of AYUSH
- Koppikar SJ, Jagtap SD, Devarshi PP, Jangle NM, Awad VB, Wele AA, et al. Triphala, an Ayurvedic formulation improves the antioxidant status on TNBS induced IBD in rats. Europ J Integr Med 2014;6(6):646-56.
- Jeganathan NS, Kannan K. HPTLC method for estimation of ellagic acid and gallic acid in Triphala churanam formulations. Res J Phytochem 2008;2(1):1-9.
- Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol 2006;5(11).
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 1996;239(1):70-6.
[Crossref] [Google Scholar] [Pub Med]
- Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Techno 1995;28(1):25-30.
- Öztürk H, Kolak U, Meric C. Antioxidant, anticholinesterase and antibacterial activities of Jurinea consanguinea DC. Rec Nat Prod 2011;5(1):43.
- Bustanji Y, Al-Masri IM, Mohammad M, Hudaib M, Tawaha K, Tarazi H, et al. Pancreatic lipase inhibition activity of trilactone terpenes of Ginkgo biloba. J Enzyme Inhib Med Chem 2011;26(4):453-9.
[Crossref] [Google Scholar] [Pub Med]
- Rajan K, Praveen K, Vaibhav R, Gauranga SR. Physicochemical evaluation of Triphala churna. Int J Pharm Sci. 2015;1(1):71-4.
- Okello EJ, Leylabi R, McDougall GJ. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food Funct 2012;3(6):651-61.
[Crossref] [Google Scholar] [Pub Med]
- Tang XC, Han YF. Pharmacological profile of huperzine A, a novel acetylcholinesterase inhibitor from Chinese herb. CNS Drug Rev 1999 Sep;5(3):281-300.
- Chamundeeswari D, Kanimozhi P, Kumar V, Reddy C. Formulation and evaluation of Churna for digestive property. Sri Ramachandra J Med. 2007;39.
- Irshad M, Zafaryab M, Singh M, Rizvi M. Comparative analysis of the antioxidant activity of Cassia fistula extracts. Int J Med Chem 2012;2012:1-6.
[Crossref] [Google Scholar] [Pub Med]
- Ahmed D, Khan MM, Saeed R. Comparative analysis of phenolics, flavonoids and antioxidant and antibacterial potential of methanolic, hexanic and aqueous extracts from Adiantum caudatum leaves. Antioxidants 2015;4(2):394-409.
[Crossref] [Google Scholar] [Pub Med]
- Meng GE, Tian YC, Yang Y, Shi J. Evaluation of DPPH free radical scavenging activity of various extracts of Ligularia fischeri in vitro: A case study of Shaanxi region. Indian J Pharm Sci 2016;78(4):436-42.
- Singh VK, Murali B, Koshy R, Narayanan DBA. Determination of similarities/dissimilarities of processed botanicals. Cutting Edge 2018;9-14.
- Pathompak P, Charoenchai L, Monton C. The cholesterol esterase inhibition and total phenolic content of aqueous extract of triphala of and modified triphala formulas. Bull Health Sci Techn 2015;13(2):25-30.