- *Corresponding Author:
- P. J. Das
Animal Genetics Laboratory, ICAR-National Research Centre on Pig, Rani, Assam 781015, India
E-mail: drpranabjyotidas@gmail.com
| Date of Received | 14 October 2021 |
| Date of Revision | 28 April 2022 |
| Date of Acceptance | 25 January 2023 |
| Indian J Pharm Sci 2023;85(1):64-75 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study explores the natural bioactive metabolites, antioxidant and antibacterial activities present in the host plants of Antheraea assamensis (muga silkworm) viz. Machilus bombycina (som), Litsea polyantha (sualu), Litsea salicifolia (dighloti) and Litsea citrata (mejankari). The methanolic extracts of leaves of these plants were screened for their phytochemical analysis and free radical scavenging activity using 2,2-diphenyl- 1-picrylhydrazyl as the free radical. Phytochemical analysis revealed the presence of flavonoids, phenolic compounds, carotenoids, saponins, coumarins, terpenoids, tanins, cardiac glucosides and xanthoproteins in the said plants. The results of 2,2-diphenyl-1-picrylhydrazyl scavenging activity revealed Litsea polyantha to exhibit stronger antioxidant efficiency compared to the rest. Besides these, the plant extracts were investigated for their antibacterial assessment against Gram-positive Staphylococcus aureus and Streptococcus suis and Gram-negative Pasteurella multocida and Escherichia coli bacteria found in pig. All the plant extracts were validated to possess antibacterial activity with variable potency. Litsea citrata was the most effective extract retarding microbial growth of both Gram-positive and Gram-negative bacteria, followed by Litsea salicifolia, Litsea polyantha and Machilus bombycina. The present findings underscore the fact that indigenous food plants of muga silkworm are fortified with antioxidant and antibacterial efficiency, which might pave to the development of novel herbal pharmacological compounds for the medication of pig diseases.
Keywords
Antibacterial property, 2,2-diphenyl-1-picrylhydrazyl, Litsea citrata, Litsea polyantha, Litsea salicifolia, Machilius bombycina, phytochemicals
Concerns involving food safety and food control are mounting in today’s market place over the globe. The threat posed by drug-resistant pathogens has resulted in the increasing momentum in research and development of effective alternative medications. The primary cause behind refers to many commonly used antibiotics that have become less and less proficient against certain illnesses due to their frequent use ultimately forcing for the emergence of drugresistant bacteria. Even after repeated efforts from pharmaceuticals to produce many new antibacterials over the years, resistance to these drugs has become a global menace[1]. The emergence and spread of multidrug-resistant bacterial pathogens have substantially endangered the current antibacterial prophylaxis[2]. Such sort of antimicrobial resistance is becoming a fatal cause of mortality in humans and other livestock. In India, pig husbandry is very much predominant and northeast India is hailed as one of the major hotspots of pig domestication. This ground for pork as an important component of the food basket for consumers in Eastern and Northeastern states of India. Streptococcus suis (S. suis), Staphylococcus aureus (S. aureus), Pasteurella multocida (P. multocida) and Escherichia coli (E. coli) are some of the major pathogens affecting porcine animals, triggering economic loss in the pig industry. The exploitation of antibiotics in the treatment of bacterial diseases in swine causes side effects to the health of the animal as well as hampers its meat quality. The present scenario necessitates innovation of alternative herbal therapies with strong remedial properties[2]. Plant-based products have constantly engrossed attention in humankind owing to their versatile applications. Scientific investigations focusing screening of antibacterial and antioxidant activities of plants have shown plants as a representative potential source of new anti-infective agents[2-8] for the treatment of several ill-fated diseases. The innumerable therapeutic and natural phytochemicals contained in them proved to be beneficial in the treatment of diverse diseases namely diabetes, heart disorders, chronic inflammatory disorders, tumorigenesis disorders etc[9]. Antioxidants are molecules, which inhibit or quench free radical reactions and avert oxidative cellular damage[10]. Intake of dietary antioxidants is the simplest way to reduce the development of induced oxidative stress pathologies[8,11]. A variety of synthetic antioxidants is commonly available in the food industry for food safety purposes. Nevertheless, the use of such compounds has been restricted by the regulatory authorities owing to their long-term toxicity and carcinogenicity[12]. Probe concerning phytochemical repertoire of varied plants and their parts have been executed in enormous in the past in response to its low side effects and cost effectiveness[6-10]. Identification of phytochemical essentials namely flavonoids, alkaloids, carotenoids, tannins, phenolic compounds etc.[6] is of paramount importance to discover and develop herbal therapeutic drugs with enhanced efficacy to replace synthetic antibacterials and antioxidants for use in food and medical purposes.
Silkworm in northeast India occupies an imperative hold in socio-economic existence of the common people[13]. Indian golden silkmoth Antheraea assamensis Helfer (muga silkworm) is an indigenous, monotypic and semi-domesticated species geographically confined only to Brahmaputra valley of the northeastern biome of India, especially Assam. It is polyphagous and feeds on a wide variety of food plants out of which som (Machilus bombycina (M. bombycina)) and soalu (Litsea polyantha (L. polyantha)), synonyms (Litsea monopetala) function as the primary host plants while dighloti (Litsea salicifolia (L. salicifolia)) and mejankori (Litsea citrate (L. citrate)) serves as the secondary host plants. Geo-climatic conditions like high humid temperate, climate and forest vegetation of its primary and secondary host plants are the main factors accountable for its geographical isolation. Unlike mulberry silkworm, muga silkworm culture is an outdoor rearing tradition practiced by the livelihood of northeast particularly Assam throughout generations as a part of their ethnicity. Previously, investigations focusing Litsea polyantha have cited about its innumerable medicinal properties such as antioxidant, anti-microbial, anti-bacterial, antifungal, anti-hyperglycemic, anti-atherothrombosis and anti-diarrheal[14-17]. Mulberry, the host plant of mulberry silkworm (Bombyx mori) has been widely exploited and cited to have armoured with antimicrobial and antioxidant properties[18-20]. The current research is aimed to discover comparative screening of various phytochemical components and the antioxidant perspective present in leaves of muga food plants, which can be utilized for the formulation of potential medicines for the treatment of several diseases. The study also explores to uncover the antibacterial properties of the host plants against four important bacterial organisms of pig viz. Grampositive S. aureus and S. suis and Gram-negative P. multocida and E. coli. The present findings might, therefore, be useful to devise new and innovative herbal drugs for the treatment of bacterial diseases affecting the swine industry and causing economic depredation to the same.
Materials and Methods
Preparation of plant leaf extract:
Leaves of host plants of muga and mulberry were procured from different locations in and around Guwahati, Assam. Mulberry leaf has been used as a reference in the current investigation. The collected leaves were washed with tap water and subsequently sun-dried. The dried leaves were ground and extracted in 100 % methanol in the ratio 1:10, at 50° for 72 h. The extracts were filtered and later concentrated under reduced pressure to obtain a viscous semi-solid mass for further downstream applications.
Sample preparation for phytochemical screening:
A qualitative phytochemical screening of muga host plants to detect the presence of essential phytoconstituents, such as flavonoids, tannins, saponins, terpenoids, carotenoids, coumarins, phenolic compound, cardiac glycosides, xantho protein and quinones were carried out using standard biochemical procedures as reported previously[21].
2,2-diphenyl-1picrylhydrazyl (DPPH) method of total antioxidant capacity assessment:
Free radical scavenging activity of methanolic extract of plant samples was determined by the previously described method by Brand-Williams et al.[22]. The assay is based on the determination of the concentration of DPPH methanolic solution, after adding antioxidants. The solution of DPPH in methanol (6×10-5 M) was prepared just before Ultra-Violet (UV) measurements. The samples of different concentrations were added to DPPH solution in 1:1 ratio followed by vortexing. The reaction was allowed to take place in the dark at room temperature for 30 min. Absorbance at 517 nm was measured at different time intervals. Decreasing intensity of the purple colour was taken as increasing scavenging activity. The inhibition (%) of radical scavenging activity was calculated using the following equation.
Inhibition (%)=[(A0-A)/A0]×100
Where A0 is the absorbance of DPPH in the absence of the sample and A is the absorbance of DPPH in the presence of the sample
Collection and isolation of bacteria:
Tissue samples from pigs were collected from different pig farms, slaughter house and pork markets in and around Guwahati, Assam. The samples were collected aseptically in the collection container under the chilled condition for further processing. The bacterial samples were incubated in their respective broth, overnight at 37° and subsequently plated and streaked on nutrient agar, which was followed by staining, biochemical tests and polymerase chain reaction as per standard protocol.
Bacterial strains:
The antibacterial efficiency of each plant extract was evaluated against S. aureus, S. suis, P. multocida and E. coli. The bacterial strains were used from the repository of Animal Health Lab, ICAR-NRC on Pig, Rani, Assam.
In vitro evaluation of antibacterial activity by tube method:
The antimicrobial activity of the plant extracts were studied in vitro using the macro dilution method[23]. Four different concentrations of each plant extract viz. 100, 200, 500 and 1000 mg/ml were prepared from the stock solution. 1 ml of broth culture of each of the four bacterial strains containing approximately 1×108 CFU/ml was transferred to five sterile tubes containing the plant extract in different concentrations as mentioned above and incubated at 37° for 24 h. Antimicrobial activity of all the five plant extracts along with positive (bacteria and broth) and negative control (only broth) against the four bacteria was determined by observing the turbidity of the broth.
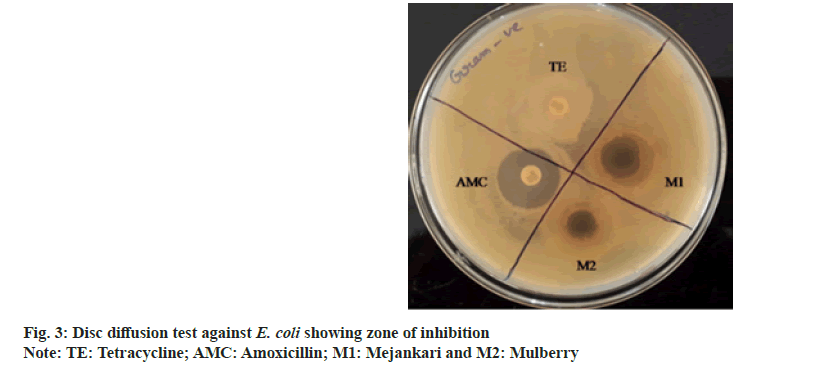
Disc diffusion test:
Disc diffusion method[23] was performed to compare the bacterial growth inhibition by the plant extracts and other commercially available broad-spectrum antibiotics. In this method, sterile discs impregnated with 500 mg/ml concentration of the plant extracts was dried in incubator and used in the test. Broth culture of the four bacteria containing approximately 1×108 CFU/ml was uniformly spread separately over sterile Muller Hinton agar plates and discs containing the plant extract along with commercially available antibiotic discs namely amoxicillin and tetracycline. The samples and antibiotic discs were placed at a uniform distance and incubated for 24 h at 37°. After incubation, the zone of inhibition surrounding the discs was measured by using Hi zone antibiotic scale; (Hi-Media Laboratories Pvt. Ltd., Mumbai, India).
Gas Chromatography-Mass Spectroscopy (GC-MS) analysis:
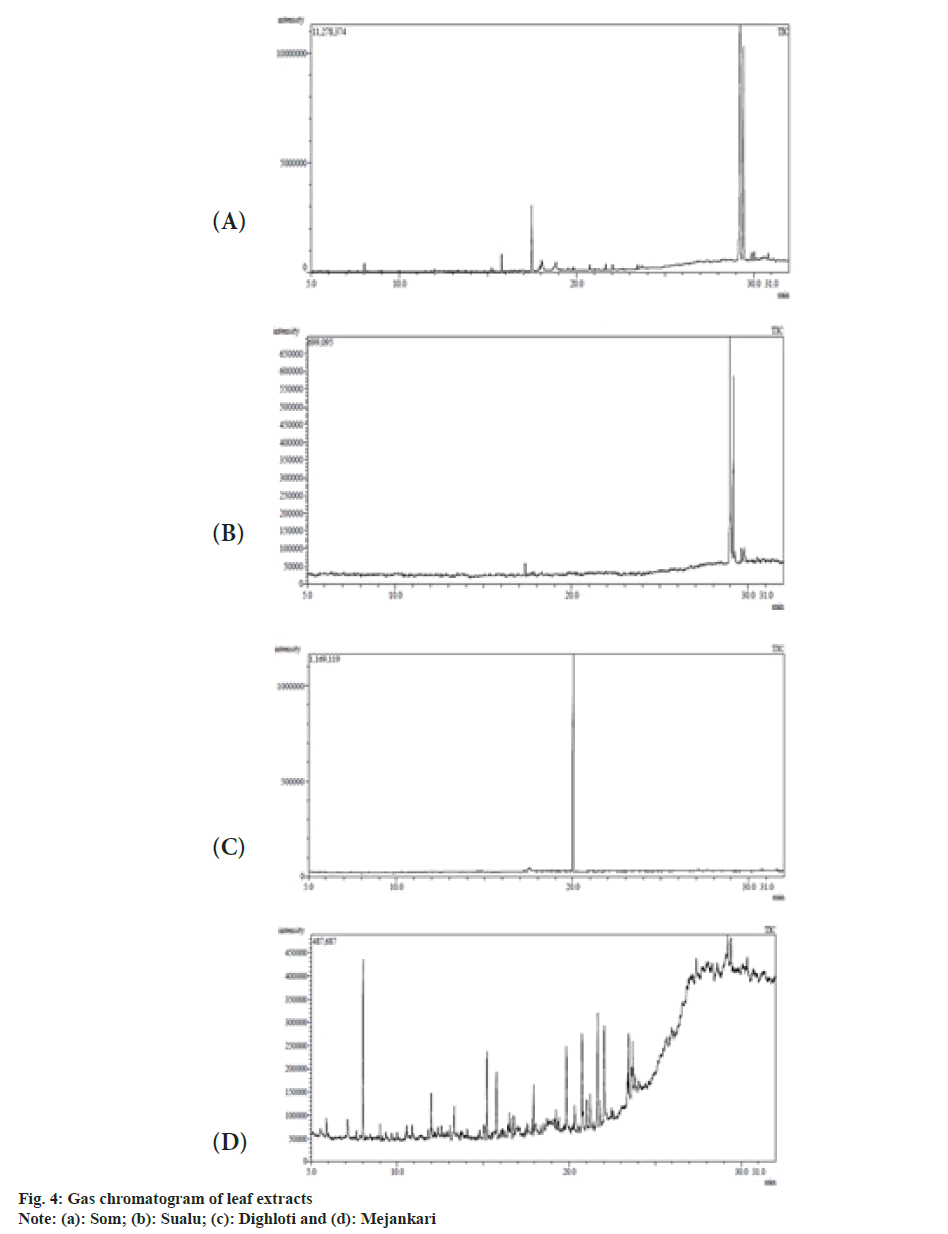
The extracts were filtered through a 0.2 μm filter for GC-MS analysis performed in a Perkin Elmer Clarus 680/600 unit fitted with Elite 5MS column (30 m×0.25 mm, 0.25 μm film thickness). The injector and detector temperatures were maintained at 300°. The samples were injected in the split mode, using pressurecontrolled helium as carrier gas at a linear velocity 37.2 cm/s (at 50°). The oven temperature was programmed from 50° (after 2.5 min) to 150° at 15°/min. Again temperature was increased up to 200° at 3°/min. The final temperature was programmed to 300° at 8°/min and held for 8 min. The mass spectrometer (Clarus 600; single quad) was operated in the Electron Ionization (EI) mode at 70 eV with a source temperature of 200° and a continuous scan from mass to charge ratio (m/z) 50 to 600. The peaks were identified by matching the mass spectra with the National Institute of Standards and Technology (NIST) library, USA.
Results and Discussion
Preliminary phytochemical screening as shown in Table 1 confirms the presence or absence of arrays of phytochemicals in our plants of interest. Bioactive metabolites specifically flavonoids, tannins, terpenoids, carotenoids and phenolic compounds were discovered to be present in all the extracts. Whereas, the evidence of quinone in the extracts haven’t been witnessed in this study. On the other hand, metabolites like coumarin, cardiac glycoside and xanthoprotein were found to be absent only in dighloti extract.
| S No. | Phytochemicals | M. bombycina (som) | L. polyantha (sualu) | L. salicifolia(dighloti) | L. citrate (mejankari) | M. alba (mulberry) |
|---|---|---|---|---|---|---|
| 1 | Flavonoid | + | + | + | + | + |
| 2 | Tannin | + | + | + | + | + |
| 3 | Saponin | + | + | + | + | + |
| 4 | Terpenoid | + | + | + | + | + |
| 5 | Carotenoid | + | + | + | + | + |
| 6 | Coumarin | + | + | - | + | - |
| 7 | Phenolic compounds | + | + | + | + | + |
| 8 | Cardiac glycoside | + | + | - | + | + |
| 9 | Xantho Protein | + | + | - | + | + |
| 10 | Quinone | - | - | - | - | - |
Table 1: List of Phytochemical Constituents Present in Som, Sualu, Dighloti, Mejankari and Mulberry Leaf Extract Solution
Note: (+): Present and (-): Absent
The DPPH analysis is a widely used method to evaluate the free radical scavenging action of plant extracts. The method is based on the reduction of methanolic DPPH solution in the presence of antioxidants resulting in the formation of non-radical DPPH-H by the reaction. The findings of this study ascertained the antioxidant efficiency of muga host plants by inhibiting DPPH radical activity. Percentage inhibition of DPPH is presented in Table 2. DPPH quenching activity of sualu leaf extract was found to be highest among all followed by dighloti and som extract. The inhibition produced by mejankari extract was significantly less compared to the rest.
| Extract material | Percentage Inhibition (%) | ||||
|---|---|---|---|---|---|
| 40 (µl/ml) | 80 (µl/ml) | 120 (µl/ml) | 160 (µl/ml) | 200 (µl/ml) | |
| Som | 64.61±0.48 | 67.82±1.02 | 70.50±0.41 | 71.84±0.84 | 75.87±0.91 |
| Sualu | 76±0.52 | 80.83±0.61 | 95.84±1.16 | 96.21±0.53 | 96.64±0.30 |
| Dighloti | 72.11±1.04 | 89.72±0.64 | 92.67±0.82 | 93.26±0.27 | 93.85±0.61 |
| Mejankari | 11.12±0.67 | 12.69±0.30 | 14.20±0.44 | 17.04±0.29 | 18.63±0.15 |
| Mulberry | 70.23±0.55 | 78.86±0.36 | 84.75±0.63 | 90.46±0.61 | 93.45±0.38 |
| Ascorbic acid | 77.44±0.61 | 82.03±1.09 | 96.32±0.53 | 97.18±0.32 | 97.72±0.27 |
Table 2: Percentage Inhibition of Dpph by the Muga Host Plant Extracts
Note: (SD=n±3)
The results of antibacterial activity of the plant extracts against Gram-positive S. aureus and S. suis and Gram-negative P. multocida and E. coli by macro dilution method in four different concentrations of the extract is given in Table 3. The plant extracts at lower concentrations viz. 100 and 200 mg/ml were unable to inhibit the growth of all the four bacteria whereas the plant extracts at 500 and 1000 mg/ml could inhibit the growth of bacteria as indicated by the absence of turbidity in the tubes (fig. 1).
| Plant extracts Concentration (mg/ml) |
Growth of the bacterial species in broth | |||
|---|---|---|---|---|
| P. multocida | E. coli | S. suis | S. aureus | |
| 100 | Turbidity present | Turbidity present | Turbidity present | Turbidity present |
| 200 | Turbidity present | Turbidity present | Turbidity present | Turbidity present |
| 500 | Turbidity present | Turbidity present | No turbidity | No turbidity |
| 1000 | No turbidity | No turbidity | No turbidity | No turbidity |
| Broth with bacterial culture (Positive control) | Turbidity present | Turbidity present | Turbidity present | Turbidity present |
| Only Broth (Negative control ) | No turbidity | No turbidity | No turbidity | No turbidity |
Table 3: List of Plant Extracts Showing Antibacterial Action against S. Aureus, S. Suis, P. Multocida and E. Coli at Different Doses
Note: (-ve means no growth in the broth)
Fig. 1: In vitro antibacterial analysis of the plant extracts against gram positive and gram negative bacteria
Note: (a): S. suis (500 mg/ml); (b): P. multocida (1000 mg/ml); (A): Positive control; (B): Negative control; (C): Mejankari extract; (D): Dighloti extract; (E): Sualu extract; (F): Som extract and (G): Mulberry extract
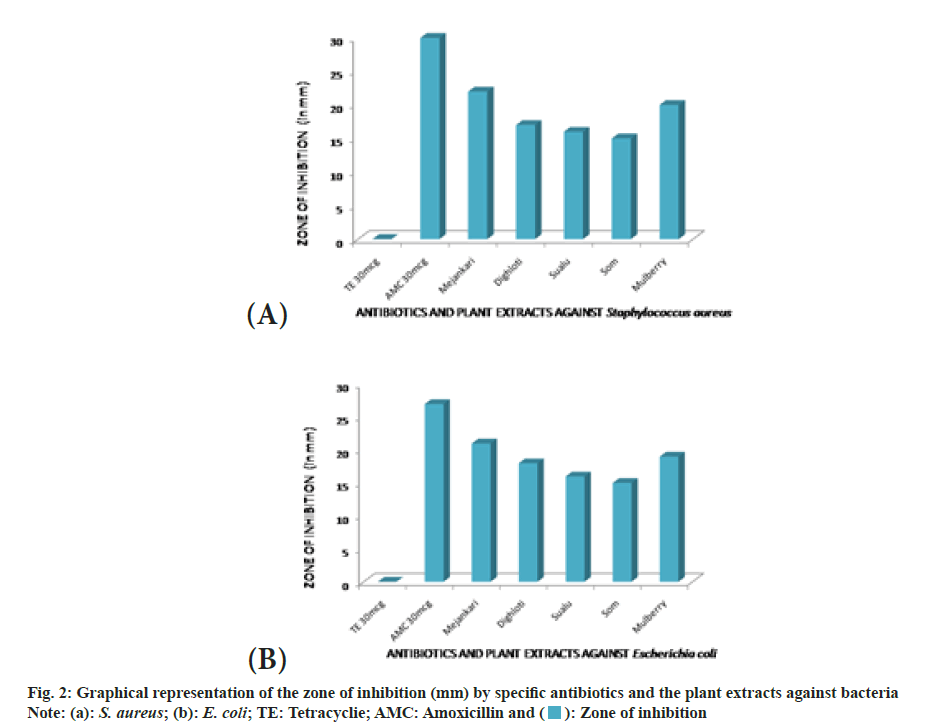
The extracts were investigated to assess their antibacterial dominance against Gram-positive S. aureus and Gram-negative E. coli using zone of inhibition method. The result validated that the crude extract possesses antimicrobial activity in comparison with standard drug amoxicillin and tetracycline. As per the observations, all plant extracts were potentially effective in suppressing bacterial growth with variable efficiency. This antimicrobial activity of the crude extract might be attributed due to the presence of phytochemical essentials mainly flavonoids, tannins and phenolic compounds in the plant leaves[24]. The extract of Litsea citrata (L. citrata) was the most effective retarding microbial growth of both Grampositive and Gram-negative bacteria, followed by L. salicifolia, L. polyantha and M. bombycina. Inhibitory effect of L. citrata was found to be more compared to extracts of Morus alba (M. alba). The results of the disc diffusion test showing the zone of inhibition produced by the antibiotics and the plant extracts against two bacterial species are shown in fig. 2. In the present study, amoxicillin showed slightly higher zone of inhibition against the Gram-negative bacteria than the zones produced by the plant extracts of mejankari and mulberry at 1000 mg/ml (fig. 3). Whereas tetracycline which is the most commonly used antibiotic against respiratory tract infection in pigs was found to be resistant showing no zone of inhibition. Similarly, in Gram-positive bacteria amoxicillin showed a higher zone of inhibition compared to the extracts of mejankari and mulberry while tetracycline showed no zone of inhibition.
The GC-MS analysis of methanolic extracts of the host plants as listed in Table 4-Table 7, discovered the presence of diverse medicinal compounds of volatile nature belonging to different chemical families. Fig. 4 shows the GC-MS spectrum of the investigated plant extracts. Nine major compounds were discovered from M. bombycina, among which the compounds possessing pharmaceutical importance are caryophyllene (1.75 %), 1,6,10-dodecatrien- 3-ol,3,7,11-trimethyl (6.51 %), furan,2,5-bis(3,4- dimethoxyphenyl)tetrahydro-3,4-dimethyl (1.12 %), cardenolide (1.11 %), phenol 4,4'-methylenebis[2-(1,1-dimethylethyl)-6-methyl (33.82 %), d-Mannitol, 1-decylsulfonyland thiazolo[3,2-a]benzimidazol- 3(2H)-1,2-(2-fluorobenzylideno)-7,8-dimethyl (40.34 %). Caryophyllene (1.75 %), 1,6,10-dodecatrien-3- ol,3,7,11-trimethyl (6.51 %) and furan, 2,5-bis(3,4- dimethoxyphenyl) tetrahydro-3,4-dimethyl (1.12 %) were also identified from L. polyantha but in different quantities. Other compounds with medicinal potency identified from L. polyantha were beta carotene (0.11 %), phenol, 2,2'-methylenebis[6-methoxy-3-(2-propenyl) (33.30 %), coniferyl aldehyde (40.41 %), 5-formyl- 2,3,3',4'-tetramethoxystilbene (1.51 %), benzoic acid, 2,6-bis[(trimethylsilyl)oxy]-, trimethylsilyl ester (3.02 %) and t-Butylphosphonic acid, di-TMS derivative (0.30 %). From Litsea salisifolia identified compounds having medicinal significance are 1,3-benzoxazol- 5-amine, 4,6-dibromo-2-phenyl (1.42 %), methyl- 4-thio.alpha.d-arabinofuranoside (1.61%), benzyl benzoate (83.15 %), 4'-Apo-.beta.psi.-carotenoic acid- carotene (1.43 %) and oxymetholone (1.65 %). Adding to the same, constituents with therapeutic value screened from L. citrata leaf extract are mainly eremophilane (0.19 %), thiazolo[3,2-a]benzimidazol- 3(2H)-one, 2-(2-fluorobenzylideno)-7,8-dimethyl (2.18 %), caryophyllene (3.96 %) digitoxin (1.09 %) and benzenepropanoic acid (1.56 %).
| Peak | Retention time (min) | Area % | Compound name | Activity |
|---|---|---|---|---|
| 1 | 8.021 | 1.03 | Benzene, 1,3-dichloro- | Organic compound |
| 2 | 15.775 | 1.75 | Caryophyllene | Antibacterial, Antifungal |
| 3 | 17.474 | 6.51 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl- | Antimicrobial, Antioxidant, Antifungal, Anticancer |
| 4 | 18.828 | 1.11 | Cardenolide | Cardiac glycosides, Anticancer |
| 5 | 24.03 | 0.29 | d-Mannitol, 1-decylsulfonyl- | Antimicrobial |
| 6 | 29.235 | 40.34 | Thiazolo[3,2-a]benzimidazol-3(2H)-one, 2-(2-fluorobenzylideno)-7,8-dimethyl | Antimicrobial |
| 7 | 29.422 | 33.82 | Phenol, 4,4'-methylenebis[2-(1,1-dimethylethyl)-6-methyl- | Phenolic |
| 8 | 29.48 | 0.22 | Benzoic acid, 2,3-bis[(trimethylsilyl)oxy]-, trimethylsilyl ester | Antibacterial, Antifungal |
| 9 | 29.879 | 1.23 | 2-(2-Acetoxy-3-methoxyphenyl)-3-methoxy-4H-chromen-4-one | Not specific activity reported |
| 10 | 30.043 | 1.41 | 5,12d-Ethano(furo[2,3,4-mn]oxepino[2,3,4-ed]anthracen-9-ol-2-one) | Dye |
| 11 | 30.824 | 1.12 | Furan, 2,5-bis(3,4-dimethoxyphenyl)tetrahydro-3,4-dimethyl- | Anticancer, Antifungal, Antimicrobial etc |
Table 4: List of Active Compounds Present in Som Leaf Extract
| Peak | Retention time (min) | Area % | Compound name | Activity |
|---|---|---|---|---|
| 1 | 17.35 | 2.9 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- | Antimicrobial, Antifungal, Anticancer |
| 2 | 17.719 | 0.99 | Diethyl Phthalate | Plasticizer |
| 3 | 17.89 | 0.48 | Caryophyllene oxide | Antibacterial, Antifungal |
| 4 | 18.774 | 0.11 | Beta Carotene | Antioxidant |
| 5 | 28.978 | 40.41 | Coniferyl aldehyde | Phenylpropanoid compound |
| 6 | 29.153 | 33.3 | Phenol, 2,2'-methylenebis[6-methoxy-3-(2-propenyl) | Phenolic compound |
| 7 | 29.238 | 1.51 | 5-formyl-2,3,3',4'-tetramethoxystilbene | Anticancer |
| 8 | 29.63 | 3.82 | 9-Oxabicyclo[4.3.0]non-2-ene-4,5-dicarboxylic acid, 8-hydroxy-1,2,7,7-tetramethyl-, dimethyl ester | No specific activity reported yet |
| 9 | 29.785 | 3.02 | Benzoic acid, 2,6-bis[(trimethylsilyl)oxy]-, trimethylsilyl ester | Antifungal, Antiseptic |
| 10 | 30.2 | 0.3 | t-Butylphosphonic acid, di-TMS derivative | Antiretroviral, antimalarial, Antihypertensive etc |
| 11 | 30.516 | 0.85 | Furan, 2,5-bis(3,4-dimethoxyphenyl)tetrahydro-3,4-dimethyl-, | Antifungal, Antimicrobial, Disinfectant, etc |
Table 5: List of Active Compounds Present in Sualu Leaf Extract
| Peak | Retention time (min) | Area % | Compound name | Activity |
|---|---|---|---|---|
| 1 | 8.854 | 1.16 | Propene-1,2,3-tricarboxylic acid | Organic compound |
| 2 | 12.289 | 1.42 | 1,3-Benzoxazol-5-amine, 4,6-dibromo-2-phenyl- | Antibacterial, Antifungal, Anticancer, Anti-inflammatory etc. |
| 3 | 17.455 | 1.48 | Diethyl Phthalate | Plasticizer |
| 4 | 18.926 | 1.43 | 4'-Apo-.beta.psi.-carotenoic acid- carotene | Antioxidant |
| 5 | 19.46 | 1.61 | Methyl-4-thio.alpha.d-arabinofuranoside | Antimycobacterial |
| 6 | 20.016 | 83.15 | Benzyl Benzoate | Used against scrabies, insect repellent |
| 7 | 21.545 | 1.65 | Oxymetholone | Used in treatment of anaemia |
Table 6: List of Active Compounds Present in Dighloti Leaf Extract
| Peak | Retention time (min) | Area % | Compound name | Activity |
|---|---|---|---|---|
| 1 | 27.31 | 0.19 | Eremophilane | Antimicrobial, Anticancer, Immunomodulatory |
| 2 | 29.217 | 2.18 | Thiazolo[3,2-a]benzimidazol-3(2H)-one, 2-(2-fluorobenzylideno)-7,8-dimethyl | Antimicrobial |
| 3 | 23.402 | 1.65 | 9,12-Octadecadienoic acid, methyl ester | Used in paints, oils |
| 4 | 21.815 | 1.56 | Benzenepropanoic acid | Phenylpropanoid compound |
| 5 | 20.321 | 1.47 | Heneicosane | Pheromone |
| 6 | 15.778 | 3.96 | Caryophyllene | Antibacterial, Antifungal |
| 7 | 15.048 | 0.62 | 2-Propenoic acid | Used in textile industry |
| 8 | 11.82 | 0.57 | Azulene | Naphthalene |
| 9 | 26.825 | 1.09 | Digitoxin | Cardiac glycoside |
Table 7: List of Active Compounds Present in Mejankari Leaf Extract
As cited by Varadarajan et al.[25] the secondary metabolites and other chemical constituents of medicinal plants account for their medicinal value. They possess various biological therapeutics which provide the scientific base for the use of herbs in traditional medicine in many ancient communities[26]. The important bioactive essentials investigated in our research might be the key drivers responsible for the anticipated pharmacological properties of the muga food plants.
The absorbance of DPPH solution with methanolic extracts of muga food plants was found to be decreased with increased concentration confirming the free radical scavenging activity of the test extracts. Flavonoids, tannins, phenolics, terpenoids and carotenoids are a major group of compounds that act as primary antioxidants or free radical scavengers[27,28]. The flavonoids and phenolic compounds are known to hold antioxidant power due to the presence of hydroxyl groups in their structures and their contribution to defence system against the oxidative damage due to endogenous free radicals is extremely important[29,30]. The antioxidant activity of the studied extracts can be credited to the incidence of identi?ed phytochemicals detected in the samples. Scavenging activity is found to be directly proportional to the concentrations of extracts. The results unveiled that food plants of muga silkworm mainly sualu and dighloti have better antioxidant potency compared to other plants including mulberry.The results also discovered that all plant extracts were potentially effective in suppressing bacterial growth with variable potency. This antimicrobial activity of the crude extract might be attributed due to the presence of phytochemical essentials primarily flavonoids, tannins and phenolic compounds in the leaves[24]. The extract of mejankari leaf was the most effective retarding microbial growth of both Gram-positive and Gramnegative bacteria, followed by dighloti, sualu and som. Inhibitory effect of mejankari extract was found to be more compared to extracts of mulberry. The study also disclosed and confirmed that the Gram positive and Gram negative bacteria used in this study has become drug-resistant against tetracycline, the most commonly used drug against respiratory disorders in swine.
The GC-MS analysis authenticated profuse presence of compounds with potent medicinal supremacy in the investigated food plants. Based on prior literature the compounds identified are reported to be manifested with pharmaceutical values like antimicrobial, antifungal, anti-viral, anti-oxidant, anti-cancer, antiretroviral etc, which may contribute to the healing potential of muga host plants. Caryophyllene is reported to have antibacterial and antifungal activity by Sabulal et al.[31] 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl also termed as nerolidol is proposed to have multi-facet pharmacological properties such as anti-microbial, anti-biofilm, anti-oxidant, antifungal, anti-parasitic, skin-penetration enhancer, skin-repellent, anti-nociceptive, anti-inflammatory and anti-cancer[32]. Cardenolide compounds are evidenced to have anticancer activity by Krishna et al.[33] Diverse biological properties have been associated with thiazolo[3,2-a]benzimidazole derivatives in the passing decades, including antibacterial, antifungal, anti-inflammatory, anti-ulcer, anti-viral, anthelmintic and anti-cancer activity. Moreover, thiazolo[3,2-a] benzimidazole derivatives are well known as plateletactivating factor antagonists and neoplasm inhibitors. Some thiazolo[3,2-a]benzimidazole derivatives inhibit H+/K+-ATPase and gastric secretion and are thus useful as anti-ulcer agents. Furthermore, thiazolo[3,2-a] benzimidazol-1-oxide shows gastric antisecretory activity[34]. Importance of furan based compounds as studied by Chandrashekarachar et al.[35], have cited to have loaded with innumerable biological activities like anti-cancer, anti-bacterial, anti-fungal etc. Countless studies throughout the years have confirmed carotenoids and their derivatives as an established source of rich antioxidants[36]. Correspondingly phenolic compounds are too regarded for their potent antioxidant nature. Antioxidant significance of phenolic compounds can be attributed to their ability to chelate metal ions involved in the production of free radicals[37,38].
Coniferyl aldehyde and benzenepropanoic acid belong to the family of phenylpropanoid compound. Phenylpropanoids form a large class of phenolic compounds. They are derived from cinnamic acid/pcoumaric acid, which on the other hand is obtained from the amino acid phenylalanine catalyzed from the enzyme phenylalanine ammonia-lyase. This enzyme is the branch-point enzyme between the shikimate pathway and phenylpropanoid metabolisms[39]. The phenylpropanoid pathway serves as a rich source of metabolites in plants, is required for the biosynthesis of lignin, and serving as a starting point for the production of many other important compounds, such as the flavonoids, coumarins and lignans[40].
According to Xu[41] 5-formyl-2,3,3',4'- tetramethoxystilbene holds significant anticancer efficiency and has been investigated to induce apoptosis and reduce the viability of paclitaxel and cisplatin-resistant osteosarcoma cells. Benzoic acid has long been used as a strong antimicrobial agent in food preservatives[42]. Furthermore, benzoic acid also possesses antibacterial effectiveness against Bacillus subtilis I, Bacillus megaterium I, Bacillus sphaericus, Bacillus polymyxa, S. aureus I, S. aureus II, S. aureus III (Gram-positive), E. coli I, E. coli II and E. coli III (Gram-negative)[43,44]. Benzoic acid in its undissociated form exhibits various antibacterial and antifungal activities[45]. It is also investigated to be powerful against another Gram-negative bacterium, Listeria monocytogenes at 1000 μl/ml concentration[46]. Phosphonic acid and its derivatives are well known to have actions like antiretroviral, anti-malarial, antihypertensive etc. Benzoxazol compounds and benzoic acid are benzamide analogues that are established to possess a countless number of pharmacological activities like antibacterial, antifungal, antidepressant, antiseptic etc.[47]
Arabinofuranoside compounds carry mycobacterial growth inhibition activities[48]. Glycolipids containing an arabinofuranoside trisaccharide inhibited the growth of Mycobacterium smegmatis (M. smegmatis). Both arabinofuranoside trisaccharide and lipidic portion were essential for the biological activity[48]. It was shown further that arabinomannan glycolipids inhibited the sliding motility and biofilm formation of M. smegmatis[49]. Benzyl benzoate identified from L. salicifolia in our present study is an established drug used against scabies in animals and is also exploited as an insect repellant[50,51]. Oxymetholone extract is conventionally used for the treatment of anaemia[52]. Eremophilane identified in L. citrata is probed to possess antimicrobial, anticancer and immunomodulatory function by Yuyama et al.[53].
Digitoxin is a cardiac glycoside widely known for treating congestive heart failure[54]. It is also evidenced to function as a promising anticancer agent when used at therapeutic concentrations[55]. Thiourea, 1-[2-(2-benzylphenoxy)ethyl]-3-(O-totyl) are volatile organic compounds with antifungal property[56]. Pregn-4-ene-3,20-dione, 17,21-dihydroxy-, bis(Omethyloxime) is inspected to have anti-inflammatory function[57]. d-Mannitol, 1-decylsulfonyl compound is examined to have anti-microbial activity by Alagammal et al.[58]. In addition to the abovementioned, compounds with various industrial importances like anthracene, diethyl phthalate, 2-propenoic acid etc. have also been detected in the current study.
The use of herbal therapeutics in replace of synthetic drugs for treatment of diverse diseases and disorders has become a bourgeoning area of research. The present work is the first study reporting the comparative screening of primary and secondary host plants of muga silkworm and identification of their rich phytochemical inventory. The phytoconstituents of profound interest discovered in this study suggest the fortified antioxidant and antibacterial potentiality of muga food plants. The results of DPPH scavenging activity uncovered L. polyantha to exhibit strong antioxidant efficiency compared to other food plants. Besides these, the host plants have also revealed anti-bacterial efficacy against Gram-positive and Gram-negative bacteria. Among the food, plants investigated L. citrata was evidenced to possess greater antibacterial inhibition compared to the rest in opposition to both Gram-positive S. aureus and Gram-negative E. coli. Compiling the above findings it can be accomplished that food plants of muga silkworm are equipped with antioxidant and antibacterial competence, which can be developed as natural alternative therapeutics against a plethora of diseases. The study does indicate that further exploration for isolation, characterization and structure elucidation of bioactive compoundsin the mentioned plants may contribute to the discovery of noble drug initiatives.
Acknowledgement:
The authors gratefully acknowledge the financial support from the Department of Biotechnology- Research Associateship Program in Biotechnology and Life Sciences, Govt. of India. The authors are also thankful to the Director, ICAR-NRC on Pig, Assam, India for granting permission to avail the facilities to carry out the research.
Conflicts of interests:
The authors declare that there is no conflict of interest.
References
- Adwan G, Mhanna M. Synergistic effects of plant extracts and antibiotics on Staphylococcus aureus strains isolated from clinical specimens. J Sci Res 2008;3(3):134-9.
- Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. Afr J Biotechnol 2008;7(12):1797-1806.
- Kumari S, Deori M, Elancheran R, Kotoky J, Devi R. In vitro and in vivo antioxidant, anti-hyperlipidemic properties and chemical characterization of Centella asiatica (L.) extract. Front Pharmacol 2016;7:400.
- Vijyalakshmi R, Ravindran R. Preliminary comparative phytochemical screening of root extracts of Diospyrus ferrea (Wild.) Bakh and Arva lanata (L.) Juss. Ex Schultes. Asian J Plant Sci Res 2012;2:581-7.
- Doss A. Preliminary phytochemical screening of some Indian medicinal plants. Anc Sci Life 2009;29(2):12-6.
[Google Scholar] [PubMed]
- Pandey P, Mehta R, Upadhyay R. Physico-chemical and preliminary phytochemical screening of Psoralea corylifolia. Arch Appl Sci Res 2013;5(2):261-5.
- Raphael E. Phytochemical constituents of some leaves extract of Aloe vera and Azadirachta indica plant species. Global Adv Res J Environ Sci Toxicol 2012;1(2):14-7.
- Kumari SK, Sridevi V, Lakshmi MC. Studies on Phytochemical screening of aqueous extract collected from fertilizers affected two medicinal plants. J Chem Biol Phys Sci 2012;2(3):1326-32.
- Salvamani S, Gunasekaran B, Shukor MY, Bakar MZ, Ahmad SA. Phytochemical investigation, hypocholesterolemic and anti-atherosclerotic effects of Amaranthus viridis leaf extract in hypercholesterolemia-induced rabbits. RSC Adv 2016;6(39):32685-96.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39(1):44-84.
[Crossref] [Google Scholar] [PubMed]
- Kharat SS, Kumkar PB, Rajpure SR, Sonawane KS. Qualitative phytochemical screening of Gnidia glauca (Fresen) Gilg. plant extract. Int J Pharm Bio Sci 2013;4(4):144-8.
- Gülçin ?, Elias R, Gepdiremen A, Chea A, Topal F. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: Cepharanthine and fangchinoline. J Enzyme Inhib Med Chem 2010;25(1):44-53.
[Crossref] [Google Scholar] [PubMed]
- Tikader A, Vijayan K, Saratchandra B. Muga silkworm, Antheraea assamensis (Lepidoptera: Saturniidae)-an overview of distribution, biology and breeding. Eur J Entomol 2013;110(2):293-300.
- Biswas NN, Acharzo AK, Anamika S, Khushi S, Bokshi B. Screening of natural bioactive metabolites and investigation of antioxidant, antimicrobial, antihyperglycemic, neuropharmacological, and cytotoxicity potentials of Litsea polyantha Juss. Ethanolic root extract. Evid Based Complement Alter Med 2017;2017.
[Crossref] [Google Scholar] [PubMed]
- Hasan MF, Iqbal MA, Uddin MS. Antibacterial and antifungal activity of Litsea monopetala leaves on selected pathogenic strains. Eur J Med Plant 2016;12(4):1-8.
- Nasrin F, Hakim ML. In vivo antidiarrheal study of ethanolic extracts of Mikania cordata and Litsea monopetala leaves. Bangladesh J Pharmacol 2015;10(3):562-5.
- Poonia BS, Sasmal D, Mazumdar PM. Anti-diarrheal activity of methanol extract of Litsea polyantha bark in mice. Fitoterapia 2007;78(3):171-4.
[Crossref] [Google Scholar] [PubMed]
- Zou Y, Liao S, Shen W, Liu F, Tang C, Chen CY, et al. Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in Southern China. Int J Mol Sci 2012;13(12):16544-53.
[Crossref] [Google Scholar] [PubMed]
- Arabshahi-Delouee S, Urooj A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem 2007;102(4):1233-40.
- Thabti I, Elfalleh W, Tlili N, Ziadi M, Campos MG, Ferchichi A. Phenols, flavonoids, and antioxidant and antibacterial activity of leaves and stem bark of Morus species. Int J Food Prop 2014;17(4):842-54.
- Sofowora AE. The State of Medicinal Plants in Nigeria, University of Ibadan, Ibadan, Nigeria. 1993.
- Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 1995;28(1):25-30.
- Thornsberry C. NCCLS standards for antimicrobial susceptibility tests. Lab Med 1983;14(9):549-53.
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999;12(4):564-82.
[Crossref] [Google Scholar] [PubMed]
- Varadarajan P, Rathinaswamy G, Asirvatahm D. Antimicrobial properties and phytochemical constituents of Rheo discolor. Ethnobot Leaflet 2008;12:841-5.
- Rehab AH, Amira AE. Plants secondary metabolites: The key drivers of the Pharmacological actions of medicinal plants. Herbal Med 2018;10:11-30.
- Behera S, Babu SM, Ramani YR, Choudhury PK, Panigrahi R. Phytochemical investigation and study on antioxidant properties of Ocimum canum hydro-alcoholic leaf extracts. J Drug Deliv Ther 2012;2(4):122-8.
- Potterat O. Antioxidants and free radical scavengers of natural origin. Curr Org Chem 1997;1(4):415-40.
- Saggu S, Sakeran MI, Zidan N, Tousson E, Mohan A, Rehman H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem Toxicol 2014;72:138-46.
[Crossref] [Google Scholar] [PubMed]
- Razali N, Razab R, Junit SM, Aziz AA. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale). Food Chem 2008;111(1):38-44.
- Sabulal B, Dan M, Kurup R, Pradeep NS, Valsamma RK, George V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006;67(22):2469-73.
[Crossref] [Google Scholar] [PubMed]
- Chan WK, Tan LT, Chan KG, Lee LH, Goh BH. Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 2016;21(5):529.
[Crossref] [Google Scholar] [PubMed]
- Krishna AB, Manikyam HK, Sharma VK, Sharma N. Plant cardenolides in therapeutics. Int J Indig Med Plants 2015;48:1871-96.
- Al-Rashood KA, Abdel-Aziz HA. Thiazolo [3, 2-a] benzimidazoles: Synthetic strategies, chemical transformations and biological activities. Molecules 2010;15(6):3775-815.
[Crossref] [Google Scholar] [PubMed]
- Chandrashekarachar D, Kesagudu D. Impotrtance of furan based compounds and their biomedical applications: An overview. Indo Am J Pharm Res 2017;7(1):7541-9.
- Paiva SA, Russell RM. β-carotene and other carotenoids as antioxidants. J Am Coll Nutr 1999;18(5):426-33.
[Crossref] [Google Scholar] [PubMed]
- Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr 2001;21(1):381-406.
[Crossref] [Google Scholar] [PubMed]
- Croft KD. The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad of Sci 1998;854(1):435-42.
[Crossref] [Google Scholar] [PubMed]
- Tan KH, Nishida R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J Insect Sci 2012;12(1):56.
[Crossref] [Google Scholar] [PubMed]
- Fraser CM, Chapple C. The phenylpropanoid pathway in Arabidopsis. Arabidopsis Book 2011;9:e0152.
[Crossref] [Google Scholar] [PubMed]
- Xu H. (Z)-3, 4, 3', 5'-tetramethoxystilbene, a natural product, induces apoptosis and reduces viability of paclitaxel-and cisplatin-resistant osteosarcoma cells. J Cancer Res Therap 2016;12(4):1261-5.
[Crossref] [Google Scholar] [PubMed]
- Chipley JR. Sodium benzoate and benzoic acid. In: Antimicrobials in food 1983:11-35.
- Pundir RK, Jain P. Evaluation of five chemical food preservatives for their antibacterial activity against bacterial isolates from bakery products and mango pickles. J Chem Pharm Res 2011;3(1):24-31.
- Sofos JN, Beuchat LR, Davidson PM, Johnson EA. Naturally occurring antimicrobials in food. Regul Toxicol Pharmacol 1998;28(2):71-2.
[Crossref] [Google Scholar] [PubMed]
- Leesmith J. General Microbiology Laboratory. Kasetsart University Publishing. 2005:58-60.
- Rajashekhara E, Suresh ER, Ethiraj S. Thermal death rate of ascospores of Neosartorya fischeri ATCC 200957 in the presence of organic acids and preservatives in fruit juices. J Food Prot 1998;61(10):1358-62.
[Crossref] [Google Scholar] [PubMed]
- Asif M. Pharmacological potential of benzamide analogues and their uses in medicinal chemistry. Mod Chem Appl 2016;4(4):1-0.
- Naresh K, Bharati BK, Jayaraman N, Chatterji D. Synthesis and mycobacterial growth inhibition activities of bivalent and monovalent arabinofuranoside containing alkyl glycosides. Org Biomol Chem 2008;6(13):2388-93.
- Naresh K, Bharati BK, Avaji PG, Jayaraman N, Chatterji D. Synthetic arabinomannan glycolipids and their effects on growth and motility of the Mycobacterium smegmatis. Org Biomol Chem 2010;8(3):592-9.
[Crossref] [Google Scholar] [PubMed]
- Burns DA. Diseases caused by arthropods and other noxious animals. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s textbook of dermatology, 2. 8th ed. Wiley-Blackwell; 2010. p. 38-41.
- Shaikh J. Benzyl Benzoate. In: Wexler P, editors. Encyclopedia of Toxicology, 1. 2nd ed. Elsevier; 2005. p. 264-5.
- Pavlatos AM, Fultz O, Monberg MJ, Vootkur A. Review of oxymetholone: A 17α-alkylated anabolic-androgenic steroid. Clin Ther 2001;23(6):789-801.
[Crossref] [Google Scholar] [PubMed]
- Yuyama KT, Fortkamp D, Abraham WR. Eremophilane-type sesquiterpenes from fungi and their medicinal potential. Biol Chem 2017;399(1):13-28.
[Crossref] [Google Scholar] [PubMed]
- Arispe N, Diaz JC, Simakova O, Pollard HB. Heart failure drug digitoxin induces calcium uptake into cells by forming transmembrane calcium channels. Proc Natl Acad Sci 2008 Feb 19;105(7):2610-5.
[Crossref] [Google Scholar] [PubMed]
- Elbaz HA, Stueckle TA, Tse W, Rojanasakul Y, Dinu CZ. Digitoxin and its analogs as novel cancer therapeutics. Exp Hematol Oncol 2012;1(1):1-0.
[Crossref] [Google Scholar] [PubMed]
- Cheon DM, Jang DS, Kim HY, Choi KS, Choi SK. Detection of antifungal endolichenic fungi and antifungal compound. Korean J Microbiol 2013;49(2):165-71.
- Al-Rubaye AF, Kadhim MJ, Hameed IH. Determination of bioactive chemical composition of methanolic leaves extract of Sinapis arvensis using GC-MS technique. Int J Toxicol Pharmacol Res 2017;9(2):163-78.
- Alagammal M, Soris PT, Mohan VR. GC-MS analysis of Polygala rosmarinifolia Wights and Arn. J Appl Pharm Sci 2012;2(4):188-90.

 ): Zone of inhibition
): Zone of inhibition