- *Corresponding Author:
- Ming Yuan
Department of Urology, Beijing Water Resources Hospital, Haidian, Beijing 100036, China
E-mail: gaohong@cfau.edu.cn
| This article was originally published in a special issue, “Innovations in Biomedical Research and Drug Development” |
| Indian J Pharm Sci 2023:85(3) Spl Issue “117-123” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Renal transplantation, as an effective treatment for end-stage renal disease, has many clinical complications, among which delayed graft function recovery, as one of the common postoperative complications, is also a major risk factor affecting the short-term and long-term efficacy of renal transplantation. Therefore, reasonable use of delayed graft function after renal transplantation to promote renal function recovery is of great significance for the prognosis of these patients. Based on this, 62 patients who underwent renal transplantation and developed delayed graft function in our hospital from June 2017 to December 2019 were selected as the research objects. According to the random color method, they were divided into three groups; diosmin group (n=20), ulinastatin group (n=21) and combination group (n=21). To observe the effects of different medication regimens on renal injury markers and renal ultrasound related indexes in patients with delayed graft function and to lay a theoretical foundation for guiding clinical medication and improving prognosis of delayed graft function recovery patients. After comparing the general data of the three groups, it was found that there was no significant difference (p>0.05), indicating that there was no difference in medication results due to personal factors. The levels of serum creatinine, cystatin and blood urea nitrogen in the ulinastatin group after treatment were significantly better than those before treatment. The levels of renal injury markers in the combined group were significantly better after treatment than before treatment. According to the ultrasound results, there was no significant difference in the renal ultrasound indexes between the diosmin and ulinastatin groups after treatment, while the renal ultrasound indexes of the combined group were better than those of the other two groups. It can be concluded from the results that diosmin combined with ulinastatin can significantly reduce renal injury in patients with delayed graft function and can effectively improve renal function in such patients, which has a positive effect on improving prognosis.
Keywords
Renal transplantation, graft function, renal injury markers, renal ultrasound, diosmin, ulinastatin
Allogeneic renal transplantation is one of the most effective measures to save the lives of patients with chronic end-stage renal disease. It has a long history of 67 y and successful renal transplantation can restore the metabolic and endocrine functions of the kidney[1]. Since August 5th 1968, the United States has passed the Harvard criteria for the discrimination of brain death, legally recognizing the legal use of medical extraction of cadaveric organs for transplantation and promoting the steady development of clinical allogeneic kidney transplantation[2]. However, complications after renal transplantation are inevitable in clinical practice, among which Delayed Graft Function (DGF) is one of the complications after renal transplantation. According to data statistics in different regions and transplant centers, the incidence of DGF after cadaveric renal transplantation is about 20 %-50 % and the incidence of DGF after living renal transplantation is about 10 %[3]. According to relevant studies, the risk factors affecting the occurrence of DGF include renal source factors, recipient factors and other factors, and the clinical manifestations are often urine volume change, renal transplant site discomfort and other symptoms[4]. At the same time, the laboratory biochemical indicators of DGF patients also show slow or unimproved renal function[5]. Therefore, how to prevent DGF and improve, and restore graft function as soon as possible after DGF is of great clinical significance for renal transplant patients[6]. Ulinastatin has the functions of inhibiting protease activity, stabilizing lysosomal membrane and scavenging oxygen free radicals, which can significantly reduce the body damage caused by multiple hydrolases and inflammatory mediators caused by surgery, trauma and hypoxia in tissues and organs, and improve renal function by protecting renal tubules[7]. Diosmin is a vascular protection and capillary stabilizer, which can reduce venous dilatation and venous blood stasis, normalize capillary wall permeability and enhance resistance[8]. However, the combination of the two drugs in the treatment of patients with DGF has not been reported in most studies. Based on this, 62 patients with DGF were selected as the research objects in this study to observe the effect of different medication regimens on improving renal injury markers and renal ultrasound indexes in such patients, without providing a therapeutic basis for further improving the prognosis of patients with DGF.
Materials and Methods
General information:
A total of 62 patients with DGF after renal transplantation in our hospital from June 2018 to December 2021 were randomly divided into three groups; diosmin group (n=20), ulinastatin group (n=21) and combination group (n=21). Different drug treatment regimens were used to observe each group. All subjects had the right to know the medication regimen in this study and those with contraindications, low compliance and combined immune dysfunction were excluded.
Method:
Grouping method: The grouping method used in this study was random color method. 20 pink, 21 red and 21 blue cards were prepared, with pink representing the diosmin group, red representing the ulinastatin group and blue representing the combined group. All the cards were put into an airtight box and the patients who agreed to participate in this study were asked to draw them by themselves at the time of treatment. According to the color of the cards drawn by each patient, they were included into different groups.
Drug regimen for each group: All patients did not recover graft function or were diagnosed with DGF 3 d to 7 d after renal transplantation. The delayed recovery of graft function was determined according to the clinical manifestations, biochemical examination results and color ultrasound examination of the graft kidney. Hemodialysis was performed according to the doctor’s advice. Using a Belang dialysis machine. Bicarbonate dialysate and alum film dialyzer or acetic acid film dialyzer, the dialysis time was 2.5 h~4 h. In the diosmin group, oral diosmin alone was used as adjuvant therapy (produced by Wuhan Mayinglong Pharmaceutical Co., Ltd., approval number: Sinopharmed H20066737), 1.5 g each time for 5 d starting from the 1st d after operation. In the ulinastatin group, ulinastatin (Guangdong Tempu Biochemical Medicine Co., Ltd., batch number: 20180422/20191125, specification: 100 000 units) was injected intravenically. The method of administration was as follows; 0.5 million units ulinastatin was mixed with 0.9 % sodium chloride injection 50 ml and 20 000 U/h was continuously pumped once a day for 3 consecutive treatments days; the combined group was treated with oral diosmin combined with intravenous ulinastatin after operation, with the same usage and dosage as the above two groups. The renal injury markers and renal ultrasound indexes of all patients were observed and recorded before treatment and 15 d after treatment.
Detection of renal injury markers: The fasting venous blood of the subjects in the morning was 3 ml and the levels of Serum creatinine (Scr), Retinol-Binding Protein (RBP), Beta 2-Microprotein (β2-MG) and Cystatin C (CysC) of the subjects were detected by immunoturbidimetry. The detection instrument was DxC80 automatic biochemical analyzer (produced by Bateman-Coulter Company, United States of America (USA)) the reagent boxes, standards and quality control products were provided by Fujian Maixin Biotechnology Development Co., Ltd. The rate method was used to detect the level of Blood Urea Nitrogen (BUN) in patients. The kit was provided by Wuhan Bode Bioengineering Co., Ltd. The spring morning urine of the patient was collected, and the Urine Micro Albumin (UMAlb) level was measured by immune scattering turbidimetry. The detection instrument was AU3640 automatic biochemical analyzer (produced by Olympus Company, Japan).
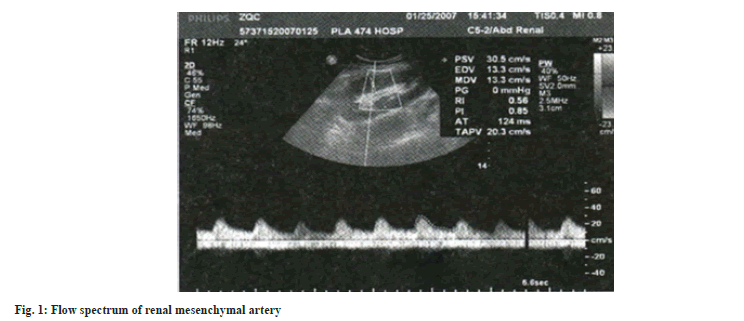
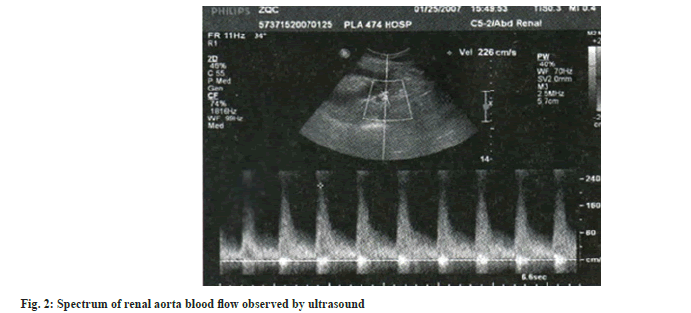
Renal ultrasound examination: After 15 d of treatment, renal ultrasound was performed on all patients. The patients were placed in the supine position and the surface of the transplanted kidney was fully exposed. The size of the transplanted kidney, cortical thickness, calculi and collection system dilatation were measured first. Color Doppler was used to observe the shape, course and blood flow filling of the intra renal artery and spectrum Doppler was used to measure the blood flow spectrum. After local amplification, the sampling volume was located in the Magnetic Resonance Angiography (MRA) of the renal hilum, the Segonal Artery in the Renal Sinus (SRA) and the Interlobar Artery (IRA) of the renal column. The sampling volume was set to 2-4 mm and the angle between the blood flow and the acoustic beam was <60°, the subjects were asked to hold their breath after inhalation and 3-5 consecutive spectral images with consistent morphology and clear shape were recorded. The maximum lumen Diameter (D) of the arteries at each level was measured and the Peak Systolic Velocity (PSV), End-Diastolic Velocity (EDV), Resistance Index (RI) and Pulse Index (PI) were recorded automatically and calculated.
Statistical processing:
Statistical Package for the Social Sciences (SPSS) 25.0 software was used for data analysis. The count data were expressed as rate and Chi-square (χ²) test and the measurement data were expressed as mean±standard deviation. One-way variance test was used to compare multiple groups’ p<0.05 was considered significant.
Results and Discussion
The comparison of clinical information and kidney sources among the three groups showed that there was no significant difference in the above aspects, indicating that there was no significant deviation in postoperative treatment results caused by the above factors, as shown in Table 1.
| Diosmin group (n=20) | Ulinastatin group (n=21) | Combined group (n=21) | χ²/F | p | |
|---|---|---|---|---|---|
| Age (years) | 46.80±10.78 | 51.76±6.74 | 46.71±9.71 | 2.053 | 0.137 |
| Gender | 0.420 | 0.811 | |||
| Man | 11 (55.00) | 10 (47.62) | 12 (57.14) | ||
| Woman | 9 (45.00) | 11 (52.38) | 9 (42.86) | ||
| BM I (kg/m2) | 24.64±2.52 | 23.36±2.50 | 23.70±2.62 | 1.377 | 0.260 |
| Kidney source | 0.160 | 0.923 | |||
| DCD | 16 (80.00) | 16 (76.19) | 17 (80.95) | ||
| LDK | 4 (20.00) | 5 (23.81) | 4 (19.05) | ||
| Kidney derived blood group | 1.378 | 0.967 | |||
| A | 4 (20.00) | 5 (23.81) | 6 (28.57) | ||
| B | 6 (30.00) | 5 (23.81) | 4 (19.05) | ||
| O | 7 (35.00) | 6 (28.57) | 7 (33.33) | ||
| AB | 3 (15.00) | 5 (23.81) | 4 (19.05) | ||
| Warm ischemic time (min) | 5.73±0.35 | 5.71±0.37 | 5.65±0.29 | 0.339 | 0.714 |
| Cold ischemic time (h) | 6.24±3.23 | 6.22±2.99 | 6.96±2.96 | 0.389 | 0.679 |
| HLA match points | 0.593 | 0.744 | |||
| 0~1 | 9 (45.00) | 7 (33.33) | 8 (38.10) | ||
| 2~3 | 11 (55.00) | 14 (66.67) | 13 (61.90) | ||
Table 1: Comparison of clinical information among the three groups.
By observing the changes of renal injury markers in the three groups before and after treatment, it could be found that only CysC in the diosmin group had significant changes before and after treatment, while the levels of Scr, CysC and BUN in the ulinastatin group were significantly better after treatment than before treatment. The levels of renal injury markers in the combined group were significantly better after treatment than before treatment. It can be seen from the results in Table 2 that diosmin combined with ulinastatin can effectively improve renal injury markers in DGF patients.
| Diosmin group (n=20) | Ulinastatin group (n=21) | Combined group (n=21) | F | p | |
|---|---|---|---|---|---|
| Scr (µmol/l) | |||||
| Before | 112.89±9.27 | 111.94±9.06 | 116.77±7.61 | 1.824 | 0.17 |
| After | 112.09±8.16 | 104.38±9.25* | 94.25±7.62* | 23.464 | <0.001 |
| RBP (mg/l) | |||||
| Before | 75.60±6.99 | 76.19±6.24 | 75.51±7.01 | 0.062 | 0.94 |
| After | 78.85±5.65 | 77.78±7.90 | 69.24±6.62* | 12.439 | <0.001 |
| β2-MG (mg/l) | |||||
| Before | 3.38±0.86 | 3.26±0.77 | 3.50±0.88 | 0.447 | 0.642 |
| After | 3.46±0.73 | 3.67±0.79 | 2.49±0.73* | 14.806 | <0.001 |
| CysC (mg/l) | |||||
| Before | 1.59±0.29 | 1.60±0.34 | 1.51±0.35 | 0.455 | 0.636 |
| After | 1.51±0.34* | 1.18±0.31* | 1.10±0.23* | 10.722 | <0.001 |
| BUN (mmol/l) | |||||
| Before | 3.74±0.48 | 3.29±0.55 | 3.23±0.74 | 0.617 | 0.617 |
| After | 3.39±0.66 | 2.89±0.52* | 2.75±0.56* | 6.813 | <0.002 |
| UmALB (mg/l) | |||||
| Before | 59.16±4.74 | 57.72±5.22 | 57.92±5.76 | 0.444 | 0.644 |
| After | 60.37±4.25 | 59.01±6.13 | 51.45±6.04* | 15.488 | <0.001 |
Note: (*) indicates statistical significance compared with pre-treatment.
Table 2: Changes of Renal Injury markers in the three groups before and after medication.
Table 3 shows the renal ultrasound indexes of the three groups after treatment, from which we can see that there is no significant difference in the maximum lumen diameter of MRA, SRA and IRA among the three groups. In addition, the renal ultrasound indexes of the combined group are significantly better than those of the diosmin and ulinastatin groups. From the comparison of diosmin and ulinastatin groups, the levels of the two groups were mostly close after treatment. Fig. 1 and fig. 2 shows renal ultrasound imaging data.
| Diosmin group (n=20) | Ulinastatin group (n=21) | Combined group (n=21) | F | p | |
|---|---|---|---|---|---|
| MRA | |||||
| D (cm) | 0.53±0.02 | 0.52±0.02 | 0.52±0.02 | 1.474 | 0.237 |
| PSV (cm/s) | 68.04±7.07 | 69.17±5.65 | 73.46±8.21 | 3.398 | 0.04 |
| EDV (cm/s) | 20.04±1.83 | 21.17±2.19 | 26.90±3.03 | 48.182 | <0.001 |
| RI | 0.71±0.05 | 0.67±0.04 | 0.63±0.04 | 19.971 | <0.001 |
| PI | 1.34±0.21 | 1.23±0.18 | 1.20±0.15 | 2.207 | 0.021 |
| SRA | |||||
| D (cm) | 0.38±0.04 | 0.38±0.03 | 0.38±0.03 | 0.089 | 0.915 |
| PSV (cm/s) | 46.44±5.66 | 45.19±5.42 | 50.99±6.17 | 5.877 | 0.009 |
| EDV (cm/s) | 13.83±1.08 | 13.99±1.03 | 18.77±1.77 | 91.034 | <0.001 |
| RI | 0.70±0.04 | 0.69±0.06 | 0.60±0.04 | 24.925 | <0.001 |
| PI | 1.26±0.13 | 1.26±0.14 | 1.02±0.07 | 28.939 | <0.001 |
| IRA | |||||
| D (cm) | 0.26±0.03 | 0.25±0.03 | 0.25±0.02 | 1.625 | 0.206 |
| PSV (cm/s) | 29.78±2.80 | 28.83±4.12 | 32.77±3.86 | 6.701 | 0.002 |
| EDV (cm/s) | 11.59±1.08 | 11.35±1.02 | 13.07±1.12 | 15.679 | <0.001 |
| RI | 0.68±0.06 | 0.70±0.05 | 0.59±0.06 | 26.763 | <0.001 |
| PI | 1.31±0.17 | 1.31±0.14 | 1.08±0.12 | 17.030 | <0.001 |
Table 3: Changes of Renal Ultrasound indexes in three groups after medication.
DGF is one of the most common complications after renal transplantation, mainly manifested as sudden oliguria, anuria or transient polyuria and increased Scr. Due to the scarcity of organ sources, the standards for kidney donors are becoming wider and wider, and the incidence of delayed recovery of transplanted kidney is also on the rise[9]. Studies have shown that the causes of DGF mainly include donor factors and recipient factors, among which the poor quality of donor kidney can lead to a significant increase in the incidence of DGF after surgery, followed by donor age, gender, creatinine level, diabetes, hypertension, improper kidney retrieval and repair, etc., which will become the predisposing factors of DGF[10]. Data have shown that the risk of DGF in recipients is doubled when the donor is older than 55 y, so the donor criteria should be strictly controlled. In addition, Donation after Circulatory Death (DCD) donors inevitably experience a long warm ischemia time during the declaration of death and subsequent organ acquisition, and prolonged ischemia time is a high risk factor for DGF[11]. From the perspective of receptor factors, intraoperative and early postoperative hypotension is one of the most common prerenal factors of DGF[12]. Arterial stenosis and vascular blockage of the transplanted kidney lead to decreased blood perfusion of the transplanted kidney, resulting in a series of pathophysiological changes and renal tissue damage, which is generally mainly acute tubular injury. Such patients have a higher probability of DGF after surgery[13]. In this study, after comparing the above related risk factors among the three groups of patients, it was shown that there was no significant difference in DGF risk factors and clinical data comparison among the three groups, which ensured the rigor and credibility of the following study.
In the results of this study, we can conclude that diosmin alone has no obvious effect on improving renal injury indexes in patients with DGF, while ulinastatin alone has significant effect on some indexes in patients with DGF, but the combination of the two drugs can significantly improve renal injury related markers in patients with DGF. After analysis, the author concluded that ulinastatin is a broad-spectrum protease inhibitor with strong enzymatic activity and has inhibitory effects on various hydrolytic enzymes such as granulocyte elastase and trypsin[14]. In addition, ulinastatin is also a lysosomal membrane stabilizer, which can stabilize lysosomal membrane, inhibit lysosomal release and myocardial inhibitory factor production and improve microcirculation. It can also remove free radicals and inhibit the release of inflammatory mediators[15]. Thus the renal blood flow is reduced to prevent shock; improve glomerular function; protect kidney tubules, reduce the release of a variety of enzymes and it can inhibit the secretion of diuretic hormone and aldosterone, thereby playing a role in reducing renal injury[16]. As a micro granular and purified flavonoids intravenous active drug, diosmin has been proved to have a good therapeutic effect on lymphedema in clinical practice[17]. And it can also by increasing the number of lymphatic lymphatic’s open quantity, set up to promote lymphatic peristalsis, increase the backflow, so although the separate application for improving DGF markers of kidney injury in patients with no significant role, but because after joint ulinastatin promotes lymphatic peristalsis, so can effectively enhance the effect of ulinastatin[8].
And from the point of ultrasonic kidney index, separate application ulinastatin and diosmin to improve the function of the kidney index has no obvious difference, but the combined use of more can significantly improve renal artery blood flow velocity, reduce the resistance and so on, is mainly due to the diosmin can; prolonged to adrenal hormone in the venous wall, improve the vein tension, promoting venous return[18]; increase the red blood cell flow rate, lead to the decrease of blood viscosity, prevent capillary blockage[19]; it can inhibit the release of inflammatory transmitters in the body, reduce local inflammatory reaction, reduce damage to local capillaries and maintain their permeability[20]. Through these mechanisms, the circulation of local blood and lymphatic fluid is promoted, which is manifested as the improvement of renal ultrasound indexes. At the same time, the renal ultrasound indexes of patients in the combination group were significantly better than those in the single drug group. The specific reasons are as described in the above paragraph and will not be described here. In conclusion, diosmin combined with ulinastatin can effectively improve the renal injury-related indicators and renal ultrasound function parameters in patients with DGF, which is of great significance for promoting the prognosis of such patients.
Funding:
This work was supported by the “Twelfth Five-Year Plan” Medical Science Development Foundation of People's Liberation Army (PLA) (11MS012).
Conflict of interests:
The authors declared no conflict of interests.
References
- Koken ZO, Karahan S, Tuncbilek Z, Celik SS. Nursing diagnoses and interventions in kidney transplant recipients: A retrospective study. Transplant Proc 2019;51(7):2321-3.
[Crossref] [Google Scholar] [PubMed]
- Voora S, Adey DB. Management of kidney transplant recipients by general nephrologists: Core curriculum 2019. Am J Kidney Disease 2019;73(6):866-79.
[Crossref] [Google Scholar] [PubMed]
- Kharfan-Dabaja MA, Kumar A, Ayala E, Aljurf M, Nishihori T, Marsh R, et al. Standardizing definitions of hematopoietic recovery, graft rejection, graft failure, poor graft function and donor chimerism in allogeneic hematopoietic cell transplantation: A report on behalf of the American society for transplantation and cellular therapy. Transplant Cell Ther 2021;27(8):642-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Fu Q, Liu J, Li J, Deng R, Wu C, et al. Risk factors and outcomes of prolonged recovery from delayed graft function after deceased kidney transplantation. Renal Fail 2020;42(1):792-8.
[Crossref] [Google Scholar] [PubMed]
- Dunn MA, Rogal SS, Duarte-Rojo A, Lai JC. Physical function, physical activity and quality of life after liver transplantation. Liver Transplant 2020;26(5):702-8.
[Crossref] [Google Scholar] [PubMed]
- Hashim E, Yuen DA, Kirpalani A. Reduced flow in delayed graft function as assessed by IVIM is associated with time to recovery following kidney transplantation. J Magn Reson Imaging 2021;53(1):108-17.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Peng C, Zhang Z, Shi J, Lin Y, Gu L, et al. Intravenous infusion of ulinastatin attenuates acute kidney injury after cold ischemia/reperfusion. Int Urol Nephrol 2019;51(10):1873-81.
[Crossref] [Google Scholar] [PubMed]
- Cazaubon M, Benigni JP, Steinbruch M, Jabbour V, Gouhier-Kodas C. Is there a difference in the clinical efficacy of diosmin and micronized purified flavonoid fraction for the treatment of chronic venous disorders? Review of available evidence. Vasc Health Risk Manag 2021;17:591-600.
[Crossref] [Google Scholar] [PubMed]
- Zhou J, Yu X, Su T, Wang S, Yang L. Critically ill, tubular injury, delayed early recovery: Characteristics of acute kidney disease with renal oxalosis. Renal Fail 2021;43(1):425-32.
[Crossref] [Google Scholar] [PubMed]
- Bromberg JS, Weir MR, Gaber AO, Yamin MA, Goldberg ID, Mayne TJ, et al. Renal function improvement following ANG-3777 treatment in patients at high risk for delayed graft function after kidney transplantation. Transplantation 2021;105(2):443-50.
[Crossref] [Google Scholar] [PubMed]
- Montagud-Marrahi E, Molina-Andújar A, Rovira J, Revuelta I, Ventura-Aguiar P, Piñeiro G, et al. The impact of functional delayed graft function in the modern era of kidney transplantation–A retrospective study. Transplant Int 2021;34(1):175-84.
[Google Scholar] [PubMed]
- Mohamed R, Crislip GR, McLarnon S, Wei Q, O’Connor PM, Sullivan JC. Persistent vascular congestion in male spontaneously hypertensive rats contributes to delayed recovery of renal function following renal ischemia perfusion compared with females. Clin Sci 2022;136(11):825-40.
[Crossref] [Google Scholar] [PubMed]
- Ajlan B, Maghrabi Y, Mokhtar G, Baeesa S. Timing of ventriculoatrial shunt removal on renal function recovery of patients with shunt nephritis: Case report and systematic review. Clin Neurol Neurosurg 2022;218:107279.
[Crossref] [Google Scholar] [PubMed]
- Chen F, Zhu J, Wang W. Ulinastatin attenuates lps-induced inflammation and inhibits endoplasmic reticulum stress–induced apoptosis in renal tubular epithelial cells via regulation of the TLR4/NF-κB and Nrf2/HO-1 pathways. Inflammation 2021;44(6):2323-32.
[Crossref] [Google Scholar] [PubMed]
- Li T, Ji X, Liu J, Guo X, Pang R, Zhuang H, et al. Ulinastatin improves renal microcirculation by protecting endothelial cells and inhibiting autophagy in a septic rat model. Kidney Blood Press Res 2022;47(4):256-69.
[Crossref] [Google Scholar] [PubMed]
- Hang CC, Guo YH, Li CS, Wang S. Effects of ulinastatin on renal perfusion evaluated by Doppler ultrasonography in a porcine model of septic shock. Exp Ther Med 2021;22(5):1324.
[Crossref] [Google Scholar] [PubMed]
- Zheng Y, Zhang R, Shi W, Li L, Liu H, Chen Z, et al. Metabolism and pharmacological activities of the natural health-benefiting compound diosmin. Food Function 2020;11(10):8472-92.
[Crossref] [Google Scholar] [PubMed]
- Abogresha NM, Mohammed SS, Hosny MM, Abdallah HY, Gadallah AM, Greish SM. Diosmin mitigates cyclophosphamide induced premature ovarian insufficiency in rat model. Int J Mol Sci 2021;22(6):3044.
[Crossref] [Google Scholar] [PubMed]
- Fattori V, Rasquel-Oliveira FS, Artero NA, Ferraz CR, Borghi SM, Casagrande R, et al. Diosmin treats lipopolysaccharide-induced inflammatory pain and peritonitis by blocking NF-κB activation in mice. J Nat Prod 2020;83(4):1018-26.
[Crossref] [Google Scholar] [PubMed]
- Tekeli MY, Eraslan G, Çakır Bayram L, Soyer Sarıca Z. Effect of diosmin on lipid peoxidation and organ damage against subacute deltamethrin exposure in rats. Environ Sci Pollut Res 2021;28(13):15890-908.
[Crossref] [Google Scholar] [PubMed]