- *Corresponding Author:
- Tejal Rawal
Institute of Pharmacy, Nirma University, S. G. Highway, Ahmedabad-382 481, India
E-mail: tejalsharma123@gmail.com
| Date of Submission | 02 February 2015 |
| Date of Revision | 14 December 2015 |
| Date of Acceptance | 02 February 2016 |
| Indian J Pharm Sci,2016;78(1):8-16 |
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
After 50 years drought, several drugs are looming from the pipeline to combat tuberculosis. They will serve as a boon to the field that has been burdened with primitive, inadequate treatments and drug-resistant bacterial strains. From the decades, due to lack of interest and resources, the field has suffered a lot. Learning from the flaws, scientists have renovated their approaches to the finding of new antitubercular drugs. The first line drugs take about six months or more for the entire treatment. The second line remedy for resistant-tuberculosis requires daily injections which carry severe side effects. Drug resistance remains a constant menace because patients stop the medication once they start feeling better. So new drugs are required to be explored which are effective against tuberculosis especially drug resistant tuberculosis. These drugs need to work well with other drugs as well as with antivirals used for the treatment of human immunodeficiency virus. It is also very important to be considered that the treatments need to be cheap, as tuberculosis primarily affects people more in the developing countries. Further, new drugs must cure the disease in short span of time than the current six to nine month regimen. Recently a few new and potent drugs such as bedaquiline, delamanid, teixobactin have been evolved which may serve as a nice step forward, with a better outcome. Teixobactin, a new antibiotic has been found to have promising action against resistant strains, is also under consideration.
Keywords
Tuberculosis, latent, bedaquiline, teixobactin
Introduction
Several potent drugs have been available for the treatment of tuberculosis (TB) since past 70 years. However, poor management of tuberculosis continues to take a heavy count of human lives [1,2]. The disease has become prevalent in countries like Africa, India, China and Russia [3-5]. In India nearly 500,000 people die every year due to this dreadful disease [6-10]. In the early times, in the absence of any cure, infected patients faced slow and painful death. Many patients died unattended in infirmaries [11-13].
There are about 10 million new tuberculosis cases added every year worldwide [14]. About 2 million people succumb to this life threatening disease [15]. Some of the main factors which has contributed to this death dealing disease are the absence of basic sanitary facilities and the emigration of people from rural to urban crowded slums [16-18].
Tuberculosis is caused by rod shaped, aerobic, acid fast, gram positive bacteria named Mycobacterium tuberculosis which was first identified by a German Physician, Robert Koch, in 1882 (Table 1) [6,7]. The bacterium exists in both latent and active forms.
| Years | Milestones |
|---|---|
| 1882 | Robert Koch isolated and cultivated the bacteria responsible for TB |
| 1920 | French Microbiologist A. Calmette and his veterinarian student, C. Guerin developed BCG vaccine |
| 1943 | Dr. Selman Waksman developed streptomycin as a drug cure for TB |
| 1944 | J. Lehman isolated para amino salicylic acid and later discovered isoniazid |
| 1959 | Dr. Melly Ellen Avery discovered the role of surfactant in keeping the air sacs of the lungs open |
| 1966 | Italian PietroSensi isolated rifamycin S from a fungus |
| 1970 | TB treatment course was introduced employing 4 drugs for 6–9 months |
| 1989 | HIV was identified and people became more vulnerable to TB |
| 2005 | USFDA recognized improved diagnostic tests based on the DNA of Mycobacterium tuberculosis |
BCG: Bacillus Calmette–Guérin, TB: tuberculosis, USFDA: United States Food and Drug Administrative
Table 1: Milestones In Tuberculosis
Latent Tuberculosis (Dormant Form)
About 2 billion people are carrying M. tuberculosis in the dormant form worldwide. Out of this, only 1% will develop active form of the disease during their life time. The development of the disease takes place whenever the immunity goes down due to various factors like old age, HIV/AIDS. It becomes
difficult to understand how the bacteria survive for months and years without multiplying or showing any signs of the disease. However the bacteria remains noninfectious during its dormant form but this form continues to be an unlimited pool of infection. Several diagnostic tests have been available to detect latent infection. The antiquated tuberculin test for latent TB is not authentic as it gives false positive results. A small amount of TB proteins is injected under the top layer of the skin of the inner forearm. Appearance of red bumps within 2 days after injection indicates the presence of tuberculosis infection. IFNγ release assays (IGRAs) can be used as an alternative to this age old tuberculin test. These are based on the fact that T-lymphocytes when exposed to specific antigens will release IFNγ [19-25].
Current Treatment Regime
WHO in 1970 recommended a drug regime consisting of four first line drugs- isoniazid (INH), rifampicin (RFM), pyrazinamide (PZ) and ethambutol (ETB), detailed in Table 2 [13]. This regime can be used for the effective treatment of sensitive, non resistant strains of M. tuberculosis. There are two phases of drug administration. Initially during the intensive or rigorous phase of the first two months of the treatment, all the above four drugs are administered on alternate days followed by the continuous phase of next 4-6 months in which only two drugs, rifampicin and isoniazid are administered (Table 3). The above four drugs are effective for the following sub populations of bacteria (a) active growers that are killed by isoniazid (b) periodic metabolic bursts killed by rifampicin (c) low metabolic activity state-susceptible to pyrazinamide. However no drug is effective for dormant bacilli [26-28].
| Drug | Year of invention | Mode of action |
|---|---|---|
| Isoniazid | 1952 | Inhibits cell wall synthesis |
| Rifampicin | 1968 | Nucleic acid synthesis |
| inhibitor with weak effect | ||
| Pyrazinamide | 1952 | Inhibits membrane energy |
| metabolism with weak effect | ||
| Ethambutol | 1961 | Inhibits cell wall synthesis |
Table 2: Invention Of First Line Drugs And Their Mode Of Action
| Phase | Drugs | Time |
|---|---|---|
| Intensive or rigorous | INH + RIF + PZ + ETB | First 2 months |
| Continuous | INH + RIF | 4-6 months |
INH: Isoniazid, RFM: rifampicin, PZ: pyrazinamide, ETB: ethambutol
Table 3: Dose Regime
Hindered bioavailability of rifampicin
Rifampicin and isoniazid interact in the acidic medium. Rifampicin degrades to form 3-formylrifampicin which reacts with isoniazid to form inactive hydrazone of rifampicin. The interaction results in about 20-30% reduction in the bioavailability of rifampicin. To overcome this issue, many research studies are going on [29-32].
Poor patient complaisance
The above drug regime has many flaws which have led to the poor patient complaisance. The patient has to face severe side effects such as nausea and hepatotoxicity. For this reason the patients do not remain stick to the regime. Only a highly disciplined patient can stick to the regime till the end of the treatment. For this purpose WHO has launched DOTS (Directly observed treatment short course) programme. A patient has to visit a TB clinic on alternate days for about 6-9 months and the formulation is put into the mouth and visually confirmed that patient has swallowed the drug. However, in India DOTS has not been very successful. India has the burden of having maximum number of TB patients of about 2 million out of which total number of deaths is 5 lakh per year. About 70% of TB patients are aged between 15-54 years. The poor patient compliance is a major factor which results in the development of resistance to first line drugs. The development of drug resistance is a constant threat as patients stop taking their medicines once they feel better [33-35].
Persistent form of Mycobacterium tuberculosis
All the TB drugs are effective as long as the M. tuberculosis remains in the multiplying phase. However, isoniazid kills over 90% of the mycobacteria in the first two days of the treatment. But still the combination of two drugs rifampicin and isoniazid takes about 6-9 months to kill the persistent form of the microorganism. Soon after the multiplying phase, the bacteria enters a persistent phase in which it stops multiplying and undergoes hibernation. As in case of latent phase, there is no diagnostic test for the detection of persistent form of bacteria. Rifampicin is the only drug which is effective against the persistent M. tuberculosis but has weak activity which elongates the duration of the treatment for several months [36,37].
Disease recurrence (relapse of the disease)
After a few months of ceasation of the drug combination treatment, there are chances of relapse of the persistent form into active form. The disease may even develop in more nasty form i.e., drug-resistant tuberculosis (DR-TB). There is no biomarker available to confirm the complete absence of persistent form in the host [38].
Drug-resistant tuberculosis
Mismanagement of the antitubercular drugs may lead to the development of drug resistance. The time duration for treatment is 6-9 months and symptoms of disease usually disappear after 2-3 months of the treatment. Hence patient may discontinue the medication which is major cause for the drug resistance development. Sometimes it also occurs in the patients who delay the regular administration of the drugs or do not take all the four prescribed drugs. Patients may also develop tuberculosis again after the treatment in the past. DR-TB is communicable through air from one person to another. DR-TB occurs in many forms such as multi-drug resistant tuberculosis, extensively drug resistant tuberculosis and totally drug resistant tuberculosis [38].
Multidrug-resistant tuberculosis
Multidrug-resistant tuberculosis (MDR-TB) is the disease in which the strains of M. tuberculosis become non responsive to the two most effective first line drugs. Several injectables like streptomycin, amikacin, kanamycin and oral fluoroquinolones like moxifloxacin, gatifloxacin can also be given as an alternate therapy but these are highly toxic, less efficacious and very expensive. Apart from these, the duration of the treatment may extend upto 18-24 months instead of 6-9 months. The faulty treatment regimen and unreliable diagnostic tests are also the major factors which may lead to the development of resistance. Only 1% of the cases are on proper treatment but still have poor outcomes [39-41].
Extensively drug-resistant tuberculosis
Patients with extensively drug-resistant tuberculosis (XDR-TB) are resistant to rifampicin, isoniazid, and fluoroquinolones and at least one injectable antibiotic. It is mainly widespread in the countries like Africa, Asia and Soviet Union having majority of cases of HIV/AIDS. XDR-TB has the highest mortality and morbidity rates if co-infected with HIV/AIDS.
Totally drug-resistant tuberculosis
It is the worst form of tuberculosis. It is a condition in which the patients become resistant to all the first and second line drugs ultimately leading to death [42].
Tuberculosis as an obstreperous disease
TB is very challenging essentially due to several reasons (a) poor detection rate as in India it takes about 6 months or more to detect the resistant strains, (b) co-infection with HIV/AIDS (c) enhanced transmission in overcrowded slums, hospitals and prisons, (d) malnutrition, poverty and poor healthcare system, (e) increasing cases of diabetes due to foetal malnutrition, (f) poor patient complaisance and (g) absence of biomarkers to monitor the success of the treatment and complete eradication of the disease. The above reasons presuppose the need of new drugs for the complete and successful treatment of DR-TB [43,44].
Prevalence of tuberculosis in HIV/AIDS patients
Outburst of majority of the cases of TB has been reported among the immunocompromised patients. In immunocompromised patients, the progress of the disease is rapid and fiery leading to high death rates. In this critical situation, there is an urge for new potent agents which must tackle both the dreadful diseases. However, from practical point of view, immunocompromised patients are not considered suitable for controlled trial studies [45-48].
Evolving New Drugs For Tuberculosis
Several antitubercular drugs have been in the use for five to six decades. Eventually, M. tuberculosis has developed resistance to rifampicin and isoniazid. The incidence of TB is high in countries like India, China, former USSR states, while in some African states it is the highest due to the prevalence of HIV/AIDS. Second line drugs can be administered but they are very expensive and their duration of treatment is about 2 long years. These drugs are required to be administered in very high doses which lead to the development of toxicity [49-56].
Barriers in the discovery of new drug for tuberculosis
The barriers in the discovery of new drugs for TB are numerous like (a) limited knowledge about the basic biology of M. tuberculosis, (b) unavailability of animal models for the screening of new drugs against latent and persistent forms, (c) the compatibility of the drug molecule with rifampicin and isoniazid, (d) unavailability of biomarkers to evaluate the efficacy of new drugs, (e) a new drug is always tested in combination and not as a single drug, (f) the drug must not induce or inhibit CYP-450 enzyme [57,58].
Basic biology of Mycobacterium tuberculosis
The drug uptake by cell wall of M. tuberculosis is very slow as the microorganism is a clever adversary. Its ability to hunker down and survive under stress is very trickier. The bacterium has an outer lipid bilayer which provides protection to the organism and is hard to penetrate. This outer bilayer is the thickest biological membrane known and has very low permeability. Additionally the fatty acid cell wall acts as a forbidding barrier towards diffusion of hydrophilic and hydrophobic compounds thus enhancing the chances of survival of the organism [59].
New Drug Molecules Undergoing Clinical Trials
There is an urge to develop novel and effective drugs against TB. Several drugs have been discovered which are under clinical trials (Table 4) [60-65].
| Drug | Class | Status | Mechanism |
|---|---|---|---|
| GatifloxacinFluoroquinolones Currently | Inhibits DNA gyrase, DNA | ||
| in Phase-III | replication | ||
| MoxifloxacinFluoroquinolones Currently | Inhibits DNA replication | ||
| in Phase-III | and transcription | ||
| Bedaquiline | Diarylquinoline | Phase-II | Inhibits ATP synthesis |
| Delamanid | Nitroimidazole | Phase-III | Inhibits protein and |
| mycolic acid synthesis | |||
| PA-824 | Nitroimidazole | Currently | Inhibits cell wall synthesis |
| in Phase-II | by releasing nitrous oxide | ||
| LL-3858 | Pyrrole | Currently | Information related to |
| in Phase-I | molecular mechanism | ||
| currently in Phase-I trials | |||
| Rifalazil | Rifamycin | Currently | Exhibits long acting oral |
| derivative | in Phase-II | activity against bacteria | |
| Teixobactin | A new class | Yet to | Act by targeting lipid |
| antibiotic | begin the | molecules which serve as | |
| trials | building blocks of cell wall | ||
ATP: Adenosine triphosphate
Table 4: Current Status Of New Drugs In Clinical Trials
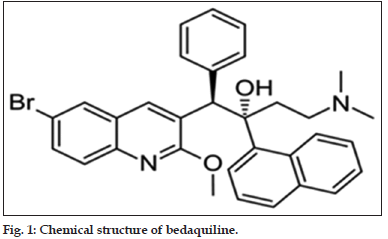
Bedaquiline (TMC 207)
Chemically it is a diarylquinoline compound (fig. 1). It is currently in Phase II but soon to enter Phase III. It has a long terminal half-life. The drug has excellent activity against susceptible TB. It does not show any cross resistance to the present drugs. It exhibits a potent sterilization effect and is active against both the fast and slow growing M. tuberculosis with a novel mechanism of action of inhibiting bacterial ATP synthase. It is highly potent as compared to isoniazid and rifampicin. Further in the fed conditions, the serum concentration of the drug increases by two fold when compared to fasting condition. It gets metabolized by Cytochrome P450 3A4 (CYP3A4) enzyme. Thus the serum concentration of the drug gets reduced upto 50% in presence of rifampicin which is a CYP3A4 inducer [65-67].
Nitroimidazoles derivatives
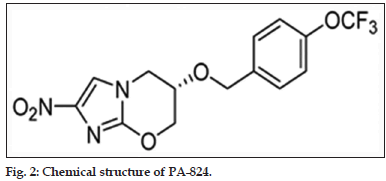
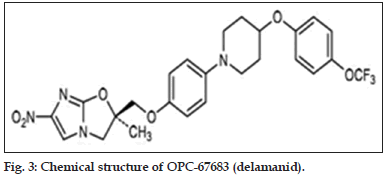
Nitroimidazole derivatives were synthesized in 1970s. Amongst several nitroimidazoles, some were found to have good anti mycobacterial properties. Of these, CG-17341 was developed as the lead compound. But later it was found to be mutagenic. Two nitroimidazole drugs, PA-824 and OPC-67683 (Delamanid, figs. 2 and 3) have been further developed and are under clinical studies.
PA-824, nitroiimdazoloxazine
The drug will soon complete Phase II clinical trials. It is effective against normal as well as resistant strains of bacteria. It has high in vitro and in vivo activity and does not exhibit cross resistance to any other TB drugs in use. It is basically a prodrug which has been found to show good activity against dormant form along with rifampicin, isoniazid and pyrazinamide. The compound also has a long half-life. It acts by two mechanisms, the cell wall synthesis inhibition and respiratory poisoning in the bacterium. However, replacement of isoniazid or pyrazinamide with PA- 824 may lead to the relapse of the disease after 6 months [68-71].
Delamanid (OPC-67683)
The drug has recently entered Phase III clinical study. The drug exhibited excellent in vitro activity with no cross resistance with any first line antiTB drug. It does not get metabolized by any of the CYP enzymes. The drug also has a long half-life. It also exhibits powerful activity which may be due to the release of reactive nitrogen species [71].
Fluoroquinolones
The fluoroquinolones are also found to have powerful activity against resistant strains. They mainly act by inhibiting DNA gyrase. Currently, two fluoroquinolones gatifloxacin and moxifloxacin are presently entering Phase III study [72-75].
LL-3858
The drug is currently in Phase I. It has a pyrrole alkaloid nucleus. It is also an analogue of Isoniazid. It also exhibited a potential activity against MDR-TB in mice [75].
Oxazolidinones
They serve as synthetic antibacterial compounds which are active orally. Linezolid, an oxazolidinone compound got approved in 2000 for the treatment of drug resistant gram positive bacteria. Linezolid is a potent oxazolidinone which can be used for MDR-TB. It acts by inhibiting protein synthesis. It mainly interferes with the formation of the initiation complex. It does not exhibit any cross resistance with the first line drugs. The main target of linezolid is bacterial ribosome (23S RNA in 50S ribosomal subunit). It also exhibits a highly potent activity. Sutezolid (PNU-100480) and AZD-5847 are also chemically related to linezolid. In the recent studies sutezolid is found to be more active than linezolid in recent studies. AZD-5847 has also been recognized for its anti-TB properties. The main concern is that it may lead to hematological issues [76,77].
Ethylenediamine derivatives
SQ-109, an ethylenediamine derivative is also a potent antiTB compound with the similar structure to the first line drug ethambutol. The mode of action is not completely clear, but it has been found to inhibit lipid/cell wall synthesis [78].
Rifalazil (KRM-1648)
It is a rifamycin derivative which is under Phase II clinical trials. It has been found to be a promising candidate which exhibits long acting oral activity against the bacteria in both animal and human models [79,80].
Caprazamycin-B
A promising liponucleoside antibiotic which has been developed in Japan is Cpprazamycin-B. The drug exhibited bactericidal activity specifically against Mycobacterium species including resistant strains. It mainly inhibits the synthesis of mycobacterial cell wall. The therapeutic efficacy was found to be moderate in mice models [81,82].
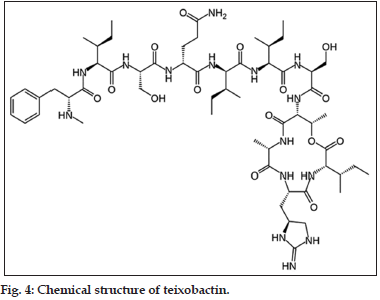
Teixobactin
Teixobactin, A new class powerful antibiotic that kills drug resistant bacteria, which has been reported in January 2015 as a promising compound for its activity against drug resistant Gram positive pathogens (fig. 4). It exhibits a unique mechanism of action by targeting the lipid molecules which are required by the bacteria to build their cell walls. It has been reported that teixobactin has such a rapid activity that it does not allow the resistance development in bacteria. Teixobactin has been commended as the first new antibiotic drug against resistant bacteria for the last 30 years. It hasn’t been tested in human beings yet but has been found to overcome the development of resistance with the present antibiotic drugs. It has also been found to strike multiple targets in the bacteria. It also showed be potent activity against resistant tuberculosis in rat models. Lipid II is the precursor of peptidoglycan synthesized in the cytoplasm and lipid III is the precursor of wall teichoic acids (WTA). Teixobactin has the ability to bind to both these lipids to form stoichiometric complexes. These complexes serve as an obstruction in the formation of functional cell envelope [83-86].
New Drug Delivery Strategies For Antitubercular Drugs
The techniques of drug delivery also determine the efficacy of a treatment. New drug delivery systems have been evolved to reduce the degradation and loss of the drug, to enhance the bioavailability and to reduce the toxic effects. Pulmonary drug delivery systems are gaining utter importance currently due to their numerous advantages like large surface area for absorption, high permeability and good blood supply. New strategies for drug targeting to alveoli are achieving a great attention as the alveolar epithelium has been shown to serve as a prominent site for the absorption of various therapeutics. The formulations such as liposomes, solid lipid nanoparticles, nanoemulsions, polymeric micelles are gaining utmost importance due to their potential advantages to target the drug to specific site [87-93].
Nanotechnology And Tuberculosis Treatment
Upgraded research has paved the way for the development of novel drug delivery systems based on nanotechnology. Reduction of the particle size to nanoscale improves the solubility of the poorly soluble drugs [94-100].
Nanoemulsions and nanosuspensions
The drop size of nanoemulsions lies between 10 and 100 mm. These are thermodynamically stable oil-in-water dispersions which provide the advantages of ease of large scale production and spontaneous generation. Nanosuspensions are colloidal dispersions of drugs which have been stabilized with surfactants. Ahmed et al. developed nanoemulsions of rifampicin which indicated a great potential of intravenous delivery of this antitubercular drug [101].
Liposomes/niosomes
Liposomes are lipidic bimembrane structures produced by cholesterol hydration, phospholipids while niosomes additionally contain nonionic surfactants. Niosomal encapsulation of pyrazinamide has been done by El-Ridy et al. in order to achieve sufficient macrophage targeting and to overcome drug resistance [102].
Polymeric and nonpolymeric nanoparticles
The polymeric and nonpolymeric nanoparticles have been evolved as modes for targeting and stabilization. Extensive studies of antitubercular drugs like rifampicin, isoniazid, pyrazinamide entrapped in the105 poly DL-lactide coglycolide or chitosan nanoparticles have been done [103-105].
Polymeric micelles
Polymeric micelles contain polymers and surfactants which form spherical structure above the critical micelle concentration. The self-assembling of amphiphilic polymers lead to the generation of these polymeric micelles [106].
Nanotechnology has lead to the development of inhaled drug delivery system with potential merits such as direct drug delivery to the site of infection, avoiding the first pass metabolism, reducing the dose of the drug and hence reducing the toxicity of the drug. The nanoparticles get swamped by the alveolar macrophages, thus leading to the direct release of antiTB drugs into the alveolar macrophages. This alveoli targeting strategy is playing a prominent role in attacking the TB bacilli. The various nanoformulations can be converted into dry powder to potentiate their merits [107].
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Central TB Division. Revised National Tuberculosis Control Programme. Guidelines on Programmatic Management of Drug Resistant TB (PMDT) in India. New Delhi: Central TB Division,Directorate General of Health Services, Ministry of Health and Family Welfare; 2012.
- World Health Organization. TB. A Global Emergency. WHO Report on the TB Epidemic. WHO/TB/94.177. Geneva: World Health Organization; 1994.
- Ginsberg AM, Spigelman M. Challenges in tuberculosis drug research and development. Nat Med 2007;13:290-4.
- Specter M. Deadly misdiagnosis. New Yorker 2010;15:48-53.
- Harries AD, Maher D, Nunn P. Practical and affordable measures for the protection of health care workers from tuberculosis in low-income countries. Bull World Health Organ 1997;75:477-89.
- Dye C, Williams BG. The population dynamics and control of tuberculosis. Science 2010;328:856-61.
- Murray JF. A century of tuberculosis. Am J RespirCrit Care Med 2004;169:1181-6.
- Raviglione M, Marais B, Floyd K, Lönnroth K, Getahun H, Migliori GB, et al. Scaling up interventions to achieve global tuberculosis control: Progress and new developments. Lancet 2012;379:1902-13.
- Meya DB, McAdam KP. The TB pandemic: An old problem seeking new solutions. J Intern Med 2007;261:309-29.
- Chakraborty AK. Epidemiology of tuberculosis: Current status in India. Indian J Med Res 2004;120:248-76.
- Sharma SK, Mohan A. Tuberculosis: From an incurable scourge to a curable disease – Journey over a millennium. Indian J Med Res 2013;137:455-93.
- Lönnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, Glaziou P, et al. Tuberculosis control and elimination 2010-50: Cure, care, and social development. Lancet 2010;375:1814-29.
- Rennie J. Unromantic killer. Sci Am 2009;300:8-9.
- Tomioka H, Namba K. Development of antituberculous drugs: Current status and future prospects. Kekkaku 2006;81:753-74.
- Okada M, Kobayashi K. Recent progress in mycobacteriology. Kekkaku 2007;82:783-99.
- Espinal MA. The global situation of MDR-TB. Tuberculosis (Edinb) 2003;83:44-51.
- Hyman CL. Tuberculosis: A survey and review of current literature. CurrOpinPulm Med 1995;1:234-42.
- Huebner RE, Castro KG. The changing face of tuberculosis. Annu Rev Med 1995;46:47-55.
- Pai M. Alternatives to the tuberculin skin test: Interferon-gamma assays in the diagnosis of Mycobacterium tuberculosis infection. Indian J Med Microbiol 2005;23:151-8.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. CurrOpin Infect Dis 2009;22:174-82.
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance.N Engl J Med 2010;363:1005-15.
- Small PM, Pai M. Tuberculosis diagnosis – Time for a game change. N Engl J Med 2010;363:1070-1.
- Gutierrez-Lugo MT, Bewley CA. Natural products, small molecules, and genetics in tuberculosis drug development. J Med Chem 2008;51:2606-12.
- Lin PL, Flynn JL. Understanding latent tuberculosis: A moving target. J Immunol 2010;185:15-22.
- Hauck FR, Neese BH, Panchal AS, El-Amin W. Identification and management of latent tuberculosis infection. Am Fam Physician 2009;79:879-86.
- Dover LG, Coxon GD. Current status and research strategies in tuberculosis drug development. J Med Chem 2011;54:6157-65.
- Chan ED, Iseman MD. Current medical treatment for tuberculosis. BMJ 2002;325:1282-6.
- Arora VK, Gupta R. DOTS strategy in India – The challenges. Pulm Med 2002;8:19-27.
- Shishoo CJ, Shah SA, Rathod IS, Savale SS, Kotecha JS, Shah PB. Stability of rifampicin in dissolution medium in presence of isoniazid. Int J Pharm 1999;190:109-23.
- Shishoo CJ, Shah SA, Rathod IS, Savale SS, Vora MJ. Impaired bioavailability of rifampicin in presence of isoniazid from fixed dose combination (FDC) formulation. Int J Pharm 2001;228:53-67.
- Shishoo CJ, Shah SA, Rathod IS, Savale SS. Impaired bioavailability of rifampicin from fixed dose combination (FDC) formulation with isoniazid. Indian J Pharm Sci 2001;63:443-9.
- Singh S, Mariappan TT, Sharda N, Kumar S, Chakraborti AK. The reason for an increase in decomposition of rifampicin in the presence of isoniazid under acid conditions. Pharm PharmacolCommun 2000;6:405-10.
- Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: A systematic review of qualitative research. PLoS Med 2007;4:e238.
- Jaggarajamma K, Sudha G, Chandrasekaran V, Nirupa C, Thomas A, Santha T, et al. Reasons for non-compliance among patients treated under Revised National Tuberculosis Control Programme (RNTCP), Tiruvallur district, South India. Indian J Tuberc 2007;54:130-5.
- Bayer R, Wilkinson D. Directly observed therapy for tuberculosis: History of an idea. Lancet 1995;345:1545-8.
- Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, et al. Biomarkers and diagnostics for tuberculosis: Progress, needs, andtranslation into practice. Lancet 2010;375:1920-37.
- Furin JJ, Johnson JL. Recent advances in the diagnosis and management of tuberculosis. CurrOpinPulm Med 2005;11:189-94.
- Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med 2012;367:931-6.
- Keshavjee S, Farmer PE. Picking up the pace - Scale-up of MDR tuberculosis treatment programs. N Engl J Med 2010;363:1781-4.
- Loddenkemper R, Sagebiel D, Brendel A. Strategies against multidrug-resistant tuberculosis. EurRespir J Suppl 2002;36:66s-77s.
- Cole ST, Telenti A. Drug resistance in Mycobacterium tuberculosis. EurRespir J Suppl 1995;20:701s-13s.
- Arya N, Raut MK, Tekale SG, Shishoo CJ, Jain KS. Tuberculosis: New drug discovery pipelines. Austin J Anal Pharm Chem 2014;1:1-9.
- Pai M, Minion J, Sohn H, Zwerling A, Perkins MD. Novel and improved technologies for tuberculosis diagnosis: Progress andchallenges. Clin Chest Med 2009;30:701-16, viii.
- Davies PD. Tuberculosis: The global epidemic. J Indian Med Assoc 2000;98:100-2.
- Iseman MD. Tuberculosis therapy: Past, present and future. EurRespir J Suppl 2002;36:87s-94s.
- Sacks LV, Behrman RE. Developing new drugs for the treatment of drug-resistant tuberculosis: A regulatory perspective. Tuberculosis (Edinb) 2008;88(Suppl 1):S93-100.
- Murray JF. Tuberculosis and HIV infection worldwide. Pneumologie 1995;49Suppl 3:653-6.
- Benakappa A, Benakappa N, Benakappa DG. Management of tuberculosis. Indian J Pediatr 1995;62:557-63.
- Nagarajan K. Nitrobicyclicimidazoles with potent antitubercular activity. CurrSci 2000;79:933-4.
- Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 2000;405:962-6.
- Lamichhane G, Freundlich JS, Ekins S, Wickramaratne N, Nolan ST, Bishai WR. Essential metabolites of Mycobacterium tuberculosis and their mimics. MBio 2011;2:e00301-10.
- Swindells S. New drugs to treat tuberculosis. F1000 Med Rep 2012;4:12.
- Nunn A, Phillips PP, Abubakar I. Treatment of pulmonary tuberculosis. CurrOpinPulm Med 2013;19:273-9.
- Lienhardt C, Vernon A, Raviglione MC. New drugs and new regimens for the treatment of tuberculosis: Review of the drug development pipeline and implications for national programmes. CurrOpinPulm Med 2010;16:186-93.
- Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Global tuberculosis drug development pipeline: The need and the reality. Lancet 2010;375:2100-9.
- Rivers EC, Mancera RL. New anti-tuberculosis drugs with novel mechanisms of action. Curr Med Chem 2008;15:1956-67.
- Zumla A, Hafner R, Lienhardt C, Hoelscher M, Nunn A. Advancing the development of tuberculosis therapy. Nat Rev Drug Discov 2012;11:171-2.
- Kaneko T, Cooper C, Mdluli K. Challenges and opportunities in developing novel drugs for TB. Future Med Chem 2011;3:1373-400.
- Engelhardt H, Heinz C, Niederweis M. A tetramericporin limits the cell wall permeability of Mycobacterium smegmatis. J BiolChem 2002;277:37567-72.
- van den Boogaard J, Kibiki GS, Kisanga ER, Boeree MJ, Aarnoutse RE. New drugs against tuberculosis: Problems, progress, and evaluation of agents in clinical development. Antimicrob Agents Chemother 2009;53:849-62.
- Zumla AI, Gillespie SH, Hoelscher M, Philips PP, Cole ST, Abubakar I, et al. New antituberculosis drugs, regimens, and adjunct therapies: Needs, advances, and future prospects. Lancet Infect Dis 2014;14:327-40.
- Lienhardt C, Raviglione M, Spigelman M, Hafner R, Jaramillo E, Hoelscher M, et al. New drugs for the treatment of tuberculosis: Needs, challenges, promise, and prospects for the future. J Infect Dis 2012;205Suppl 2:S241-9.
- Lougheed KE, Taylor DL, Osborne SA, Bryans JS, Buxton RS. New anti-tuberculosis agents amongst known drugs. Tuberculosis (Edinb) 2009;89:364-70.
- Rivers EC, Mancera RL. New anti-tuberculosis drugs in clinical trials with novel mechanisms of action. Drug Discov Today 2008;13:1090-8.
- Makarov V, Manina G, Mikusova K, Möllmann U, Ryabova O, Saint-Joanis B, et al.Benzothiazinones kill Mycobacterium tuberculosis by blocking Arabinan synthesis. Science 2009;324:801-4.
- Rustomjee R, Diacon AH, Allen J, Venter A, Reddy C, Patientia RF, et al. Early bactericidal activity and pharmacokinetics of thediarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother 2008;52:2831-5.
- Tam CM, Yew WW, Yuen KY. Treatment of multidrug-resistant and extensively drug-resistant tuberculosis: Current status and futureprospects. Expert Rev ClinPharmacol 2009;2:405-21.
- Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, et al. PA-824 kills nonreplicatingMycobacteriumtuberculosis by intracellular NO release. Science 2008;322:1392-5.
- Manjunatha U, Boshoff HI, Barry CE. The mechanism of action of PA-824: Novel insights from transcriptional profiling. CommunIntegr Biol 2009;2:215-8.
- Palomino JC, Martin A. TMC207 becomes bedaquiline, a new anti-TB drug. Future Microbiol 2013;8:1071-80.
- Matteelli A, Carvalho AC, Dooley KE, Kritski A. TMC207: The first compound of a new class of potent anti-tuberculosis drugs. Future Microbiol 2010;5:849-58.
- Andries K, Verhasselt P, Guillemont J, Göhlmann HW, Neefs JM, Winkler H, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005;307:223-7.
- Gillespie SH, Kennedy N. Fluoroquinolones: A new treatment for tuberculosis? Int J Tuberc Lung Dis 1998;2:265-71.
- Sulochana S, Rahman F, Paramasivan CN. In vitro activity of fluoroquinolones against Mycobacterium tuberculosis. J Chemother 2005;17:169-73.
- Moadebi S, Harder CK, Fitzgerald MJ, Elwood KR, Marra F. Fluoroquinolones for the treatment of pulmonary tuberculosis. Drugs 2007;67:2077-99.
- Diekema DJ, Jones RN. Oxazolidinone antibiotics. Lancet 2001;358:1975-82.
- Schecter GF, Scott C, True L, Raftery A, Flood J, Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2010;50:49-55.
- Sacksteder KA, Protopopova M, Barry CE 3rd, Andries K, Nacy CA. Discovery and development of SQ109: A new antitubercular drug with a novel mechanism of action. Future Microbiol 2012;7:823-37.
- Rothstein DM, Shalish C, Murphy CK, Sternlicht A, Campbell LA. Development potential of rifalazil and other benzoxazinorifamycins. Expert OpinInvestig Drugs 2006;15:603-23.
- Kutlin A, Kohlhoff S, Roblin P, Hammerschlag MR, Riska P. Emergence of resistance to rifampin and rifalazil in Chlamydophilapneumoniaeand Chlamydia trachomatis. Antimicrob Agents Chemother2005;49:903-7.
- Kaysser L, Lutsch L, Siebenberg S, Wemakor E, Kammerer B, Gust B. Identification and manipulation of the caprazamycin gene cluster lead to new simplified liponucleoside antibiotics and give insights into the biosynthetic pathway. J BiolChem 2009;284:14987-96.
- Igarashi M, Nakagawa N, Doi N, Hattori S, Naganawa H, Hamada M. Caprazamycin B, a novel anti-tuberculosis antibiotic, from Streptomycessp. J Antibiot (Tokyo) 2003;56:580-3.
- Knapton S. First new antibiotic in 30 years discovered in major breakthrough. Telegraph 07 Jan 2015. Available on http://www. telegraph.co.uk/news/science/science-news/11331174/First-new-antibiotic-in-30-years-discovered-in-major-breakthrough.html. [Last accessed on 2015 Jan 14].
- Connor S. Teixobactin discovery: Scientists create first new antibiotic in 30 years- and say it could be the key to beating superbug resistance. Independent 08 Jan 2015. Available on http://www.independent.co.uk/ life-style/health-and-families/health-news/first-new-antibiotic-in-30-years-could-be-key-to-beating-superbug-resistance-9963585.html. [Last accessed on 2015 Jan 14].
- Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, et al. A new antibiotic kills pathogens without detectable resistance.Nature 2015;517:455-9.
- Yong E. A new antibiotic that resists resistance. Phenomena 07 Jan 2015. Available on http://phenomena.nationalgeographic. com/2015/01/07/antibiotic-resistance-teixobactin/. [Last accessed on 2015 Jan 14].
- Degim IT, Celebi N. Controlled delivery of peptides and proteins. Curr Pharm Des 2007;13:99-117.
- Sangwan S, Agosti JM, Bauer LA, Otulana BA, Morishige RJ, Cipolla DC, et al. Aerosolized protein delivery in asthma: Gammacamera analysis of regional deposition and perfusion. J Aerosol Med 2001;14:185-95.
- Scheuch G, Siekmeier R. Novel approaches to enhance pulmonary delivery of proteins and peptides. J PhysiolPharmacol 2007;58 Suppl 5(Pt 2):615-25.
- Siekmeier R, Scheuch G. Systemic treatment by inhalation of macromolecules – Principles, problems, and examples. J Physiol Pharmacol 2008;59(Suppl 6):53-79.
- Loira-Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev 2014;75:81-91.
- Paranjpe M, Müller-Goymann CC. Nanoparticle-mediated pulmonary drug delivery: A review. Int J MolSci 2014;15:5852-73.
- Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem 2009;17:2950-62.
- Shegokar R, Al Shaal L, Mitri K. Present status of nanoparticle research for treatment of tuberculosis. J Pharm PharmSci 2011;14:100-16.
- Misra A, Hickey AJ, Rossi C, Borchard G, Terada H, Makino K, et al.
- Inhaled drug therapy for treatment of tuberculosis. Tuberculosis (Edinb) 2011;91:71-81.
- Sosnik A, CarcabosoAM, Glisoni RJ, Moretton MA, Chiappetta DA. New old challenges in tuberculosis: Potentially effective nanotechnologies in drug delivery. Adv Drug Deliv Rev 2010;62:547-59.
- Smith JP. Nanoparticle delivery of anti-tuberculosis chemotherapy as a potential mediator against drug-resistant tuberculosis. Yale J Biol Med 2011;84:361-9. Pinheiro M, Lúcio M, Lima JL, Reis S. Liposomes as drug delivery systems for the treatment of TB. Nanomedicine (Lond) 2011;6:1413-28.
- Andrade F, Videira M, Ferreira D, Sarmento B. Nanocarriers for pulmonary administration of peptides and therapeutic proteins. Nanomedicine (Lond) 2011;6:123-41
- Buxton DB. Nanomedicine for the management of lung and blood diseases. Nanomedicine (Lond) 2009;4:331-9.
- Ahmed M, Ramadan W, Rambhu D, Shakeel F. Potential of nanoemulsions for intravenous delivery of rifampicin. Pharmazie 2008;63:806-11.
- El-Ridy MS, Abdelbary A, Nasr EA, Khalil RM, Mostafa DM, El-Batal AI, et al.Niosomal encapsulation of the antitubercular drug, pyrazinamide. Drug DevInd Pharm 2011;37:1110-8.
- Azarmi S, Roa WH, Löbenberg R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv Drug Deliv Rev 2008;60:863-75.
- Rytting E, Nguyen J, Wang X, Kissel T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin Drug Deliv 2008;5:629-39.
- Pandey R, Zahoor A, Sharma S, Khuller GK. Nanoparticle encapsulated antitubercular drugs as a potential oral drug delivery system against murine tuberculosis. Tuberculosis (Edinb) 2003;83:373-8.
- Roy I, Vij N. Nanodelivery in airway diseases: Challenges and therapeutic applications. Nanomedicine 2010;6:237-44.
- Beck-Broichsitter M, Merkel OM, Kissel T. Controlled pulmonary drug and gene delivery using polymeric nano-carriers. J Control Release 2012;161:214-24.