- *Corresponding Author:
- T. Rajamohan
Department of Biochemistry, University of Kerala, Kariavattom, Thiruvananthapuram-695 581, India
E-mail: trmohan2003@rediffmail.com
| Date of Submission | 15 June 2011 |
| Date of Revision | 6 September 2012 |
| Date of Acceptance | 8 September 2012 |
| Indian J Pharm Sci, 2012, 74 (5): 397-402 |
Abstract
The present study evaluates the effect of aqueous extract of coconut haustorium on isoproterenol-induced myocardial infarction in Sprague Dawley rats. Rats were pretreated with aqueous extract of coconut haustorium (40 mg/100 g) orally for 45 days. After pretreatment, myocardial infarction was induced by injecting isoproterenol subcutaneously (20 mg/100 g body weight) twice at an interval of 24 h. Activity of marker enzymes like lactate dehydrogenase, creatinine kinaseâ??MB, aspartate transaminase and alanine transaminase were increased in the serum and decreased in the heart of isoproterenol treated rats indicating cardiac damage. These changes were significantly reduced in haustorium pretreated rats. Moreover, an increase in the activities of antioxidant enzymes and decrease in the levels of peroxidation products were observed in the myocardium of coconut haustorium pretreated rats. Histopathology of the heart of these rats showed almost normal tissue morphology. From these results, it is clear that aqueous extract of coconut haustorium possess significant cardioprotective and antioxidant properties during isoproterenol-induced myocardial infarction in rats.
Keywords
Antioxidant enzymes, cardiac markers, coconut haustorium, isoproterenol, myocardial infarction
Ailments of heart and blood vessels are collectively termed as cardiovascular diseases. Among cardiovascular diseases, myocardial infarction (MI) accounts for majority of deaths and disabilities in both developed and developing countries [1]. MI is characterized by an imbalance between coronary blood supply and myocardial demand which results in ischemia and myocardial death. Experimental and clinical observations have shown that during ischemic injury, oxidative stress produced by the generation of reactive oxygen species plays a key role in the development of MI [2]. This is evidenced by marked increase in production of lipid peroxidation products and transient inhibition of endogenous antioxidants [3‑5].
Isoproterenol (ISO) is a β‑adrenergic agonist which has been found to cause severe stress in myocardium, by increasing the heart rate, resulting in infarct‑like necrosis of heart muscle due to oxygen deficiency [6]. ISO, upon oxidation also increases oxidative stress through enhanced free radical formation and causes severe stress in the myocardium resulting in myocardial cell death [7,8]. MI induced by ISO has been reported to show metabolic and morphological changes in the heart of experimental animals similar to those seen in human myocardial ischemia [9]. Experimental induction of MI by ISO in animals is well established model to study the protective role of various cardioprotective agents.
Phytochemicals, which can suppress free radical generation and enhance endogenous antioxidant defence, may attenuate myocardial dysfunction during MI. Plant products which may be used as dietary supplement to prevent MI is gaining importance now a day because currently used synthetic drugs are not free from setbacks [10,11]. Natural foods from plant sources are very rich in antioxidants, vitamins and minerals. Dietary antioxidants are considered beneficial because they slow down the chemical process of oxidation and repair the free radical damage which is implicated in the development of myocardial damage during MI [12].
Coconut (Cocos nucifera Linn.) is a large oil seed belonging to the family of Palmae. It is largely used for culinary purposes in tropical regions of the world. Anatomically, coconut is composed of four distinct parts; the outer fibrous exocarp or husk, the highly lignified endocarp or shell, the white solid endosperm or kernel, and a large central cavity filled with liquid endosperm called coconut water. On germination the basal part of embryo, which is embedded in solid endosperm near the germinating pore of coconut, enlarges to form a cotyledonary structure called haustorium. Haustorium enlarges and fills the entire water cavity in 20‑24 weeks after germination. During this period it mobilizes nutrients in endosperm and nourishes germinating embryo [13]. Coconut kernel, water and haustorium are edible parts of coconut. Analysis of coconut haustorium (CH) revealed that it contains proteins, minerals, alkaloids, polyphenols and growth promoting substances. Proteins, minerals and polyphenols can have protective effect during ISO-induced MI. Previous studies have found that coconut kernel and coconut water rich in these bioactive components possess cardio protective properties against ISO-induced MI in rats [14,15]. So far no study reports are available regarding the protective effect of CH on ISO-induced MI and oxidative stress. Hence an attempt was made to evaluate the cardio protective activity of aqueous extract of CH during experimental MI in rats.
Materials and Methods
ISO hydrochloride was purchased from Sigma Chemical Co., St. Louis, MO, USA. All other chemicals were purchased from Sisco Research Laboratories (SRL), Mumbai, India and were of analytical grade.
Coconut haustorium
Mature coconuts (12 months from flowering) of west coast tall variety were harvested from the Kerala University Campus and dried under shade for 22 days. Nuts of identical weights were placed in horizontal plain and two‑thirds of each nut was covered with soil. Soil moisture was maintained by periodical watering. Germinated nuts were randomly taken for the experiment 15 weeks after germination. It was dehusked, broken carefully and haustorium was collected, ground and dried at 55º. It was then extracted with water (1:5, w/v) thrice. Combined filtrate was lyophilized using MODULYOD‑230 freeze dryer (Thermo Electron Corporation, Milford, MA.) and stored at −4º until use.
Experimental animals
Male albino rats (Sprague‑Dawley) weighing an average of 180‑200 g were used for the experiment. All the animals were given standard rat chow (Sai feeds, Bangalore, India) and distilled water ad libitum. Temperature of the animal house was maintained at 25±5° with alternate 12 h period of light and dark. Food intake was recorded daily, whereas bodyweights of rats were recorded weekly. Experiments were approved by the Institutional Animal Ethics Committee. The laboratory guidelines of the committee for the purpose of control and supervision of experiments on animals, India for the use and care of animals were followed throughout the experimental period.
Experimental design
Rats were divided into four groups of eight rats each. Group I and group II received normal saline and group III and IV received aqueous extract of CH (40 mg/100 g body weight) orally for 45 days. After this period, ISO (20 mg/100 g) was dissolved in normal saline and injected subcutaneously to rats of group II and IV at an interval of 24 h for 2 days to induce MI. 12 h after the second dose of ISO injection, rats were subjected to euthanasia by means of intraperitoneal injection of thiopentone sodium (>40 mg/kg body weight). Blood samples and heart were collected for the estimation of various biochemical parameters.
Biochemical estimations
Creatinine kinase‑MB (CK‑MB) [16], lactate dehydrogenase (LDH) [17], alanine transaminase (ALT) and aspartate transaminase (AST) [18] activity were determined in serum and heart. Troponin T in serum was determined by highly specific enzyme immunoassay [19]. The activities of superoxide dismutase (SOD) [20], catalase [21], glutathione peroxidase (GPx) [22], and glutathione reductase (GRd) [23] in the heart were assayed as described earlier. Reduced glutathione (GSH) and glutathione‑S‑transferase (GST) were assayed by method of Ellman [24] and Habig et al. [25]. Thio Barbituric Acid Reactive Substances (TBARS) [26] and protein carbonyls [27] in heart tissue were also estimated.
Histopathological analysis of heart
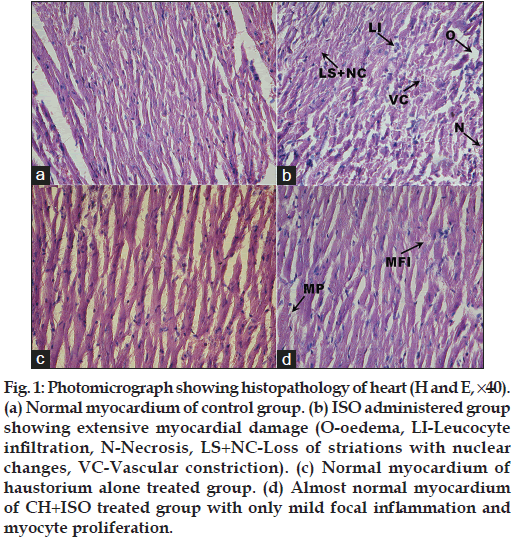
The heart tissues were rapidly dissected out, washed in saline and fixed by immersion at room temperature in 10% formalin solution. For the histological examinations, paraffin embedded tissue sections of heart (5 μm) were stained with Hematoxylin and Eosin (H and E). The tissue samples were then examined under light microscope (Zeiss Axioscope 2 plus, USA) and photographed (Canon Zoom Browser EX, Japan).
Statistical analysis
Statistical analysis was performed by one‑way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) using Statistical Package for Social Science (SPSS) version 17.0. Results were expressed as mean±SD for six rats in each group. P values<0.05 were considered significant.
Results
ISO treated rats (Group II) showed significant (P<0.05) increased activity of LDH, CK‑MB, AST, ALT and the concentration of cardiac troponin‑T in the serum compared to normal control rats (Group I). Group IV rats pretreated with aqueous extract of CH (40 mg/100 g body weight) showed significant decrease in the levels of these cardiac markers on ISO injection compared to ISO alone treated rats (Table 1). Cardiac injury markers in serum of CH fed normal rats (Group III) was significantly similar to that of normal control rats.
| Groups | LDH* | CK-MB# | AST** | ALT## | Troponin T#* |
|---|---|---|---|---|---|
| Group I | 441.20 ± 40.26a | 479.26 ± 43.74a | 93.43 ± 8.53a | 36.05 ± 3.30a | 0.01 ± 0.001a |
| Group II | 765.63 ± 69.88b | 651.29 ± 54.16b | 146.22 ± 13.31b | 81.51 ± 7.45b | 7.97 ± 0.728b |
| Group III | 440.07 ± 40.17a | 476.84 ± 43.52a | 92.88 ± 8.47a | 36.09 ± 3.29a | 0.01 ± 0.001a |
| Group IV | 463.68 ± 42.32a | 489.60 ± 44.53a | 106.44 ± 9.71c | 49.04 ± 4.47c | 3.15 ± 0.288c |
Group I=Normal, Group II=Isoproterenol, Group III=CH, Group IV=CH+isoproterenol. LDH=Lactate dehydrogenase, CK‑MB‑Creatine‑Kinase‑MB, AST=Aspartate transaminase, ALT=Alanine transaminase.*μM of NAD+liberated per minute per mg protein, #IU/L,**μM of oxaloacetate liberated per minute per mg protein, ##μM of pyruvate liberated per minute per mg protein, #*nM, Values expressed as mean±SD for six rats in each group. Values not sharing a common superscript (a, b, c) differ with each other significantly at P<0.05 (DMRT).
Table 1: Effect Of Aqueous Extract Of Coconut Haustorium On Cardiac Injury Markers In Serum
Table 2 show the activity of LDH, CK‑MB, AST and ALT in the heart of experimental rats. Decrease in the activity of these enzymes was observed in Group II compared to normal rats, while significant increase in the activity was seen in Group IV rats compared to Group II on ISO injection. Significantly similar activity of these enzymes was seen in Group I and Group III rats.
| Groups | Ldh* | Ck-Mb# | Ast** | Alt## |
|---|---|---|---|---|
| Group I | 218.83 ± 19.96a | 17.18 ± 1.57a | 442.70 ± 40.41a | 385.69 ± 35.19a |
| Group II | 112.86 ± 10.31b | 9.02 ± 0.82b | 169.31 ± 15.49b | 174.39 ± 18.63b |
| Group III | 220.34 ± 20.11a | 18.37 ± 1.68a | 443.02 ± 40.43a | 396.41 ± 26.70a |
| Group IV | 196.78 ± 17.96c | 16.24 ± 1.48c | 417.46 ± 38.09a | 309.05 ± 28.20c |
LDH=Lactate dehydrogenase, CK‑MB‑Creatine‑Kinase‑MB, AST=Aspartate transaminase, ALT=Alanine transaminase.*μM of NAD+liberated per minute per mg protein, #μM of phosphorus liberated per minute per mg protein,**μM of oxaloacetate liberated per minute per mg protein, ##μM of pyruvate liberated per minute per mg protein. Values expressed as mean±SD for six rats in each group. Values not sharing a common superscript (a, b, c) differ with each other significantly at P<0.05 (DMRT).
Table 2: Activity Of Cardiac Enzymes In Heart Tissue
SOD and catalase activity in the heart tissue of rats are shown in Table 3. Decrease in the activity was seen in Group II compared to Group I and increase in activity was seen in Group IV compared to Group II. No significant alteration of these enzyme activity was seen in Group III rats compared to Group I rats. Similar result was seen in the case of GPx, GRd, GSH and GST (Table 4).
| Groups | SOD* | Catalase# |
|---|---|---|
| Group I | 10.05 ± 0.92a | 9.44 ± 0.86a |
| Group II | 4.02 ± 0.39b | 3.13 ± 0.28b |
| Group III | 10.11 ± 0.92a | 9.58 ± 0.88a |
| Group IV | 7.93 ± 0.73c | 8.38 ± 0.77c |
*units/mg protein, #×10–3 units/mg protein. Values expressed as mean±SD for six rats in each group. Values not sharing a common superscript (a, b, c) differ with each other significantly at P<0.05 (DMRT).
Table 3: Activity Of Superoxide Dismutase Sod And Catalase In Heart Tissue
| Groups | GPx* | GRd # | GSH** | GST ## |
|---|---|---|---|---|
| Group I | 0.710 ± 0.07a | 4.30 ± 0.39a | 110.42 ± 10.08a | 7.99 ± 0.73a |
| Group II | 0.14 ± 0.01b | 2.28 ± 0.22b | 76.70 ± 8.19b | 0.94 ± 0.09b |
| Group III | 0.72 ± 0.07a | 4.36 ± 0.40a | 111.99 ± 10.21a | 8.05 ± 0.74a |
| Group IV | 0.65 ± 0.06a | 3.67 ± 0.34c | 102.65 ± 9.37a | 8.44 ± 0.77a |
GPx=Glutathione peroxidise, GRd=Glutathione reductase, GSH=Glutathione, GST=Glutathione‑S‑transferase.*,#×10–2 units/mg protein,**mM/100 g wet tissue, ##nM of CDNB‑GSH conjugate formed per min per mg protein. (CDNB‑1‑chloro‑2, 4‑dinitrobenzene). Values not sharing a common superscript (a, b) differ with each other significantly at P<0.05 (DMRT).
Table 4: Effect Of Aqueous Extract Of Coconut Haustorium On Antioxidant Status In The Heart Tissue
Peroxidation markers namely TBARS and protein carbonyls in the experimental rat heart are shown in Table 5. Group II rats showed significantly higher amount of peroxidation markers compared to normal rats. TBARS and protein carbonyls were decreased significantly in Group IV compared to Group II rats. Peroxidation markers were significantly similar in normal control and haustorium control rats.
| Groups | TBARS* | Protein carbonyl value# |
|---|---|---|
| Group I | 0.57 ± 0.06a | 1.51 ± 0.13a |
| Group II | 1.74 ± 0.15b | 4.55 ± 0.41b |
| Group III | 0.56 ± 0.05a | 1.49 ± 0.14a |
| Group IV | 0.86 ± 0.07c | 2.45 ± 0.22c |
*mM/100 g wet tissue, #nM/mg protein. Values expressed as mean±SD for six rats in each group. Values not sharing a common superscript (a, b, c) differ with each other significantly at P<0.05 (DMRT). TBARS=Thiobarbituric acid reactive substances
Table 5: Concentration Of Thiobarbituric Acid Reactive Substances And Protein Carbonyl Value In Heart Tissue
Histopathology of heart (fig. 1) of ISO administered rats showed marked oedema, vascular constriction, inflammatory infiltration of leucocytes, necrosis and loss of striations with nuclear changes while the heart of control rat showed normal myocardial fibres. Rats given haustorium alone showed no perceptible changes in the heart when compared to control rats. Heart of haustorium pretreated rats given ISO showed only mild focal inflammation compared to the heart of ISO administered rats. More over myocyte proliferation was observed in pretreated group.
Discussion
Reactive oxygen species are formed at an accelerated rate in the myocardium due to ISO administration. Myocardial cells contain enzymes like LDH, CK‑MB, AST, ALT and structural proteins like troponins which are released in blood due to cellular dysfunction and necrosis as a result of oxidative stress during MI. Leakage of these cardiac specific enzymes and troponins leads to their increased activity/ concentration in serum and hence they serve as diagnostic markers of cardiac damage. Leakage of these cardiac markers to serum results in their lower activity/concentration in heart [5,28,29]. In MI induced rats increased activity of marker enzymes were observed in serum while decreased activity was observed in heart compared to normal rats. In CH pretreated group these enzyme activities were decreased in serum and increased in myocardium compared to ISO treated control rats. The decline in enzyme levels and troponin T in serum may be due to the protective effect of CH on the heart, which had reduced the cardiac damage and thereby restricting the secretion of these enzymes and troponin T. These results clearly show that cardiac integrity is maintained in CH pretreated group on ISO administration.
Decrease in the activities of SOD, catalase, GPx, GRd and GST were observed in the heart of ISO alone treated rats. Superoxide radicals generated by ISO at the site of damage modulate SOD and catalase, resulting in decreased activities of these enzymes [30]. This can lead to the accumulation of superoxide anion which further damages the myocardium [31]. The decreased activities of GSH dependent enzymes such as GPx and GST in the heart of MI induced rats may be due to decreased GSH concentration. Inactivation of GRd leads to the oxidation of GSH leading to the formation of Glutathione disulfide (GSSG), which in turn inactivates the enzymes with sulfhydryl groups [32]. CH pretreatment maintained the activity of these antioxidant enzymes near to normal indicating better antioxidant status in treated rats.
ISO administration is associated with increased levels of lipid peroxidation as evidenced by increased levels of TBARS in the heart and this leads to oxidative damage of cell components like proteins, lipids and nucleic acids [33]. This correlates with the increased protein carbonyl value in ISO treated rats. Enhancement of lipid peroxidation products in ISO treated rats may be due to decreased levels of antioxidant system [34]. In CH pretreated rats, significant decrease in the levels of TBARS and protein carbonyl value were observed.
Histopathological examination of heart of ISO administrated control rats showed extensive cardiac damage. This correlates with the biochemical findings that in ISO administered rats cardiac markers are released to serum and peroxidation level was high indicating cardiac cell damage. Rats pretreated with aqueous extract of haustorium showed minimal histological changes when compared with that of ISO control rats. This observation indicates that CH protected the myocardium from ISO-induced injury. These findings clearly shows that CH possess significant cardio protective property.
Chemical analysis showed that CH contains pharmacologically important bio constituents like alkaloids, resins, steroids, terpenoids, aminoacids, vitamins and minerals. Functional groups in these compounds can act like antioxidants. Potassium, magnesium and amino acids like L‑arginine are cardioprotective in nature [35‑37]. The presence of these compounds might be responsible for the cardioprotective property of CH. Further investigation is being conducted to point out the major active component in CH offering cardioprotection.
Acknowledgements
Financial assistance from Council of Scientific and Industrial Research (CSIR), India, in the form of Junior Research Fellowship to Chikku A. M. is gratefully acknowledged.
References
- Agarwal VK, Basannan DR, Sinh RP, Dutt M, Abraham D, Mustafa MS. Coronary risk factors in a rural community. Indian J Public Health 2006;50:19-23.
- Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res 2000;47:446-56.
- Loeper J, Goy J, Rozensztajn L, Bedu O, Moisson P. Lipid peroxidation and protective enzymes during myocardial infarction. Clin Chim Acta 1991;196:119-25.
- Padmanabhan M, Prince PS. Preventive effect of S-allylcysteine on lipid peroxides and antioxidants in normal and isoproterenol-induced cardiotoxicity in rats: A histopathological study. Toxicology 2006;224:128-37.
- Zhou R, Xu Q, Zheng P, Yan L, Zheng J, Dai G. Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. Eur J Pharmacol 2008;586:244-50.
- Wexler BC, Greenberg BP. Protective effects of clofibrate on isoproterenol-induced myocardial infarction in arteriosclerotic and non-arteriosclerotic rats. Atherosclerosis 1978;29:373-95.
- Chattopadhyay A, Biswas S, Bandyopadhyay D, Sarkar C, Datta AG. Effect of isoproterenol on lipid peroxidation and antioxidant enzymes of myocardial tissue of mice and protection by quinidine. Mol Cell Biochem 2003;245:43-9.
- Rajadurai M, Stanely Mainzen Prince P. Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology 2007;230:178-88.
- Wexler BC. Myocardial infarction in young versus old male rats: Pathophysiologic changes. Am Heart J 1978;96:70-80.
- Jadeja RN, Thounaojam MC, Patel DK, Devkar RV, Ramachandran AV. Pomegranate (Punica granatum L.) juice supplementation attenuates isoproterenol-induced cardiac necrosis in rats. Cardiovasc Toxicol 2010;10:174-80.
- Patel DK, Desai SN, Gandhi HP, Devkar RV, Ramachandran AV. Cardio protective effect of Coriandrum sativum L. on isoproterenol induced myocardial necrosis in rats. Food Chem Toxicol 2012;50:3120-5.
- Prathapan A, Rajamohan T. Antioxidant and antithrombotic activity of tender coconut water in experimental myocardial infarction. J Food Biochem 2011;35:1501-7.
- Balachandran C, Arumughan C. Triglyceride deposition in tissues of germinating coconut (Cocous nucifera Linn.). J Am Oil Chem Soc 1995;72:1583-6.
- Anurag P, Rajamohan T. Cardioprotective effect of tender coconut water in experimental myocardial infarction. Plant Foods Hum Nutr 2003;58:1-12.
- Mini S, Rajamohan T. Cardioprotective effect of coconut kernel protein in isoproterenol administered rats. Indian J Biochem Biophys 2002;39:197-200.
- Rosalki SB. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med 1967;69:696-705.
- King J. The dehydrogenases or oxido reductase-Lactate dehydrogenase. In: Van D, editor. Practical Clinical Enzymology. London: Nostrand; 1965. p. 83-93.
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 1957;28:56-63.
- Katus HA, Remppis A, Neumann FJ, Scheffold T, Diederich KW, Vinar G, et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation 1991;83:902-12.
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 1984;21:130-2.
- Maehly AC, Chance B. The assay of catalases and peroxidases. Methods Biochem Anal 1954;1:357-424.
- Agergaard N, Jensen PT. Procedure for blood glutathione peroxidase determination in cattle and swine. Acta Vet Scand 1982;23:515-27.
- David M, Richard JS. Glutathione reductase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Weinheim, Florida: Verlag Chemie; 1983. p. 258-65.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70-7.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130-9.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.
- Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 1994;233:346-57.
- Sabeena Farvin KH, Anandan R, Kumar SH, Shiny KS, Sankar TV, isoproterenol-induced myocardial infarction in rats. Pharmacol Res 2004;50:231-6.
- Gürgün C, Ildizli M, Yavuzgil O, Sin A, Apaydin A, Cinar C, et al. The effects of short term statin treatment on left ventricular function and inflammatory markers in patients with chronic heart failure. Int J Cardiol 2008;123:102-7.
- Rajadurai M, Stanely Mainzen Prince P. Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardiotoxicity in Wistar rats: Biochemical and histopathological evidences. Toxicology 2006;228:259-68.
- Jayalakshmi R, Thirupurasundari CJ, Devaraj SN. Pretreatment with alcoholic extract of Crataegus oxycantha (AEC) activates mitochondrial protection during isoproterenol-induced myocardial infarction in rats. Mol Cell Biochem 2006;292:59-67.
- Ji LL, Stratman FW, Lardy HA. Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch Biochem Biophys 1988;263:150-60.
- Ebenezar KK, Sathish V, Devaki T. Effect of L-arginine and L-lysine on lysosomal hydrolases and membrane bound phosphatases in experimentally induced myocardial infarction in rats. Mol Cell Biochem 2003;247:163-9.
- Tauseef M, Sharma KK, Fahim M. Aspirin restores normal baroreflex function in hypercholesterolemic rats by its antioxidative action. Eur J Pharmacol 2007;556:136-43.
- Syme SL, Marmot MG, Kagan A, Kato H, Rhoads G. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: Introduction. Am J Epidemiol 1975;102:477-80.
- Rubenowitz E, Molin I, Axelsson G, Rylander R. Magnesium in drinking water in relation to morbidity and mortality from acute myocardial infarction. Epidemiology 2000;11:416-21.
- Kumar P, Goyal M, Agarwal JL. Effect of L-arginine on electrocardiographic changes induced by hypercholesterolemia and isoproterenol in rabbits. Indian Pacing Electrophysiol J 2009;9:45-52.