- *Corresponding Author:
- Juan Lu
Department of Neurosurgery, Affiliated 3201 Hospital of Xi’an Jiaotong University, Hanzhong, Shaanxi 723000, China
E-mail: rsc45678@126.com
| This article was originally published in a special issue, “Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(2) Spl Issue “166-173” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the efficacy of low molecular weight heparin calcium combined with clopidogrel in treating progressive ischemic stroke is the objective of the study. 150 patients of progressive ischemic stroke in 3201 hospital were divided into observation group and control group, 75 patients in each group. In addition to conventional therapy, the control group was treated with low molecular weight heparin calcium while the observation group was treated with low molecular weight heparin calcium and clopidogrel. The efficacy of the two groups after drug treatment, National institutes of health stroke scale score, neuropathy disability score and serum C-reactive protein level before treatment, and 7 d and 14 d after treatment was compared and analyzed. The effective rate of observation group was 86.7 % and the control group was 76.0 %. Serum C-reactive protein levels were compared between the two groups before treatment (p>0.05). The C-reactive protein values were decreased in both groups, but the C-reactive protein values in observation group and 7 d and 14 d after treatment were reduced more obviously than control group (all, p<0.05). Low molecular weight heparin combined with clopidogrel can effectively treat progressive ischemic stroke and improve the neurological function of progressive ischemic stroke patients, which is worth of clinical application.
Keywords
Progressive ischemic stroke, clopidogrel, low-molecular-weight heparin calcium, C-reactive protein
Stroke in Progression (SIP) is referred to as progressive stroke, which is due to brain vascular atherosclerosis and thrombosis, arterial stenosis and occlusion and continuous aggravation, and eventually cause local brain tissue hypoxia[1]. SIP, a disease of ischemia, softening and necrosis which often occurs among middle-aged and elderly people between 45 and 70 y old. About 5 million people die from SIP every year worldwide and about 1.1 million people die from SIP in China every year, which seriously threatens the health and life of human[2,3]. SIP lesions consist of the central necrotic area and the ischemic penumbra.
Due to the collateral circulation supply, a large number of neurons still survive. If the damaged can be removed within time at an early stage to ensure the recovery of blood flow in clinic, the brain function of the related areas can be restored. The previous medical treatment of SIP mainly used Low Molecular Weight Heparin Calcium (LMWH), but the long-term efficacy is not very effective[2]. In this study, LMWH combined with clopidogrel treated SIP has shown better clinical efficacy and the results are reported below.
Materials and Methods
General data:
150 SIP patients admitted to Affiliated 3201 hospital of Xi’an Jiaotong university from January 2021 to December 2022 were selected. All SIP patients were diagnosed by Computed Tomography (CT) scan or Magnetic Resonance Imaging (MRI) and the diagnosis met the diagnostic point of various cerebrovascular diseases issued by the Chinese Society of Neurosurgery, and did not meet the indications of thrombolysis, or the family members did not agree to thrombolysis[4]. SIP patients were randomly divided into observation group and control group with 75 patients in each group. The observation group had 50 males and 25 females of age 42~74 y, mean (56.7±11.9), disease duration of 24~72 h, mean (30.0±13.9) h, whereas the control group included 49 men and 26 women of age 45~72 y, mean (56.2~13.1) y, disease duration of 24 to 72 h, mean (27.5±12.7) h.
No significant differences was found in gender, age and disease duration between the two groups (p>0.05) and they were comparable.
Methods:
All SIP patients were routinely treated and monitored to reduce intracranial pressure, edaravone scavenging free radicals, monitored blood glucose and blood pressure, and the control group was given 5000 U/time, 2 times/d, and the observation group was given clopidogrel once daily orally 300 mg/time, 1 time/d for 14 d. C-Reactive Protein (CRP) was measured before treatment, 7 d and 14 d after treatment, and venous blood was collected early in the morning on an empty stomach.
Efficacy assessment:
National Institutes of Health Stroke Scale (NIHSS) and Neurological Disability Scores (NDS) were recorded for all patients, before treatment, 7 d and 14 d after treatment. The adverse reactions of the two groups after drug treatment were observed.
The curative effect was determined according to the clinical efficacy evaluation standards for stroke patients issued by the fourth National Cerebrovascular Disease Academic Conference[5], Cure-NIHSS score reduced by more than 90 %, clinical symptoms and signs were basically normal; significant effect-NIHSS score reduced 46 %~89 %, clinical symptoms and signs were significantly improved, muscle strength increased by grade I; Effective-NIHSS score reduced 18 %~45 %, improvement in the clinical signs and symptoms, muscle strength increased by grade I; ineffectiveness and deterioration-NIHSS score reduction of less than 18 %, no improvement or aggravation of the clinical signs and symptoms. There was no change or deterioration in muscle strength. Effective rate=(Number of cured cases+significant effect+effective cases)/total cases.
Coagulation indexes:
Prothrombin Time (PT), International Normalized Ratio (INR) of PT, Activated Partial Thromboplastin Time (APTT), Fibrinogen (Fg) and Platelet count (PLT) were measured before and after treatment.
Statistical method:
Statistical Package for the Social Sciences (SPSS) 19.0 software was used for data analysis and processing, measurement data were expressed as mean±standard deviation. T-test was used to compare both the groups and count data was expressed as percentage (%). Consequently, Chi square (χ²) test was used, where p<0.05 indicates a statistically significant difference.
Results and Discussion
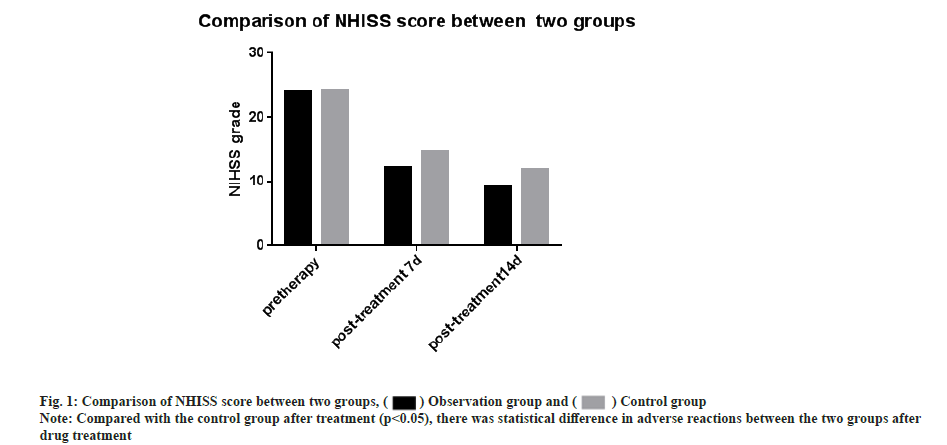
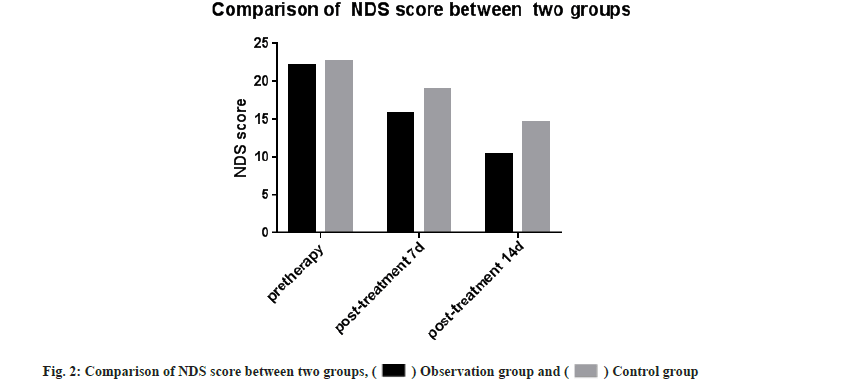
NIHSS and NDS scores were compared and there was no significant difference in NIHSS scores between the two groups before treatment (p>0.05). The NIHSS scores after 7 d and 14 d of treatment were significantly lower than the control group. The difference was significant (t-test was 2.394, 3.105, respectively and p<0.05 for all). There was no significant difference in NDS scores between the two groups before treatment (p>0.05) and the NDS scores were significantly less when compared after 7 d and 14 d of treatment with the control group. The difference was statistically significant (t-test was 3.741, 5.180, respectively and p<0.05 for all) as shown in Table 1, fig. 1 and fig. 2.
| Group | n | NIHSS grade | NDS grade | ||||
|---|---|---|---|---|---|---|---|
| Pre-therapy | Post-treatment | Pre-therapy | Post-treatment | ||||
| 7 d | 14 d | 7 d | 14 d | ||||
| Observation group | 75 | 23.96±6.85 | 12.19±2.31* | 9.12±3.10* | 22.17±2.74 | 15.72±2.40* | 10.37±2.14* |
| Control group | 75 | 24.14±5.28 | 14.68±1.62 | 11.83±2 34 | 22.59±3.62 | 18.94±3.04 | 14.66±2.91 |
Note: Compared with the control group, *p<0.05.
Table 1: Comparison of NHISS score and NDS score between the two groups (x±s).
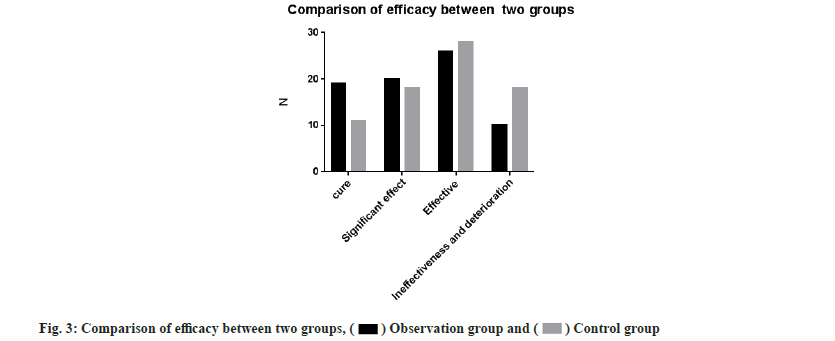
Further, efficacy between both the groups was compared. The effective rate of the observation group was 86.7 % and that of the control group was 76.0 %. This denoted that the effective rate of the observation group was significantly higher than that of the control group and the difference was statistically significant (χ2=5.637, p<0. 05) as shown in Table 2 and fig. 3.
| Group | n | Cure | Significant effect | Effective | Ineffectiveness and deterioration | Effective percentage (%) |
|---|---|---|---|---|---|---|
| Observation group | 75 | 19 | 20 | 26 | 10 | 86.7* |
| Control group | 75 | 11 | 18 | 28 | 18 | 76 |
Note: Compared with the control group, *p<0.05
Table 2: The Comparison of Efficacy between the two groups.
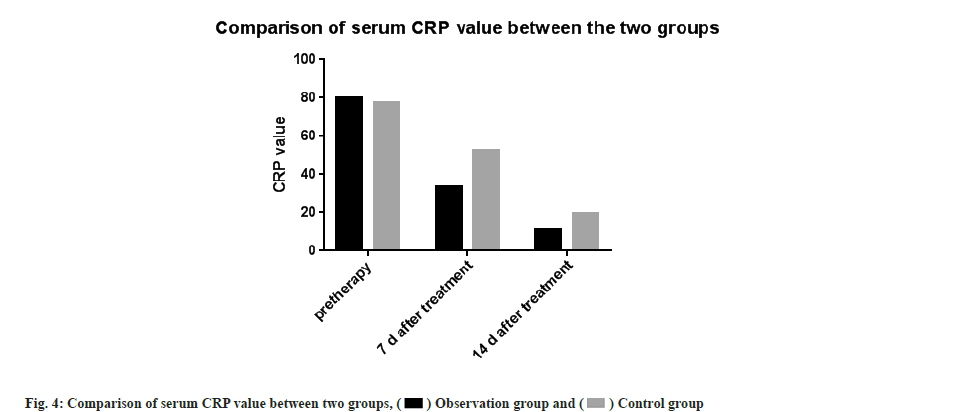
Then serum CRP levels were compared. There was no significant difference between the two groups in serum CRP levels before treatment (p<0.05). The CRP values in both groups decreased after treatment. The observation group at 7 d and 14 d after treatment decreased significantly compared with the control group, the difference was statistically significant (t-test was 9.194 and 4.612, respectively, p<0.05 for all) as shown in Table 3 and fig. 4.
| Group | n | Pre-therapy | 7 d after treatment | 14 d after treatment |
|---|---|---|---|---|
| Observation group | 75 | 79.77±6.42 | 33.52±5.62* | 10.91±2.61* |
| Control group | 75 | 77.31±5.66 | 52.25 ±4.43 | 19.53 + 3.42 |
Note: Compared with the control group, *p<0.05
Table 3: Comparison of Serum CRP between two groups (x±s, mg/l).
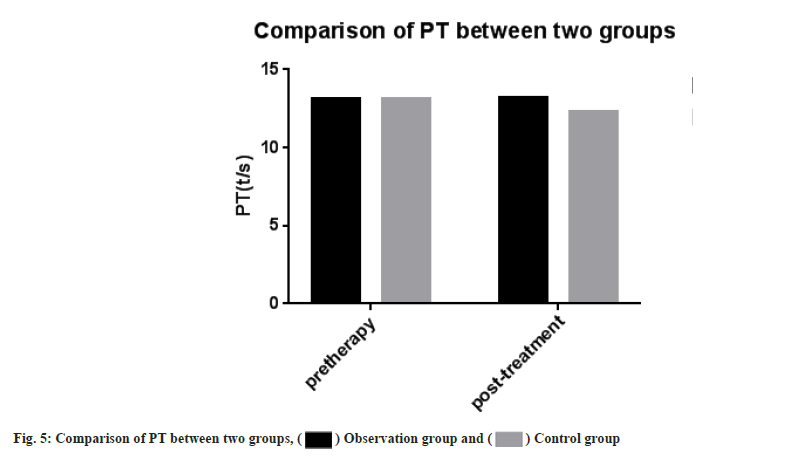
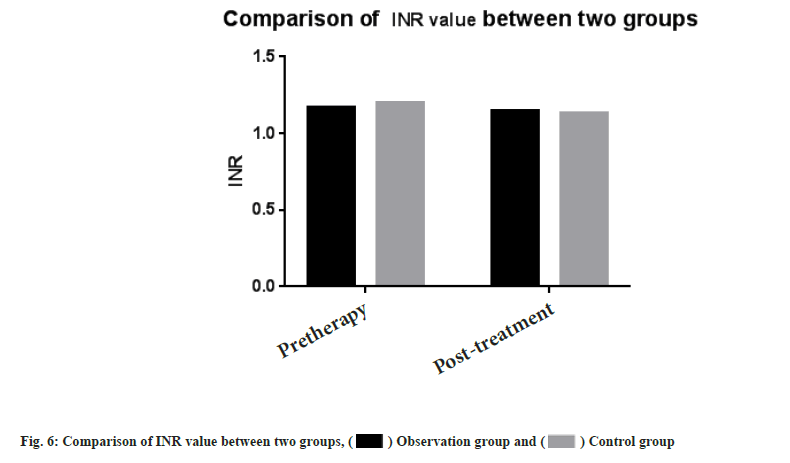
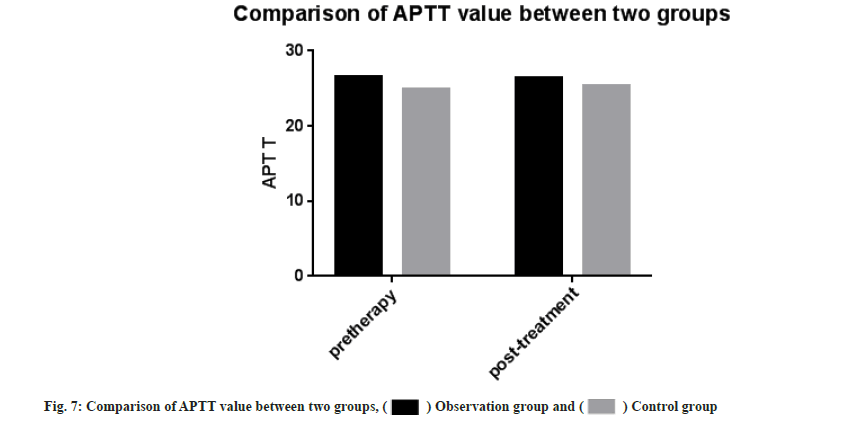
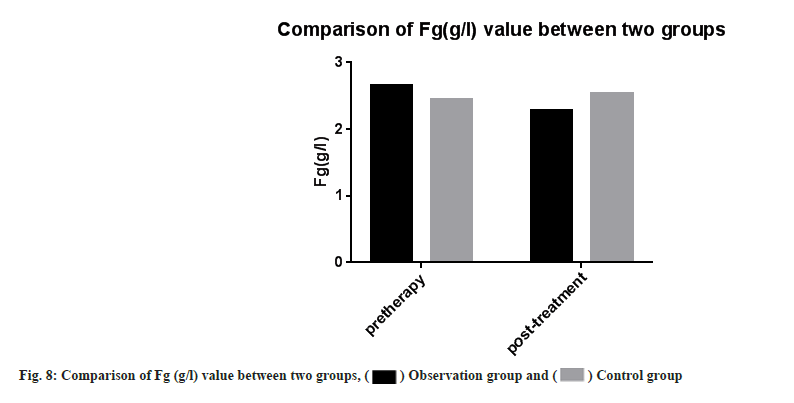
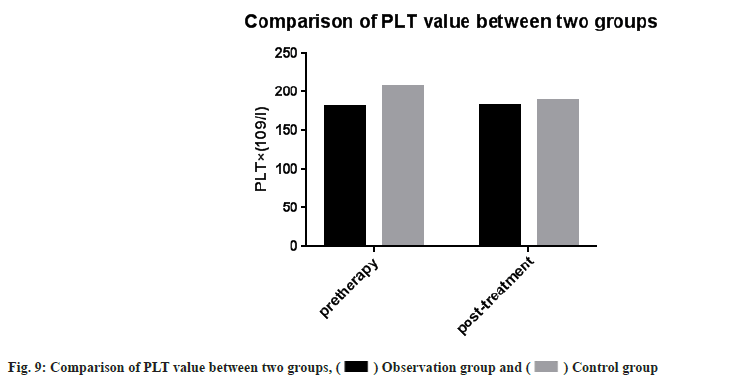
Adverse reactions and coagulation indexes between two groups before and after treatment were compared. There was no significant difference in adverse reactions between the two groups after drug treatment (p>0.05). PT, INR, APTT, Fg, PLT count were monitored in the two groups. Then we compared the coagulation indexes between two groups before and after treatment, it showed no significant difference between two groups (p>0.05) as shown in Table 4, Table 5 and fig. 5-fig. 9.
| Group | N | Abdominal pain, diarrhea | Indigestion | Constipation | Headache | Sick | Tetter | Total adverse reaction |
|---|---|---|---|---|---|---|---|---|
| Observation group | 75 | 1 (1.33) | 2 (2.66) | 1 (1.33) | 1 (1.330) | 1 (1.33) | 3 (4.00) | 9 (12.00) |
| Control group | 75 | 2 (2.66) | 0 (0) | 0 (0) | 2 (2.66) | 2 (2.66) | 2 (2.66) | 8 (10.66) |
| χ² | - | 0.341 | 2.039 | 1.011 | 0.344 | 0.344 | 0.221 | 0.071 |
| p | - | 0.557 | 0.152 | 0.316 | 0.559 | 0.559 | 0.648 | 0.79 |
Note: Compared with the control group after treatment (p>0.05) and there was no statistical difference in adverse reactions between the two groups after drug treatment
Table 4: Comparison of adverse reactions after drug treatment between two groups n (%).
| Parameters | Observation group | Control group | ||
|---|---|---|---|---|
| Pre-therapy | Post-treatment | Pre-therapy | Post-treatment | |
| PT (t/s) | 13.15±0.98 | 13.19±0.86 | 13.11±1.85 | 12.31±0.47 |
| INR | 1.17±0.10 | 1.15±0.06 | 1.20±0.19 | 1.13±0.17 |
| APTT | 26.55±3.13 | 26.47±2.95 | 24.87±1.33 | 25.40±1.43 |
| Fg (g/l) | 2.65±0.68 | 2.28±0.62 | 2.45±0.20 | 2.53±0.40 |
| PLT×(109/l) | 181.74±49.81 | 183.15±38.72 | 207.03±74.71 | 189.53±70.48 |
Note: Compared with the control group after treatment (p>0.05), there was no statistical difference in coagulation indexes between two groups after drug treatment
Table 5: The Changes of Coagulation Indexes before and after treatment were compared between two groups.
SIP refers to the nerve function or other corresponding symptoms after stroke and its pathogenesis is more complex, which may be related to multiple factors. At present, it is thought to be related to atherosclerosis in the brain and neck, and the intimal thickening and medial necrosis, causes PLT aggregation and adhesion, releases a variety of vasoconstrictor substances, and causes vasospasm and stenosis, which is progressive in course and reversible[6]. Current research shows that PLTs are an important factor causing SIP progression, inhibiting PLT aggregation is important for the treatment of SIP in conventional LMWH combined with aspirin for SIP treatment[7]. But in recent years, research shows that long-term use of aspirin reduced SIP risk by 9 % and the recurrence risk of major cardiovascular events, and the incidence of gastrointestinal adverse reactions[8]. In domestic literature, SIP was treated with oral clopidogrel 75 mg/d[9]. However, studies showed that 75 mg/d clopidogrel generally have effect 3~7 d after medication and generally used for secondary prevention, whereas 300 mg/d clopidogrel show effect 25 h after medication and the strongest effect can effectively reduce PLT aggregation in the early stage of SIP treatment, and significantly improve the prognosis[10]. The results of this study showed that the NIHSS score and NDS of the observation group 7 d and 14 d after treatment were significantly lower than the control group, the difference was statistically significant (p<0.05). The effective rate of the observation group was 86.7 % and that of the control group was 76.0 %. The effective rate of the observation group was significantly higher than that of the control group. The difference was statistically significant (p<0.05), which suggested that LMWH combined with clopidogrel could effectively treat SIP and reduce the degree of nerve injury in patients. Seners et al.[11] study shows that LMWH combined with clopidogrel can effectively reduce the NIHSS and NDS scores in patients and significantly improve the degree of nerve injury in patients. Gorgui et al.[12] showed that LMWH combined with clopidogrel can improve the neurological function of patients with SIP effectively and the long-term use can significantly improve the prognosis of patients. Li et al.[13] research found that, for the treatment of SIP, clopidogrel is effective. The patient has improved significantly during the recovery period. The results showed that, it can effectively reduce Fg level and the cervical vascular stenosis was improved. This is generally consistent with the results of this study. CRP is an acute phase protein that is widely used to monitor different inflammatory states and the therapeutic effects of inflammation. CRP can reflect the severity of SIP and the degree of brain tissue damage as shown in some studies[14]. The results of this study showed that there was no significant difference in serum CRP values before treatment (p>0.05), but the 14 d CRP values of the observation group after 7 d and 14 d of treatment decreased significantly compared with the control group, and the difference was statistically significant (p<0.05), which proved that LMWH combined with clopidogrel is effective in the treatment of SIP. However, clopidogrel resistance occurs in approximately 5 %-30 % of patients. This is called clopidogrel resistance[15], but the mechanism is still unclear.
In conclusion we found that hypertension and hyperglycemia may promote the occurrence of clopidogrel resistance to varying degrees and patients with aspirin resistance are likely to have clopidogrel resistance at the same time. Therefore, close attention should be paid to adjust the medication in time during the treatment of clopidogrel. LMWH combined with clopidogrel can effectively treat progressive ischemic stroke and improve the neurological function of progressive ischemic stroke patients, which is worth of clinical application.
Conflict of interests:
The authors declared no conflict of interest.
References
- Sherzai A, Heim LT, Boothby C, Sherzai AD. Stroke, food groups and dietary patterns: A systematic review. Nutr Rev 2012;70(8):423-35.
[Crossref] [Google scholar] [PubMed]
- Moskowitz MA, Grotta JC, Koroshetz WJ. The NINDS stroke progress review group final analysis and recommendations. Stroke 2013;44(8):2343-50.
[Crossref] [Google scholar] [PubMed]
- Qiao L, Deng Y, Ji K. An analysis of mortality of cardio-cerebrovascular disease among residents of Sichuan province during 2002 to 2010. Chin J Prev Control Chronic Noncommun Dis 2012;20(2):135-8.
- Li WH. Diagnosis of various cerebrovascular diseases. Chin J Neurol 1996;29(6):379-80.
- Li WH. Scoring criteria for the clinical neurological deficit program in stroke patients (1995). Chin J Neurol 1996;29(6):381-3.
- Taussky P, Tawk RG, Daugherty WP, Hanel RA. Medical therapy for ischemic stroke: Review of intravenous and intra-arterial treatment options. World Neurosurg 2011;76(6):S9-15.
[Crossref] [Google scholar] [PubMed]
- Grotta JC. Stroke progress review group: Summary of successes and lack of progress. Stroke 2013;44:S111-3.
[Crossref] [Google scholar] [PubMed]
- Thomson RM, Anderson DC. Aspirin and clopidogrel for prevention of ischemic stroke. Curr Neurol Neurosci Rep 2013;13:1-9.
[Crossref] [Google scholar] [PubMed]
- Liu G. Clinical effect of combined with low molecular weight heparin calcium in progressive ischemic stroke. J Hebei Med Sci Cent 2015;(11):1850-3.
- Mijajlovic MD, Shulga O, Bloch S, Covickovic‐Sternic N, Aleksic V, Bornstein NM. Clinical consequences of aspirin and clopidogrel resistance: An overview. Acta Neurol Scand 2013;128(4):213-9.
[Crossref] [Google scholar] [PubMed]
- Seners P, Baron JC. Revisiting ‘progressive stroke’: Incidence, predictors, pathophysiology, and management of unexplained early neurological deterioration following acute ischemic stroke. J Neurol 2018;265(1):216-25.
[Crossref] [Google scholar] [PubMed]
- Gorgui J, Gasbarrino K, Georgakis MK, Karalexi MA, Nauche B, Petridou ET, et al. Circulating adiponectin levels in relation to carotid atherosclerotic plaque presence, ischemic stroke risk, and mortality: A systematic review and meta-analyses. Metabolism 2017;69:51-66.
[Crossref] [Google scholar] [PubMed]
- Li LH, Ma QF, Wu LJ. Effect of clopidogrel treatment on cervical vascular stenosis and plasma fibrinogen changes in SIP patients. Chin J Biochem Pharm 2015;(9):115-7.
- van Wijk DF, Boekholdt SM, Wareham NJ, Ahmadi-Abhari S, Kastelein JJ, Stroes ES, et al. C-reactive protein, fatal and nonfatal coronary artery disease, stroke and peripheral artery disease in the prospective EPIC-Norfolk cohort study. Arterioscler Thromb Vasc Biol 2013;33(12):2888-94.
[Crossref] [Google scholar] [PubMed]
- Gorelick PB, Farooq MU. Advances in our understanding of “resistance” to antiplatelet agents for prevention of ischemic stroke. Stroke Res Treat 2013;2013:1-7.
[Crossref] [Google scholar] [PubMed]