- *Corresponding Author:
- Zhen You
Department of Biliary and General Surgery, Anhui Medical University, West China Hospital, Sichuan University, Sichuan 610041, Chengdu, China
E-mail: 18280367036@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “232-238” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the clinical efficacy and safety of ceftriaxone sodium plus Xiaoyan Lidan tablets for the treatment of acute cholecystitis. 78 acute cholecystitis individuals were consecutively selected and were divided into monotherapy group (n=36) and combination group (n=42). Monotherapy group was treated with ceftriaxone sodium while combination group were treated with Xiaoyan Lidan tablets along with ceftriaxone sodium. Clinical efficacy, resolution time of clinical symptoms/signs, hospital length of stay and the incidence of adverse reactions of the two groups were compared and recorded. Maximal long-axis and short-axis diameter of gallbladder and the gallbladder wall thickness were measured by color Doppler ultrasonography. Besides, serum indirect bilirubin and total bile acid levels were determined with the use of an automatic biochemical analyzer. Furthermore, enzyme-linked immunosorbent assay was carried out to quantify serum interleukin-6, C-reactive protein and tumor necrosis factor-alpha levels. An evidently lower overall response rate was determined in the monotherapy group compared with the combination group. Symptoms such as epigastric pain, resolution time, fever relief time, nausea and vomiting improvement time and hospital length of stay were significantly shorter in the combination group vs. monotherapy group. Combination group had lower post-treatment gallbladder tension, serum indirect bilirubin, total bile acid, interleukin-6, C-reactive protein and tumor necrosis factor-alpha levels than the monotherapy group; both the groups had similar incidence of adverse reactions. Combined effect of ceftriaxone sodium with Xiaoyan Lidan tablets is effective and safe in treating acute cholecystitis and can significantly reduce inflammatory reactions in the body, facilitating patient recovery.
Keywords
Ceftriaxone sodium, Xiaoyan Lidan, cholecystitis, bilirubin, C-reactive protein
Acute Cholecystitis (AC) is a common acute gastrointestinal disease caused by cystic duct obstruction and bacterial invasion[1]. It develops as right upper abdominal pain in most of the patients and can spread to the right shoulder and back, while in some patients it depicts complicated symptoms such as vomiting, nausea and fever[2]. AC can be divided into acute calculous cholecystitis and acalculous cholecystitis. If the disease is not effectively intervened, it will lead to multiple complications and increase the risk of death[3]. Currently, cholecystectomy is the standard treatment for AC. However, for patients at high surgical risk, urgent surgery may increase mortality[4]. Therefore, many doctors and patients choose conservative management instead[5]. Ceftriaxone is a 3rd generation cephalosporins with broad-spectrum antimicrobial activity against Gram-positive, Gram-negative, aerobic and anaerobic bacteria, which is widely used to treat a variety of infectious diseases[6]. Although Ceftriaxone Sodium (CS) is effective in treating infectious diseases, long-term use will not only develop drug resistance, but also has a serious impact on liver and kidney functioning. Xiaoyan Lidan (XYLD) tablets belong to patented Chinese medicine, which has the effects of clearing away heat, dispelling dampness and benefiting gallbladder, and is often used in the conservative treatment of cholecystitis. This study explores the clinical efficacy and safety of CS+XYLD tablets in the treatment of AC, aiming to find an effective and safe conservative treatment for patients with AC.
Materials and Methods
General information:
78 AC individuals who were admitted consecutively to our hospital from December 2021 to January 2023 were selected as the research participants. All the patients and their families have informed and signed the consent form. The hospital’s ethics committee has granted its approval for this study.
Inclusion criteria:
Patients who met the clinical diagnostic criteria for AC[7]; patients who developed the disease for the 1st time; patients having no mental disorders, no other digestive system diseases and patients who have not been enrolled for any other treatment previously were included in the study.
Exclusion criteria:
Patients who had complications such as acute pancreatitis or severe organ damage/dysfunction; patients with related contraindications to treatment, autoimmune diseases, coagulopathy or hematological system disorders and patients who have used antibiotics 1 mo prior to enrollment of this study were excluded.
Methods:
All the patients were immediately given conventional treatment such as fasting and abstained from drinking, gastrointestinal decompression, correction of electrolyte disorders, fluid infusion, etc., as well as intravenous nutrition was provided whenever necessary. Monotherapy group (n=36) was treated with 2 g/time CS injection (CSPC Zhongnuo Pharmaceutical Co., Ltd., with Saudi Food and Drug Authority (SFDA) Approval No: H13022881) which was mixed with 250 ml of 0.9 % sodium chloride solution for intravenous drip, twice a day. Similarly, combination group (n=42) was given 6 XYLD tablets (Guangdong Luofushan Sinopharm Co., Ltd., with SFDA Approval No: Z44021422), 3 times/day orally on the basis of the monotherapy group. The treatment course lasted for 4 w.
Endpoints:
Clinical efficacy: It was evaluated by grades like cure rate, marked response and non-response. Cure rate was referred to the disappearance of clinical symptoms and signs after treatment, as well as the return of the gallbladder to its normal size with smooth gallbladder wall shown by Brightness (B)-ultrasonic examination. Marked response was defined as the obvious improvement of clinical signs and symptoms after treatment with B-ultrasonic examination showing normal gallbladder size and local roughness of the gallbladder wall. Non-response corresponds to no change or even deterioration of the patient's clinical symptoms after treatment.
Overall Response Rate (ORR)=cure rate+marked response rate×100
Adverse effects: Effects such as epigastric pain, resolution time of clinical signs and symptoms, fever relief time, nausea and vomiting improvement time, and Hospital Length of Stay (HLOS) were compared between both the groups. Pre- and post-treatment of Maximal Long-Axis Diameter (MLAD) and Maximal Short-Axis Diameter (MSAD) of the gallbladder and the gallbladder wall thickness were measured using color Doppler ultrasonography. The incidence of adverse reactions was also comparatively analyzed.
Serum inflammatory indices: In addition, 5 ml of peripheral blood was collected before and after treatment in each group, centrifuged (1000 ×g) at 4° for 15 min and the supernatant was stored at -70° refrigerator until analysis. Levels of Indirect Bilirubin (IBIL) and Total Bile Acid (TBA) in blood were determined by an automatic biochemical analyzer. Besides, Enzyme-Linked Immunosorbent Assay (ELISA) was carried out to quantify Interleukin-6 (IL-6), C-Reactive Protein (CRP) and Tumor Necrosis Factor-Alpha (TNF-α) levels using the detection kits obtained from Shanghai Enzyme-linked Biotechnology Co., Ltd. The detection procedures were carried out as per the kit manuals.
Statistical analysis:
This study used Prism 6 software for data analysis and p<0.05 was considered as the minimal significance level. Count data were tested by Chi-square (χ2) and represented by n (%). Data measurement was carried out using t-test, expressed by mean±Standard Deviation (SD) (x±s).Results and Discussion
Comparison of general data such as gender, age, history of gallbladder disease, disease type, course of disease, and body temperature were compared between the two groups (p>0.05) (Table 1).
| Factors | Monotherapy group (n=36) | Combination group (n=42) | χ2/t | p |
|---|---|---|---|---|
| Gender | 0.011 | 0.915 | ||
| Male | 21 (58.33) | 25 (59.52) | ||
| Female | 15 (41.67) | 17 (40.48) | ||
| Age (y) | 0.506 | 0.477 | ||
| <60 y | 23 (63.89) | 30 (71.43) | ||
| ≥60 y | 13 (36.11) | 12 (28.57) | ||
| History of gallbladder disease | 0.403 | 0.526 | ||
| With | 9 (25.00) | 8 (19.05) | ||
| Without | 27 (75.00) | 34 (80.95) | ||
| Disease type | 0.175 | 0.676 | ||
| Acalculous cholecystitis | 4 (11.11) | 6 (14.29) | ||
| Calculous cholecystitis | 32 (88.89) | 36 (85.71) | ||
| Course of disease (h) | 6.83±4.12 | 7.50±4.94 | 0.644 | 0.522 |
| Body temperature at admission (°) | 38.13±1.13 | 38.10±1.18 | 0.114 | 0.909 |
Table 1: Comparison of General data of patients
Clinical efficacy of the drug was evaluated. ORR of monotherapy group was 61.11 %, which was low when compared with the combination group (85.71 %) and the difference was statistically significant (p<0.05) (Table 2).
| Curative effect | Monotherapy group (n=36) | Combination group (n=42) | χ2 | p |
|---|---|---|---|---|
| Cure | 9 (25.00) | 16 (38.10) | ||
| Marked response | 13 (36.11) | 20 (47.62) | ||
| Non-response | 14 (38.89) | 6 (14.29) | ||
| Overall response | 22 (61.11) | 36 (85.71) | 6.154 | 0.013 |
Table 2: Comparison of clinical efficacy of patients in two groups
Resolution time of the clinical symptoms/signs and HLOS were evaluated. Signs like epigastric pain, fever relief time, and nausea and vomiting improvement time and HLOS were significantly shorter in the combination group than in monotherapy group (p<0.05) (Table 3).
| Resolution time | Monotherapy group (n=36) | Combination group (n=42) | t | P |
|---|---|---|---|---|
| Epigastric pain (h) | 81.44±10.65 | 58.36±8.05 | 10.882 | <0.001 |
| Fever relief time (h) | 58.17±8.22 | 49.21±6.86 | 5.248 | <0.001 |
| Nausea and vomiting improvement time (h) | 45.72±6.79 | 34.89±6.48 | 7.198 | <0.001 |
| HLOS (d) | 8.58±2.66 | 6.86±1.95 | 3.286 | 0.002 |
Table 3: Resolution time of clinical symptoms/signs and HLOS of two groups
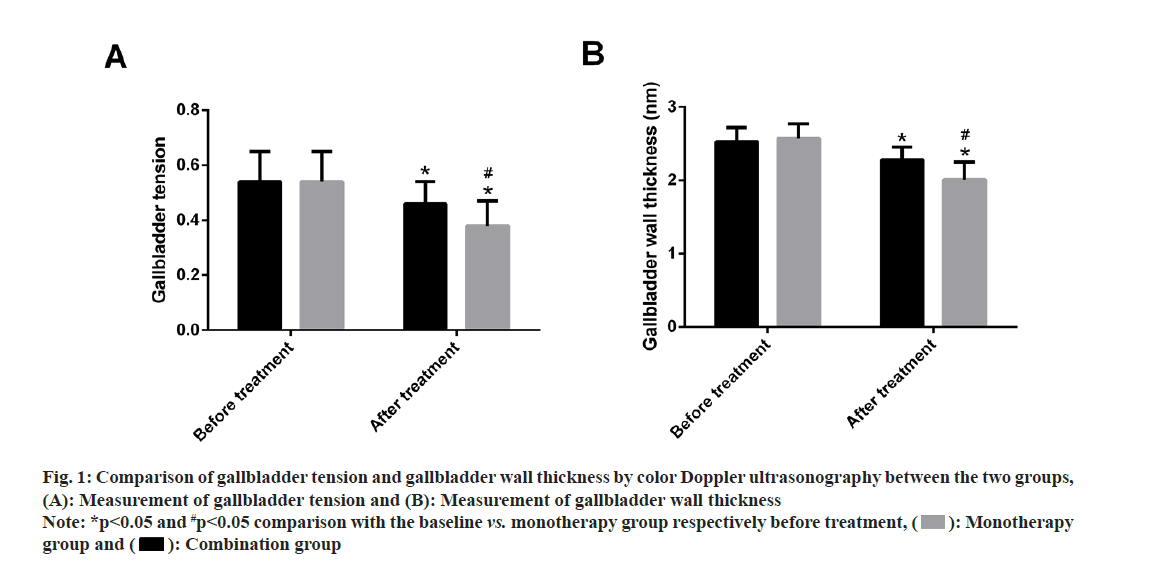
Comparison of gallbladder tension and wall thickness of the gallbladder were comparatively analyzed between the two groups. Both the groups did not differ much in pre-treatment gallbladder tension and wall thickness (p>0.05). However, marked reduction in gallbladder tension and wall thickness of the gallbladder were observed in both groups after treatment (p<0.05), with even lower values in the combination group compared with the monotherapy group (p<0.05) (fig. 1).
Fig. 1: Comparison of gallbladder tension and gallbladder wall thickness by color Doppler ultrasonography between the two groups, (A): Measurement of gallbladder tension and (B): Measurement of gallbladder wall thickness
Note: *p<0.05 and #p<0.05 comparison with the baseline vs. monotherapy group respectively before treatment, ( ): Monotherapy group and (
): Monotherapy group and ( ): Combination group
): Combination group
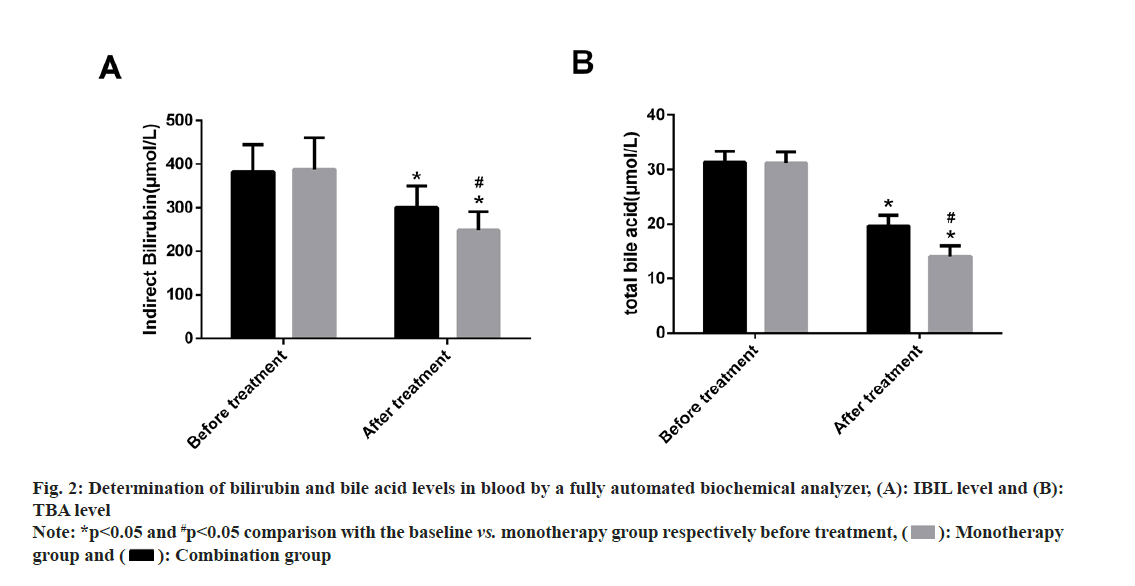
IBIL and TBA levels were examined. No statistical inter-group differences were found in pre-treatment IBIL and TBA levels (p>0.05). Both the groups showed statistically reduced levels of IBIL and TBA after treatment, with even lower levels of the two in the combination group (p<0.05). (fig. 2).
Fig. 2: Determination of bilirubin and bile acid levels in blood by a fully automated biochemical analyzer, (A): IBIL level and (B): TBA level
Note: *p<0.05 and #p<0.05 comparison with the baseline vs. monotherapy group respectively before treatment, ( ): Monotherapy group and (
): Monotherapy group and ( ): Combination group
): Combination group
Adverse reactions of patients of both the groups were compared. Both the groups experienced serious adverse reactions during treatment however, they have cured on their own. The total incidence of adverse reactions in the monotherapy group was 8.33 %, which was not significantly different from 14.29 % in the combination group (p<0.05) (Table 4).
| Adverse reactions | Monotherapy group (n=36) | Combination group (n=42) | χ2 | p |
|---|---|---|---|---|
| Nausea and vomiting | 2 (5.56) | 2 (4.76) | ||
| Abdominal pain | 0 (64.58) | 1 (2.38) | ||
| Diarrhea | 0 (35.42) | 1 (2.38) | ||
| Dizziness and headache | 1 (2.78) | 2 (4.76) | ||
| Total | 3 (8.33) | 6 (14.29) | 0.673 | 0.412 |
Table 4: Comparison of adverse reactions in two groups
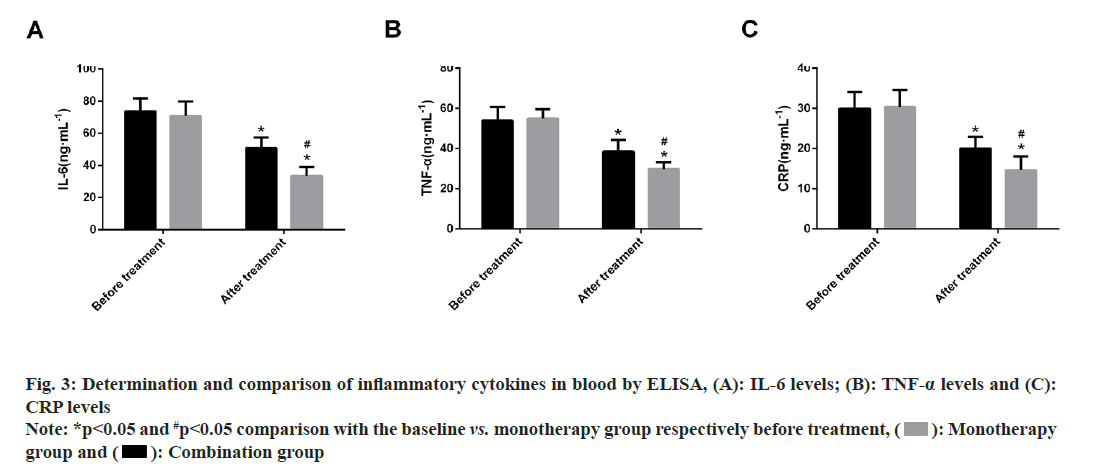
Further, inflammatory cytokine levels were compared, where no marked difference was identified between the monotherapy and combination groups in terms of pre-treatment serum IL-6, TNF-α and CRP levels (p>0.05). Significantly decreased levels of these inflammatory cytokines were found in both patient groups after therapy, with more evident reductions in the combination group (p<0.05) (fig. 3).
AC, as mentioned earlier, is usually caused by cystic duct obstruction or bacterial invasion. The pathogenic bacteria which are mainly involved to cause such infections are Escherichia coli, Klebsiella, Enterococcus and anaerobic bacteria[8]; so antibiotics are often used clinically to treat AC. Ceftriaxone is an extensively used antibiotic in clinical treatment of AC, which has the characteristics of strong antibacterial effect, good tolerance of lactamases, low toxicity and low allergic reaction[9]. CS can inhibit bacterial Deoxyribonucelic Acid (DNA) replication and controls transcription by acting on DNA gyrase, thus killing bacteria[10]. Traditional Chinese Medicine (TCM) believes that cholecystitis is a disease with pain, jaundice, liver distension, gallbladder distension and abdominal pain as the main symptoms, which is mainly caused by improper diet and yang and qi deficiency diseases from exogenous contraction with prolonged illness and injury[11]. Over years, TCM has shown its superiority in treating cholecystitis and other inflammatory diseases[12]. XYLD tablets are mainly composed of honeysuckle, folium isatidis, Radix Bupleuri, Astragalus membranaceus and Rheum officinale. Of these, honeysuckle has long been used as an antibacterial, antiviral, antioxidant and anti-inflammatory agent[13]. Radix Bupleuri is made from the dried roots of Bupleurum plants. Modern research has found that Radix Bupleuri and its active ingredients have pharmacological effects such as immunomodulation, antiviral action, liver protection and antipyresis[14]. Main components of Astragalus membranaceus are Astragalus polysaccharides, flavonoids, ginsenosides and alkaloids, among which Astragalus polysaccharides have antibacterial and antiviral properties[15]. Rheum officinale is a top medicinal plant with potent antibacterial and anti-inflammatory activities, and is considered as a good medicine for purgating heat and bowels[16]. With the continuous integration of Chinese and Western medicine in recent years, many Western medicines are used in combination with TCM in clinical practice and have played a significant role. Our research identified higher ORR with markedly shorter epigastric pain resolution, fever relief, nausea and vomiting improvement time, and HLOS in the combination group compared with the monotherapy group, with a similar incidence of adverse reactions. It shows that CS+XYLD tablets has a definite clinical effect on AC, which can effectively alleviate patients’ clinical symptoms and signs by shortening the recovery time of related diseases while ensuring high safety.
During the episode of cholecystitis, inflammation usually thickens the wall of the gallbladder, increasing the ability of the mucosa to absorb bile salts, resulting in a decrease in bile salt concentration and precipitation of stone crystals after cholesterol saturation[17]. In addition, the tension of the gallbladder wall with inflammatory thickening will be affected, which promotes the attachment and deposition of bile extravasation, forming stones that further contribute to the worsening of cholecystitis[18]. The results of this study also revealed that better gallbladder wall thickness, gallbladder tension, IBIL and TBA levels in the combination group than in the monotherapy group after treatment. It is suggested that CS+XYLD tablets can reduce the gallbladder wall thickness, enhance gallbladder tension and reduce IBIL and TBA production. Since AC is an inflammatory disease, anti-inflammation is the main direction of its treatment. The severity of AC is related to the increase of various proinflammatory cytokines (such as IL-6, TNF-α and CRP) after pancreatic acinar cell injury, and the expression of these factors increase with the severity of AC[19]. The results of this study showed that after treatment, gallbladder tension and gallbladder wall thickness in the combination group reduced significantly, compared with the monotherapy group along with the serum levels of IL-6, TNF-α and CRP. This indicates that CS+XYLD tablets work synergistically to mitigate the inflammatory response of patients, which can effectively reduce inflammation in the gallbladder and promote the recovery of the gallbladder function.
There are still some limitations in this study. This was a single-center, small-sample study and lacked follow-up observations. In addition, the influence of CS+XYLD tablets on patient prognosis and the broad applicability of the findings need to be explored and confirmed by extending the followup observation time.
Conclusively, there is a synergistic effect between CS and XYLD tablets, which has a remarkable effect in treating AC. The combination therapy can effectively alleviate patients’ clinical symptoms and signs and reduce the inflammatory reaction in vivo while ensuring high medication safety, which can be clinically popularized.
Conflict of interests:
The authors declare no conflict of interests.
References
- Cakcak IE, Kula O. Predictive evaluation of SIRI, SII, PNI, and GPS in cholecystostomy application in patients with acute cholecystitis. Ulus Travma Acil Cerrahi Derg 2022;28(7):1-7.
[Crossref] [Google Scholar] [PubMed]
- Xu R, Xu Y, Xu R. Effect of timing of laparoscopic cholecystectomy on postoperative efficacy and rehabilitation of elderly patients with acute cholecystitis. Am J Transl Res 2022;14(2):1107-13.
[Google Scholar] [PubMed]
- Yuksekdag S, Bas GU, Okan I, Karakelleoglu A, Alimoglu O, Akcakaya A, et al. Timing of laparoscopic cholecystectomy in acute cholecystitis. Niger J Clin Pract 2021;24(2):156-60.
[Crossref] [Google Scholar] [PubMed]
- Kaneta A, Sasada H, Matsumoto T, Sakai T, Sato S, Hara T. Efficacy of endoscopic gallbladder drainage in patients with acute cholecystitis. BMC Surg 2022;22(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Popowicz A, Enochsson L, Sandblom G. Timing of elective cholecystectomy after acute cholecystitis: A population-based register study. World J Surg 2023;47(1):152-61.
[Crossref] [Google Scholar] [PubMed]
- Shigemori T, Imoto I, Inoue Y, Nishiwaki R, Sugimasa N, Hamaguchi T, et al. Acute necrotizing calculous cholecystitis after treatment with ceftriaxone in an elderly patient: A case report. Surg Case Rep 2022;8(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Mayumi T, Okamoto K, Takada T, Strasberg SM, Solomkin JS, Schlossberg D, et al. Tokyo guidelines 2018: Management bundles for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 2018;25(1):96-100.
[Crossref] [Google Scholar] [PubMed]
- Costanzo ML, D'Andrea V, Lauro A, Bellini MI. Acute cholecystitis from biliary lithiasis: Diagnosis, management and treatment. Antibiotics 2023;12(3):482.
[Crossref] [Google Scholar] [PubMed]
- Karungamye P, Rugaika A, Mtei K, Machunda R. A review of methods for removal of ceftriaxone from wastewater. J Xenobiot 2022;12(3):223-35.
[Crossref] [Google Scholar] [PubMed]
- Bettuzzi T, Jourdes A, Robineau O, Alcaraz I, Manda V, Molina JM, et al. Ceftriaxone compared with benzylpenicillin in the treatment of neurosyphilis in France: A retrospective multicentre study. Lancet Infect Dis 2021;21(10):1441-7.
[Crossref] [Google Scholar] [PubMed]
- Dong ZY, Wang GL, Liu X, Liu J, Zhu DZ, Ling CQ. Treatment of cholecystitis with Chinese herbal medicines: A systematic review of the literature. World J Gastroenterol 2012;18(14):1689-94.
[Crossref] [Google Scholar] [PubMed]
- Rao K, Qin S, Yang Y, Zhan K, Wu H, Zheng H, et al. Shenling baizhu powder alleviates TNBS-induced colitis in rats by improving intestinal epithelial permeability and inhibiting inflammation through the TLR5/MyD88/NF-κB Pathway. Front Pharmacol 2022;13:1-10.
[Crossref] [Google Scholar] [PubMed]
- Ren L, Li J, Miao Z, Yan R, Li Q, Zhang R, et al. The application of gargle containing honeysuckle and semen oroxyli to reduce the pain and complications after uvulopalatopharyngoplasty. Front Pharmacol 2022;13:1-9.
[Crossref] [Google Scholar] [PubMed]
- Lv S, Zhao Y, Wang L, Yu Y, Li J, Huang Y, et al. Antidepressant active components of Bupleurum Chinese DC-Paeonia lactiflora pall herb pair: Pharmacological mechanisms. Biomed Res Int 2022:1-15.
[Crossref] [Google Scholar] [PubMed]
- Zheng Y, Ren W, Zhang L, Zhang Y, Liu D, Liu Y. A review of the pharmacological action of Astragalus polysaccharide. Front Pharmacol 2020;11:1-15.
[Crossref] [Google Scholar] [PubMed]
- Xiang H, Zuo J, Guo F, Dong D. What we already know about rhubarb: A comprehensive review. Chin Med 2020;15:1-22.
[Crossref] [Google Scholar] [PubMed]
- Lavoie B, Nausch B, Zane EA, Leonard MR, Balemba OB, Bartoo AC, et al. Disruption of gallbladder smooth muscle function is an early feature in the development of cholesterol gallstone disease. Neurogastroenterol Motil 2012;24(7):313-24.
[Crossref] [Google Scholar] [PubMed]
- Runner GJ, Corwin MT, Siewert B, Eisenberg RL. Gallbladder wall thickening. AJR Am J Roentgenol 2014;202(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Karakas DO, Yesiltas M. Validity of the Glasgow prognostic score and modified systemic inflammation score in predicting complicated cholecystitis. Hippokratia 2020;24(1):15-20.
[Google Scholar] [PubMed]
 ): Monotherapy group and (
): Monotherapy group and ( ): Combination group
): Combination group 



