- *Corresponding Author:
- W. Zhao
Department of Gastrointestinal Surgery, Ningxia Medical University General Hospital, No. 804, Shengli South Street, Xingqing District, Yinchuan City, 750004, Ningxia, China
E-mail: zhaoweiyisheng666@126.com
| This article was originally published in a special issue: Special issue on "Clinical and Experimental Studies on Drug and Intervention Repurposing in China" |
| Indian J Pharm Sci 2019:81(4)spl issue1;41-49 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In order to study the clinical efficacy of fluorouracil administered as a prodrug tegafur in combination with gimeracil and oteracil potassium (S-1) in the postoperative treatment of gastric cancer, in this study, 308 patients with clinically diagnosed gastric cancer and radical gastrectomy were followed up for data collection. The 308 patients were divided into treatment group and control group, and treated according to the experimental design. The data obtained was analysed, subjected to Χ2 test using SPSS20.0 software and the survival rate was calculated by Kaplan-Meier. The median was used to represent the survival time. The single factor analysis was performed by log-rank test. Multiple-factor analysis was performed using Cox analysis. Finally, the adverse reactions of patients with oral administration of S-1 were counted, and the clinical efficacy of the S-1 in the radical resection of gastric cancer was evaluated. The results showed that the median progress free survival of the treatment group and the control group were 7.7 and 5.4 months, respectively, and the median overall survival was 12.1 and 10.0 months, respectively (P>0.05). Adverse reactions were mainly manifested in hand-foot syndrome, gastrointestinal symptoms, bone marrow transplantation and other systemic symptoms. The study appeared to be of great significance in selecting treatment options for prolongation of survival time in patients after radical gastrectomy.
Keywords
Fluorouracil, gastric cancer, chemotherapy drugs, tegafur/gimeracil/oteracil potassium
Gastric cancer refers to one of the malignant neoplastic diseases in which epithelial cells of the gastric mucosa become cancerous. According to statistical analysis of relevant reports, gastric cancer is currently one of the four major tumors in the world, and its mortality rate is very high, ranking second in the mortality rate of neoplastic diseases[1]. In China, the number of deaths from gastric cancer is the second highest among all deaths due to malignant tumors, and the number of deaths due to gastric cancer is nearly 498 000. According to medical statistics, more than two-thirds of people are already in advanced stage when they are found or confirmed to have gastric cancer. As a consequence, many people have missed the best treatment time and lost the opportunity to prevent further spread of the disease through surgery[2]. Even though many patients with gastric cancer have not missed the opportunity for surgery, after surgery, about 69 % of patients with gastric cancer will have lesion metastasis and recurrence. In order to prolong the life of patients with advanced gastric cancer, the most common means of therapy is chemotherapy[3]. However, there is currently no uniform standard of treatment for chemotherapy. Most of the single drugs used in chemotherapy for gastric cancer are mainly confined to cisplatin, anthracyclines, fluorouracil and mitomycin C. However, the therapeutic efficiency of these drugs alone is very low and in order to improve the therapeutic effect during the postoperative stage of gastric cancer, many researchers began to study the therapeutic effect of two or more drugs in combination. The commonly used drugs are Taxol, platinum and fluorouracil[4,5].
At present, the main therapeutic drug for patients with advanced gastric cancer is fluorouracil single drugs. Among them, the most commonly used are capecitabine and tegafur/gimeracil/oteracil potassium (S-1)[6,7]. Capecitabine is a novel fluorouracil nucleoside analog, which is selectively active in tumor cells, the drug has the advantages of convenient to administer and relatively fewer adverse reactions. Clinical studies have shown that capecitabine can prolong the survival of patient. Tegafur/gimeracil/oteracil potassium (S-1) is a compound preparation of tegafur with gimeracil and oteracil potassium. In the course of use, it can reduce the gastrointestinal discomfort of patients. At present, a large number of patients with advanced gastric cancer have been treated with S-1 at home and abroad[8-10].
In summary, in this study dealing with treatment to patients with gastric cancer, the effect of a large dose of a single drug was evaluated. The single drug treatment is aimed at patients with advanced gastric cancer who have not undergone surgery. Currently, there are few studies related to fluorouracil treatment of gastric cancer patients. This study collected sample data to screen out patients with postoperative gastric cancer who conform to inclusion criteria. Informed consent was obtained from patients with gastric cancer after surgery, and the patients with gastric cancer were treated with fluorouracil combination S-1. The sample set was followed up for survival. The sample was divided into two groups, group of maintenance treatment and group of cessation treatment. By comparing the adverse reactions of S-1 in the treatment of advanced gastric cancer, the clinical data of the maintenance treatment group were statistically analyzed, and the single factor and multiple factors of survival were compared and analyzed. Finally, the clinical efficacy and effect of fluorouracil single drug S-1 on gastric cancer patients were summarized.
Materials and Methods
Case analysis of the research subjects:
The selected research subjects in this study were patients clinically diagnosed as gastric cancer, and after radical surgery, fluorouracil chemotherapy regimen was used to control patient's condition, and the sample set comprised of 450 cases. Among them, 165 patients were given single drug treatment with either capecitabine or S-1, and a total of 130 patients with complete data were obtained. Patients who underwent radical surgery without continued chemotherapy were labeled as a control group with a total of 230 patients. The selection of the sample set was based on overall consideration of the three factors, the gastric cancer TNM staging method, the gender and age of sample. In order to ensure the comprehensiveness of the sample set and the validity of the research results, the inclusion criteria for gastric cancer patients at the time of sample set selection are shown in Table 1. The standard conditions for the removal of the above samples are shown in Table 1. This study required the removal of cases that did not meet conditions specified in Table 1.
| Inclusion criteria |
| 1. The age of the patient is between 20 and 80 years old. |
| 2. The patient's pathological examination before surgery was diagnosed as gastric cancer |
| 3. Patients undergo radical surgery for gastric cancer |
| 4. Patients with clear lesion |
| 5. Index hemoglobin (HB) ≥8 g/dl, white blood cell count 4000-12000/mm3, blood platelet count (Pt) ≥100000/mm3 |
| 6. Patients with condition can survive for more than three months |
| 7. Cases with no other surgically affected |
| 8. In the maintenance chemotherapy group, after chemotherapy, capecitabine and S-1 were used for maintenance treatment. |
| 9. Taxanes, platinum, and fluorouracil were used for chemotherapy regimens |
| Exclusion criteria |
| 1. Cases that do not meet the inclusion criteria in Table 1 |
| 2. Patients with non-melanoma skin cancer and cases with carcinoma in situ of cervix |
| 3. Patients with severe liver, kidney and gastrointestinal bleeding |
| 4. Patient with a clinically significant cardiovascular disease |
| 5. Patients with ascites requiring drainage and brain metastases |
| 6. Patients with pulmonary fibrosis and interstitial pneumonia |
| 7. Case of loss of access rights |
Table 1: Sample selection
In this study, a total of 360 valid clinical data sets were collected. According to the requirements of this study, the sample data was divided into two study groups. The number of samples selected in the first group was 165 (the treatment group), and the selection criteria were patients with intraoperative implantation of fluorouracil implants. The number of samples selected in the second group was 230 (control group), and the selection criteria was whether oral S-1 was administered during surgery. Preoperative data of patients in the two groups of samples are shown in Table 2.
| Index | 1st group (treatment group) | 2nd group (control group) | t/t’ | P |
|---|---|---|---|---|
| WBC (l×109) | 6.51±1.91 | 6.52±2.14 | -0.01 | 0.98 |
| ANC (l×109) | 4.31±1.48 | 4.171±1.82 | 0.21 | 0.94 |
| HB (g/l) | 126.54±21.71 | 123.49±22.35 | -1.34 | 0.99 |
| PLT (l×109) | 258.70±81.94 | 264.70±81.94 | -0.24 | 0.90 |
| AST (U/l) | 19.62±6.47 | 18.59±6.31 | -0.11 | 0.86 |
| ALT (U/l) | 20.58±10.14 | 21.41±9.81 | -0.02 | 0.92 |
| TBIL(g/l) | 9.90±3.89 | 9.51±4.05 | 0.01 | 0.26 |
| CysC (mg/l) | 1.14±0.21 | 1.80±0.44 | -0.23 | 0.64 |
| RBP (mg/l) | 26.52±8.02 | 25.17±8.98 | 0.01 | 0.58 |
| BUN (g/l) | 5.58±1.83 | 6.01±1.54 | -0.14 | 0.29 |
| CRE (mmol/l) | 59.82±13.16 | 58.81±14.83 | 0.04 | 0.19 |
| CEA (mg/l) | 10.41±14.80 | 11.08±13.32 | 1.21 | 0.38 |
| CA19-9 (U/ml) | 12.68±9.89 | 11.89±8.80 | 0.41 | 0.36 |
| CA72-4 (U/ml) | 4.51±5.83 | 4.58±5.81 | -0.29 | 0.42 |
Table 2: Reoperative indicators of the two groups of patients (X±SD)
The preoperative index items of the two groups of patients, WBC, ANC, HB, PLT, AST, ALT, CysC, TBIL, RBP, BUN, CRE, CEA, CA19-9 and CA72-4 were compared at a p>0.05 level of significance, so according to the level of testing, there was no significant difference in the selection of samples between the two groups of samples.
Experimental treatment plan:
In this study, the induction chemotherapy regimens used in patients with gastric cancer were summarized in Table 3. In this study, seven treatment options were proposed, and different treatment options will be adopted depending on the patient's own conditions. Chemotherapy drugs were all fluorouracil single drugs.
| Treatment programs | Method of application |
|---|---|
| XELOX program (three times a week) | (OXA, oxaliplatin): 130 mg/m3, intravenous drip, d 1 |
| Xelada, capecitabine: 2000 mg/(m3·d), 2 times daily, d 1-14 | |
| Mfolfox6 program (once every 2 weeks) | (OXA, oxaliplatin): 85 mg/m3, fast intravenous infusion, d 1 |
| (5FU, fluorouracil): 400 mg/m3, fast intravenous infusion, d 1 | |
| 2400 mg/m3, 48 h pumping in | |
| SOX program: (1 time every 3 weeks) | (CF or LV, calcium leucovorin): 200 mg/m3, intravenous drip |
| (OXA, oxaliplatin): 130 mg/m3, fast intravenous infusion, d 1 | |
| DCF program: (1 time every 3 weeks) | (S-1, tegafur gimeracil and oteracil potassium): 80 mg/m3, taken twice, d 1-14 |
| (DXT, docetaxel): 75 mg/m3, intravenous drip, d 1 | |
| (cisplation, cisplatin): 75 mg/m3, intravenous drip, d 1 | |
| DF program: (1 time every 3 weeks) | (5FU, fluorouracil): 400 mg/m3, fast intravenous infusion, d 1 |
| 2400 mg/m3, 48 h pumping in | |
| (DXT, docetaxel): 75 mg/m3, intravenous drip, d 1 | |
| DX program: (1 time every 3 weeks) | (5FU, fluorouracil): 400 mg/m3, fast intravenous infusion, d 1 |
| 2400 mg/m3, 48 h pumping in | |
| DP program: (once every 3 weeks) | (CF or LV, calcium leucovorin): 200 mg/m3, intravenous drip |
| (DXT, docetaxel): 75 mg/m3, intravenous drip, d 1 | |
| Other programs | |
Table 3: Chemotherapy for patients after radical gastrectomy
Experimental materials and methods of use:
In the research of this study, oral capecitabine and S-1 were administered to patients in the maintenance treatment group. The dosage, method of administration and time of administration of the two drugs are shown in Table 4.
| Drug | Dosage, method of administration and time of administration |
|---|---|
| Capecitabine | The dose was 1250 mg/m2, 2 times a day (in the morning and evening), and the treatment was stopped for 1 w after 2 w of treatment, and 3 w was a treatment cycle. |
| S-1 | The dose was 80 mg/m2, 2 times a day (in the morning and evening), and after 2 w of continuous treatment, the drug was stopped for 1 w, and 3 w was a treatment cycle. |
Table 4: Experimental materials and methods of use
Observation indicators and evaluation criteria:
The observation indicators in this study were the median progress free survival and median survival with disease progression, median overall survival (mOS), 1-y survival, 2-y survival rate, adverse reactions with capecitabine and S-1. The above indicators were used to analyze the relationship between the clinical pathological features of patients and the prognosis of maintenance treatment. Progress free survival (PFD) refers to the period from the onset of chemotherapy until the disease progress is observed or the death of any cause occurs[11,12]. Overall survival (OS) refers to the period of time from the start of treatment to the death of the patient and has long been alive at the end of follow-up[13,14].
Follow-up and case review:
The follow-up period of this study was 2 y, and the deadline for postoperative follow-up was December 2018. By reviewing through the cases, patients with evolutionary chemotherapy were visited every three months, each visit to determine the outpatient review and determine survival. Because of changes in the patient's home address and changes in the mobile phone, 8 cases were lost.
Statistical analysis methods:
In this study, the statistical software SPSS20.0 was used to analyze the data samples. The data were analyzed by Χ2 test, Kaplan-Meier was used to calculate the survival rate, the median was used to represent the survival time, and the single factor analysis was performed by log-rank test. The multiplefactor analysis was performed using Cox. P<0.05 was considered statistically significant.
Results and Discussion
According to the needs of this study, a total of 408 cases of this study were selected by screening the hospital cases. After chemotherapy, 138 cases agreed with maintenance treatment. Due to the loss of 8 patients during the follow-up, 130 samples were collected. In this study, patients who underwent chemotherapy but did not choose to continue treatment were referred to as the cessation treatment group. Data from patients with missing follow-up were excluded, and a total of 230 patients were collected in the cessation treatment group.
A total of 360 samples were studied, with 130 patients in the maintenance treatment group and 230 patients in the cessation treatment group. The pathological features in the sample data were, gender, age, histological grade, radical operation of gastric cancer, WHO pathological classification, number of metastases, liver metastasis, and number of induction chemotherapy cycles. In the maintenance treatment group, there were 99 males and 31 females. The age distribution was mainly concentrated in 18 to 80 y old, 67 cases less than 60 y old, and 63 cases older than 60 y old. There were 57 cases of recurrence after radical gastrectomy and 73 cases did not undergo radical surgery. There were 115 cases of adenocarcinoma and 15 cases of cell carcinoma. The cases with 1-2 of metastases were 98 cases, and the cases with more than 3 metastases were 32 cases. There were 23 cases with 2 to 3 induction chemotherapy cycles, 35 cases with 4 to 5, and 72 cases with more than 6 induction chemotherapy cycles. The clinical characteristics of the sample data are shown in Table 5.
| Factor | Types | Treatment group (n=130) | Control group (n=330) | X2 | P |
|---|---|---|---|---|---|
| Gender | Male | 99 | 150 | 1.732 | 0.134 |
| Female | 31 | 180 | |||
| Age | ≤60 years old | 67 | 130 | 0.369 | 0.932 |
| >60 years old | 63 | 200 | |||
| Number of metastases | 1~2 | 98 | 191 | 2.218 | 0.413 |
| Greater than 3 | 32 | 109 | |||
| Radical surgery | Yes | 51 | 93 | 0.003 | 0.237 |
| No | 79 | 237 | |||
| Degree of tissue differentiation | High | 31 | 60 | 1.337 | 0.436 |
| Low | 99 | 270 | |||
| Pathological type | Adenocarcinoma | 115 | 210 | 0.767 | 0.264 |
| Cell carcinoma | 15 | 15 | |||
| Carcinogenesis | Lymph gland | 79 | 102 | 0.672 | 0.345 |
| Abdominal cavity | 81 | 43 | 0.634 | 0.621 | |
| Metastatic sites | Pelvic cavity | 20 | 8 | 1.329 | 1.138 |
| Liver | 7 | 12 | 0.792 | 0.734 | |
| Lung | 9 | 23 | 0.039 | 0.563 | |
| Bone | 14 | 19 | 1.884 | 0.174 | |
| Induction chemotherapy cycle | 2~3 | 23 | 45 | - | - |
| 4~5 | 35 | 65 | - | - | |
| 5~6 | 72 | 220 | - | - |
Table 5: Clinical data characteristics of sample data
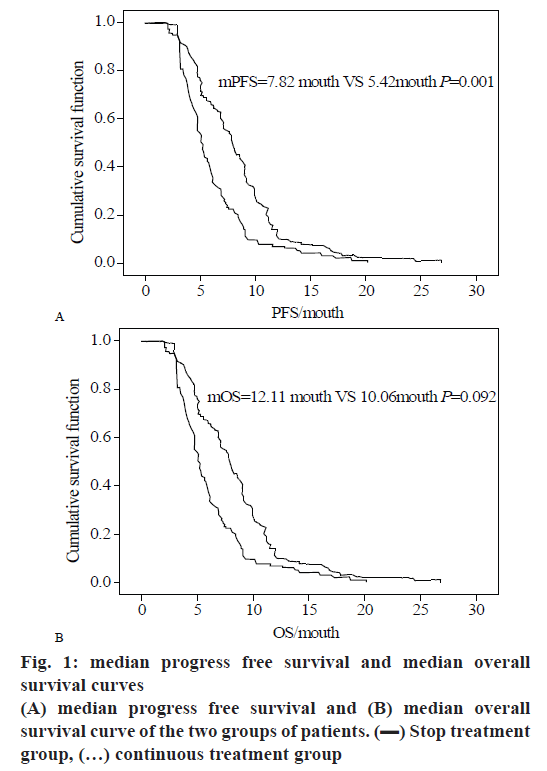
After the maintenance treatment group received 2 cycles of maintenance treatment, the median treatment cycle was 3 cycles and the average treatment cycle was 3.69 cycles. The median progress free survival in the maintenance chemotherapy group and the cessation chemotherapy group was respectively 7.70 mo (95 % CI: 4.163-9.819) and 5.40 mo (95 % CI: 2.664-7.736). Both groups of data were statistically significant (Χ2=13.382, p=0.001). The median survival time of the two groups was respectively 12.09 mo (95 % CI: 7.801-15.321) and 10.50 mo (95 % CI: 6.957-12.398). The median survival time of the group was not statistically significant (Χ2=13.382, p=0.093).
In this study, the clinical characteristics of patients and median progress free survival (mPFS) were analyzed by univariate analysis. The results are shown in Table 6. As can be observed from Table 6, there was statistical significance in clinical features. And the median progress free survival is lower in patients with liver metastasis than in patients without liver metastasis, is lower in patients with more metastases than patients with small number of metastases, and is lower in the patients with less chemotherapy cycles patients with more chemotherapy cycles.
| Clinical features | X2 | P |
|---|---|---|
| Patients without liver metastases and patients with liver metastases | 19.510 | 0.000 |
| Patients with fewer metastases and more metastases | 27.035 | 0.000 |
| Patients with more induction chemotherapy cycles and low number of chemotherapy cycles | 24.285 | 0.000 |
| Clinical features | X2 | P |
| Patients without liver metastases and patients with liver metastases | 19.372 | 0.000 |
| Patients with fewer metastases and more metastases | 19.023 | 0.000 |
| Patients with more induction chemotherapy cycles and low number of chemotherapy cycles | 23,142 | 0.000 |
Table 6: Univariate analysis of clinical features, MPFS and MOS of patients
In this study, the clinical characteristics of patients and mOS were analyzed by univariate analysis. The results are shown in Table 6. It can be observed from Table 6 that the analysis of the results of mOS was almost identical to the analysis of the results of mPFS, and both are statistically significant. In summary, Table 7 is a univariate survival analysis of clinical data of patients in the maintenance group.
| Factor | Number of cases | mPFS | mOS | |||||
|---|---|---|---|---|---|---|---|---|
| Types | Factors | Months | P | X2 | Months | P | X2 | |
| Gender | Male | 99 | 7.82 | 0.053 | 0.841 | 12.11 | 0.137 | 2.161 |
| Female | 31 | 7.71 | 11.76 | |||||
| Age | ≤60 years old | 67 | 7.61 | 0.273 | 1.146 | 12.08 | 0.356 | 0.891 |
| >60 years old | 63 | 7.82 | 12.03 | |||||
| Case type | Adenocarcinoma | 115 | 7.67 | 0.932 | 0.004 | 12.19 | 0.971 | 0.001 |
| Cell carcinoma | 15 | 6.73 | 11.56 | |||||
| Degrees of differentiation | Low | 99 | 6.65 | 0.000 | 19.472 | 12.09 | 0.000 | 19.312 |
| High | 31 | 7.73 | 12.64 | |||||
| Liver metastasis | Have | 67 | 6.12 | 0.977 | 19.489 | 10.11 | 0.422 | 0.664 |
| No | 63 | 8.96 | 13.54 | |||||
| Radical treatment | Yes | 51 | 7.72 | 0.974 | 0.001 | 12.23 | 0.421 | 0.669 |
| No | 79 | 7.72 | 12.11 | |||||
| Maintenance medication | Capecitabine | 55 | 8.3 | 0.961 | 0.003 | 12.6 | 0.000 | 23.121 |
| S-1 | 75 | 7.4 | 10.7 | |||||
| Number of metastases | 1~2 | 98 | 8.81 | 0.000 | 27.053 | 12.75 | 0.000 | 19.047 |
| >2 | 32 | 4.31 | 9.65 | |||||
| Induction chemotherapy circle | - | - | - | 0.000 | 24.319 | - | - | - |
| Number of circles | 2~3 | 23 | 4.66 | - | - | 9.72 | - | - |
| 4~5 | 35 | 5.47 | - | - | 10.45 | - | - | |
| 5~6 | 72 | 8.91 | - | - | 13.43 | - | - | |
Table 7: Univariate survival analysis of clinical data in patients in the treatment group
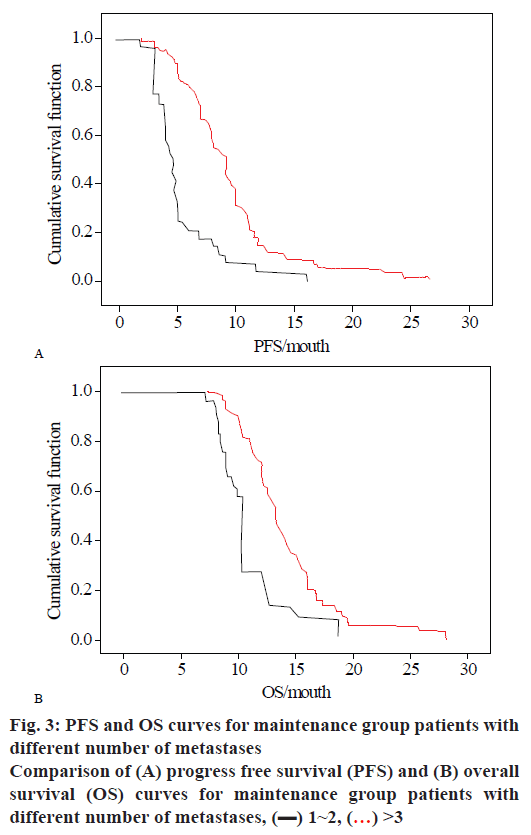
In this study, the clinical case characteristics were used as independent variables, and the PFS and OS of the patients in the sample were used as the dependent variables. Cox multivariate analysis was performed to analyze the independent influencing factors affecting patients' PFS and OS. The results are shown in Table 8, and the calculated number of liver metastases and metastases are shown in figs. 1 to 3.
| Factor | B | SE | Wald | df | Sig. | Exp(B) |
|---|---|---|---|---|---|---|
| Liver metastasis | -0.542 | 0.194 | 7.593 | 1 | 0.002 | 0.583 |
| Number of metastases | 1.006 | 0.231 | 19.342 | 1 | 0.000 | 2.731 |
| Factor | B | SE | Wald | df | Sig. | Exp(B) |
| Liver metastasis | -9.613 | 0.219 | 8.732 | 1 | 0.003 | 0.532 |
| Number of metastases | 0.923 | 0.251 | 13.734 | 1 | 0.000 | 2.523 |
Table 8: PFS and OS multivariate analysis of treatment group
Fig. 2A and B show the PFS survival curve and OS survival curve in the case of liver metastasis. It can be observed from the figure that the cumulative growth function in the treatment group decreases with time, indicating that the survival function declines slowly in the absence of liver metastasis, which shows that the survival time of gastric cancer patients is longer, and the survival crisis is generally after 5 mo. The patients without liver metastasis can basically maintain the survival time of 28 mo, while in the case of liver metastasis, the survival function begins to decline at 2.7 mo, and the survival time of patients with gastric cancer is 15 mo at maximum. Similarly, in the OS survival curve, patients with liver metastases and patients without liver metastases have almost the same survival time at the first 10 mo. After 10 mo, the survival function of patients with liver metastasis suddenly drops. The rate of decline is much higher than in patients without liver metastases. And the survival time of cases with liver metastasis was only 18 mo, and the survival time of cases without liver metastasis was 28 mo.
Figs. 3A and B are PFS survival curves and OS survival curves for the different number of metastases. It can be observed from the figure that the cumulative growth function in the treatment group decreased over time. The survival function decreased slowly when the number of metastases is 1~2, and the survival time of patients with gastric cancer was longer. Unlike the liver metastasis, there was no plateau in the case of different numbers of metastases, and the survival function decreased from the beginning. Over time, patients with a number of metastases of 1 to 2 can maintain a survival time of 28 mo, and in the case of a metastatic lesion of more than 3, the survival function of the patient also decreases at the beginning, and the maximum survival time of patients with gastric cancer was 15 mo.
In the same way, in the OS survival curve, the case where the number of metastases is 1 to 2 was the same as the case where the number of metastases is greater than 3, and the survival time of the patient in the first 8 mo was almost the same. After the 8 mo, the survival function of patients with liver metastasis suddenly decreased, and the rate of decline was much higher than that of patients without liver metastases. And the survival time of cases with liver metastasis was only 18 mo, and the survival time of cases without liver metastasis was 28 mo.
In this study, the adverse reactions were evaluated in 130 patients in the maintenance treatment group. The main adverse reactions were in the symptoms of hand-foot syndrome, digestive system symptoms, bone marrow transplantation and other systemic symptoms. The statistics are shown in Table 9.
| Adverse reactions | 0 | I | II | III | IV | III-IV % |
|---|---|---|---|---|---|---|
| Nausea and vomiting | 63 | 42 | 3 | 0 | 0 | |
| Stomatitis | 105 | 17 | 3 | 0 | 0 | |
| Abdominal pain, diarrhea | 65 | 39 | 3 | 2 | 0 | 0.83 |
| Hand and foot syndrome | 107 | 17 | 1 | 1 | 0 | 0.82 |
| Rash | 101 | 20 | 1 | 0 | 0 | |
| Chromatosis | 118 | 11 | 0 | 0 | 0 | |
| Leukopenia | 74 | 35 | 16 | 0 | 0 | 3.4 |
| Granulocyte reduction | 94 | 17 | 4 | 3 | 0 | |
| Thrombocytopenia | 78 | 30 | 10 | 0 | 0 | 0.7 |
| Liver function | 113 | 11 | 3 | 1 | 0 | |
| Renal function | 121 | 7 | 1 | 0 | 0 |
Table 9: Adverse reaction statistics of the treatment group
As can be observed from Table 6, there were 44 cases of abdominal pain and diarrhea, and the III-IV degree was 0.83 %, accounting for 45.3 % of the total adverse reactions. There were 19 cases of adverse reactions in hand-foot syndrome, with a grade III-IV of 0.83 %, accounting for 45.3 % of the total adverse reactions. There were 51 cases of adverse reactions of leukopenia, and the III-IV degree was 3.2 %, accounting for 35.3 % of the total adverse reactions. And most of the adverse reactions were concentrated in I to II degrees, indicating that most of the adverse reactions caused by oral fluorouracil single drug S-1 were tolerable to the patient. There were no chemotherapy-related deaths in the maintenance treatment group.
In this study, 308 patients with clinically diagnosed gastric cancer and radical gastrectomy were followed up for data collection. The 308 patients were divided into treatment group and control group, and treated according to the experimental design. Subsequently, the statistical data SPSS20.0 was used to analyze the data samples, then the Χ2 test was performed on the data, and the survival rate was calculated by Kaplan- Meier. The median was used to represent the survival time. The single factor analysis was performed by logrank test. Multiple-factor analysis was performed by cox analysis. Finally, the adverse reactions of patients with oral administration of S-1 were counted, and the clinical efficacy of chemotherapy with S-1 in the radical resection of gastric cancer was evaluated. From the results obtained, it was found that three factors influence median progress free survival and overall survival of gastric cancer patients (with or without liver metastasis, number of metastases, and number of induction chemotherapy cycles) were all independent factors. Patients who have undergone radical gastrectomy can effectively delay the recurrence and metastasis of the disease after using chemotherapy with S-1 for a period of time, and the survival rate improved for nearly 1 y. Adverse reactions were mainly manifested as handfoot syndrome, digestive system symptoms, bone marrow transplantation, and other systemic symptoms, but were tolerated by the patient. It can be concluded from this study that the clinical effect of chemotherapy drugs after radical gastrectomy has certain progress. This finding is of great significance for the treatment of patients after radical gastrectomy.
References
- Yang T, Aimaiti M, Su D, Miao W, Zhou B, Yusup A, et al. Enhanced efficacy with reduced toxicity of chemotherapy drug 5-fluorouracil by synergistic treatment with Abnormal Savda Munziq from Uyghur medicine. BMC Complement Altern Med 2017;17(1):201.
- Tajima Y, Shimada Y, Kameyama H, Yagi R, Okamura T, Kobayashi T, et al. Association between poorly differentiated clusters and efficacy of 5-fluorouracil-based adjuvant chemotherapy in stage III colorectal cancer. Jpn J Clin Oncol 2017;47(4):313-20.
- Jiao S, Li N, Cai S, Guo H, Wen Y. Inhibition of CYFIP2 promotes gastric cancer cell proliferation and chemoresistance to 5-fluorouracil through activation of the Akt signaling pathway. Oncol Lett 2017;13(4):2133-40.
- Kawasaki K, Takeuchi D, Kaneko T, Miura S, Kamiya J, Miyahara Y, et al. A Case of Advanced Gastric Cancer Responding to Neoadjuvant Chemotherapy with Docetaxel, Cisplatin, and 5-Fluorouracil, Leading to a Pathological Complete Response. Gan To Kagaku Ryoho 2017;44(11):1017-20.
- Sun L, Ke J, He Z, Chen Z, Huang Q, Ai W, et al. HES1 promotes colorectal cancer cell resistance to 5-fu by inducing of emt and abc transporter proteins. J Cancer 2017;8(14):2802-8.
- Takase N, Yamashita K, Sumi Y, Hasegawa H, Yamamoto M, Kanaji S, et al. Local advanced rectal cancer perforation in the middst of preoperative chemoradiotherapy: A case report and literature review. World J Clin Cases 2017;5(1):18-23.
- Sharma A, Arora M, Goyal AK, Rath G. Spray Dried Formulation of 5-Fluorouracil Embedded with Probiotic Biomass: in vitro and in vivo Studies. Probiotics Antimicrob Proteins 2017;9(3):310.
- Huang MY, Huang CM, Tsai HL, Huang CW, Hsieh HM, Yeh YS, et al. Comparison of adjuvant FOLFOX4 chemotherapy and oral UFUR/LV following adjuvant FOLFOX4 chemotherapy in patients with stage III colon cancer subsequent to radical resection. Oncol Lett 2017;14(6):6754-62.
- Du DF, Du DF, Zhou FY, Zhang LQ, Han XN, Yuan Y, et al. Abstract 764: Survival analysis for the elderly esophageal cancer patients (≥80 years) with surgery and non-surgery treatments. Cancer Res 2017;77(13 Sup):764-4.
- Mizuno T, Ebata T, Yokoyama Y, Igami T, Sugawara G, Yamaguchi J, et al. Adjuvant gemcitabine monotherapy for resectable perihilar cholangiocarcinoma with lymph node involvement: a propensity score matching analysis. Surg Today 2017;47(2):182-92.
- Karimi Dermani F, Najafi R. miR-200c as a Predictive Biomarker for 5-Fluorouracil Chemosensitivity in Colorectal Cancer. J Gastrointest Cancer 2017;49(2):102.
- Kircik L, Sung JC, Stein-Gold L, Goldenberg G. United States Food and Drug Administration Product Label Changes. J Clin Aesthet Dermatol 2017;9(1):20-29.
- Yuan L, Zhang S, Li H, Yang F, Mushtaq N, Ullah S, et al. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed Pharmacother 2018;108:184-93.
- Munemoto Y, Kanda M, Oba K, Kim HM, Takemoto H, Denda T, et al. A phase II trial to evaluate the efficacy of panitumumab combined with fluorouracil-based chemotherapy for metastatic colorectal cancer: the PF trial. Cancer Chemother Pharmacol 2018;81(9927):1-10.
 Stop treatment group,
Stop treatment group,  continuous treatment group
continuous treatment group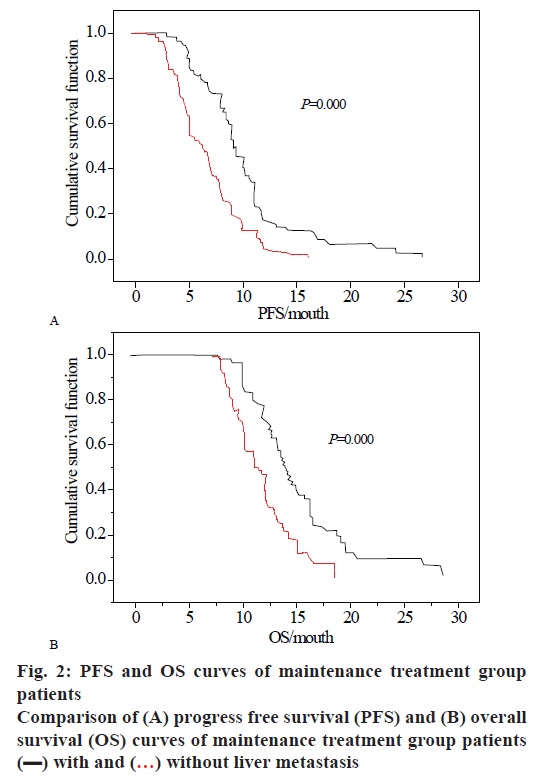
 without liver metastasis
without liver metastasis