- *Corresponding Author:
- Tao Fang
Department of Cardiovascular Medicine, Yongjia County Hospital of Traditional Chinese Medicine, Wenzhou, Zhejiang Province 325000, China
E-mail: b1210023@stu.cpu.edu.cn
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “188-195” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To observe the clinical efficacy and safety of tapazole integrated with anti-heart failure drugs in the remedy of hyperthyroidism merged with heart failure. Subjects 39 y~78 y suffered from hyperthyroidism and heart failure were enrolled, a total of 120 subjects were assigned to two subgroups according to different intervention methods. A control subgroup (n=60), which was given anti heart failure drugs intervention, and an observation subgroup (n=60), which was given tapazole intervention on the grounds of the control subgroup. The clinical efficacy, thyroid function, cardiac function, immune function and adverse reactions of the two subgroups were contrasted. In terms of the total effective rate, vs. the control subgroup (76.67 %), the observation subgroup (91.67 %) was notable higher. The concentrations of serum free triiodothyronine 3, free thyroxine 4 and N-terminal pro-brain natriuretic peptide in the observation subgroup were lower, thyroid stimulating hormone and left ventricular ejection fraction, SV, E/A were higher, the increase of cluster of differentiation 3+, cluster of differentiation 4+and cluster of differentiation 4+/ cluster of differentiation 8+ ratio in the observation subgroup was greater and cluster of differentiation 8+ declined more notablely. The remedy of hyperthyroidism and heart failure with tapazole integrated with anti-heart failure drugs is more effective. It can improve the thyroid function and heart function of subjects, enhance the body immunity, and has guaranteed safety.
Keywords
Hyperthyroidism, heart failure, tabazole, anti-heart failure therapy, cardiac function, immunity function
Hyperthyroidism is an endocrine disease mainly caused by abnormal thyroid hormone secretion. Its main clinical manifestations are hyper metabolism and increased excitability of nervous and digestive[1,2]. According to the epidemiological investigation[3], hyperthyroidism can occur at any age, with a high incidence of 30 y to 60 y old, and the majority of female subjects, with an overall incidence of about 1 %. Related studies have shown that hyperthyroidism can cause cardiomyopathy[4,5], and in severe cases can develop into heart failure. In other words, heart failure is one of the more serious complications of hyperthyroidism, which can lead to death, which should be paid attention to.
Angiotensin Receptor Neprilysin Inhibitor (ARNI), Beta Blockers (BB) and Mineralocorticoid Receptor Antagonists (MRA) are the three basic drugs for clinical remedy of heart failure with declined ejection fraction[6]. In recent years, numerous randomized researches have authenticated that Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2I) can notably benefit subjects[7,8], and the latest heart failure guidelines regard it as the fourth core remedy drug. Therefore, at present, the above four kinds of drugs are mainly used to treat heart failure, but for subjects with hyperthyroidism, the efficacy is poor.
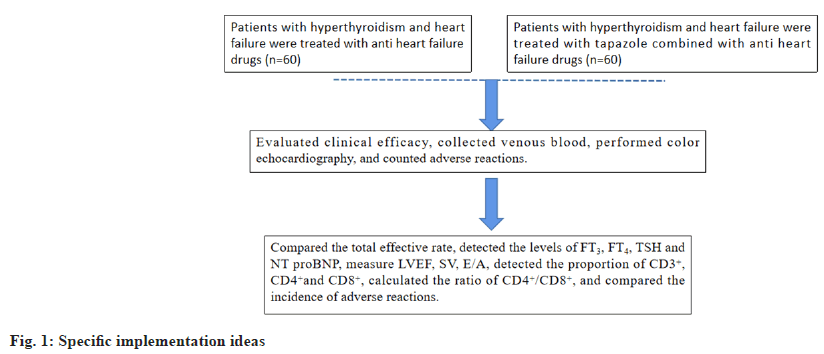
Tabazol is the main drug for clinical remedy of hyperthyroidism, but its disadvantages are slow efficacy, long remedy course and many side effects[9]. At present, the combination regimen is often used in the remedy of hyperthyroidism, but there are few studies on Tabazol integrated with anti-heart failure in the remedy of hyperthyroidism complicated with heart failure. In view of this, the author does the following research, and elaborate in detail. As shown in fig. 1 for specific implementation ideas.
Materials and Methods
Design and procedures:
This was a double-arm cohort trial with repeated measurements from January 2020 to January 2022. In this retrospective analysis trial, the anti-heart failure drugs intervention group was compared with tapazole+anti heart failure drugs intervention group in order to expect a significant improvement in the clinical efficacy, thyroid function, cardiac function, immune function. The control subgroup was anti heart failure drugs subgroup, the observation subgroup was tapazole intervention on the grounds of the control subgroup.
Clinical data:
The eligibility and exclusion criteria of patients were evaluated according to the inclusion situation. After screening, the participants were assigned according to the intervention method.
Inclusion criteria: The clinical diagnostic criteria for hyperthyroidism was conformed[10]; conformed to the diagnostic criteria for heart failure[11], and was of reduced ejection fraction type; cardiac function class II-IV; good compliance and the clinical data were complete.
Exclusion criteria: Subjects merged with severe heart, liver and kidney dysfunction; subjects merged with severe infection or blood system disease; subjects with malignant tumors and subjects with serious mental illness and those who do not tolerate the remedy drugs.
Criteria for shedding or elimination: Those who did not complete the remedy as directed by the doctor; those who were seriously ill and needed to take active measures to save them; those who withdrew voluntarily and those who were unreachable. All the subjects did not fall off. The ethics committee of the hospital approved this study.
Procedure:
Methods of drug use: Both subgroups were instructed to quit smoking and drinking, ate low sodium diet, ate less and had more meals, exercised moderately, worked and rested reasonably, and maintained a happy mood.
Control subgroup: The subjects were treated with anti-heart failure drugs. The drug regimen was ARNI (such as sacubitril valsartan sodium tablets)+BB (such as metoprolol succinate, bisoprolol, metoprolol tartrate)+MRA (such as spironolactone)+SGLT2I (such as dapagliflozin).
Observation subgroup: The subjects were given tapazole (GYZZ H11020440, Beijing Yanjing Pharmaceutical Co., Ltd., 5 mg/tablet) orally with warm water on the basis of the control subgroup, 1-2 tablets/time, 3 times/d. Both subgroups were treated continuously for 3 mo.
Measures:
Thyroid function index: 5 ml of the subjects’ venous blood from the morning before and after remedy was taken. After separation, the supernatant was taken, sub packaged and froze. The automatic biochemical analyzer was used to detect the concentrations of Free Triiodothyronine (FT3), Free Thyroid Hormone (FT4) and Thyroid Stimulating Hormone (TSH).
Cardiac function index: Before and after remedy, the Left Ventricular Ejection Fraction (LVEF), Stroke Volume (SV) per minute and ratio of mitral valve blood flow (E/A) were measured by color echocardiography, and the average value was taken for three consecutive measurements. At the same time, the supernatant after sub package was taken, and the content of N-Terminal pro Brain Natriuretic Peptide (NT-proBNP) in serum was detected by enzyme-linked immunosorbent assay.
Immunity function: Before and after remedy, 5 ml of subjects' peripheral blood was collected, and the changes of T lymphocyte subsets (including Cluster of Differentiation (CD) 3+, CD4+, CD8+) were detected by flow cytometry, and the CD4+/CD8+ ratio was calculated.
Adverse reactions: The adverse reactions of the two subgroups were counted.
Efficacy evaluation[12]:
The subject’s clinical symptoms and signs disappeared, the serum indicators TSH, FT3 and FT4 returned to normal, and the improvement concentration of cardiac function was ≥2 or returned to normal, which was marked as notablely effective.
The subject’s clinical symptoms, signs, serum indicators and cardiac function had no notable change or aggravation, which was marked as invalid. Total efficiency rate=(Notably effective+effective)/ total patients×100 %.
Statistical data:
Data was analyzed by Statistical Package for the Social Sciences (SPSS) 22.0 software. The measurement data were in accordance with normal distribution and uniform variance, expressed in (x±s). The paired sample t-test was applied for intra subgroup difference, and the independent sample t test was applied for inter subgroup difference; the counting data were expressed as n (%), and tested with Chi-Square (χ2) values. The difference was statistically notable with p<0.05.
Results and Discussion
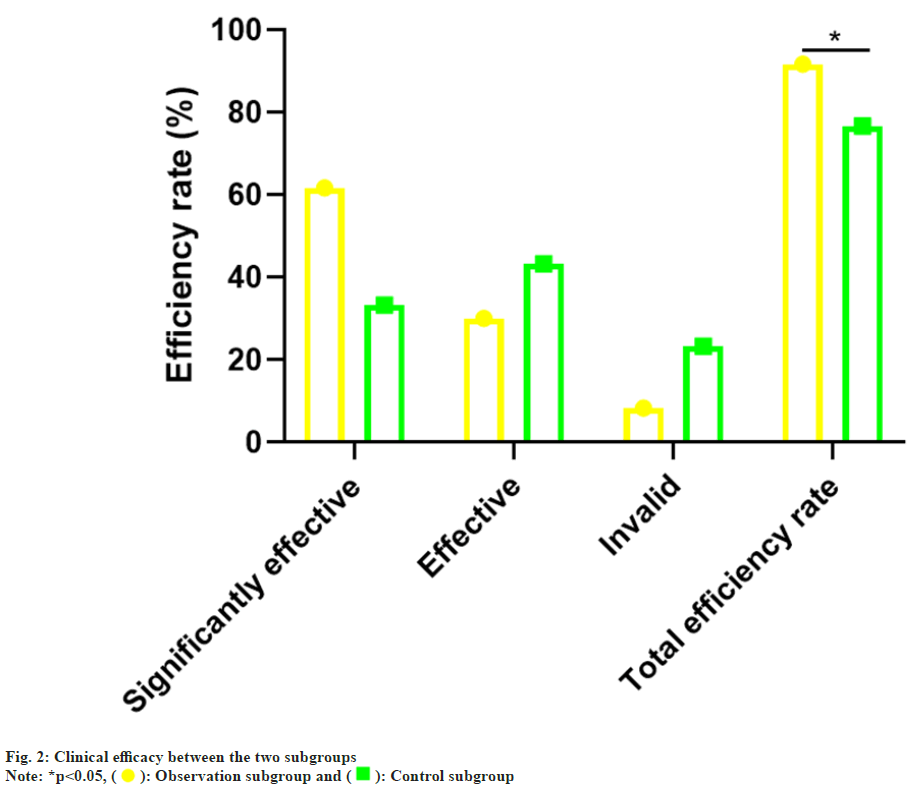
The gender, age, Body Mass Index (BMI), hyperthyroidism degree and cardiac function grade in the control subgroup and observation subgroup was no notable difference (p>0.05), which was comparable as shown in Table 1. vs. the control subgroup (76.67 %), the total effective rate of the observation subgroup (91.67 %) was notablely higher (p<0.05) as confirmed in Table 2 and fig. 2.
| Observation subgroup | Control subgroup | t/χ2 | p | |
|---|---|---|---|---|
| Gender | ||||
| Male | 18 | 15 | 0.376 | 0.539 |
| Female | 42 | 45 | ||
| Age (years) | 60.28±7.59 | 58.77±7.80 | 1.074 | 0.284 |
| Disease course (months) | 24.16±5.50 | 25.02±5.43 | 0.861 | 0.390 |
| BMI (kg/m2) | 22.47±2.15 | 22.85±2.09 | 0.981 | 0.328 |
| Hyperthyroidism degree | ||||
| Mild | 16 | 14 | 0.338 | 0.844 |
| Moderate | 38 | 41 | ||
| Severe | 6 | 5 | ||
| Cardiac function grade | ||||
| II~III | 41 | 43 | 0.158 | 0.690 |
| IV | 19 | 17 | ||
Table 1: General Information of the Two Subgroups
| Subset | n | Notably effective | Effective | Invalid | Total efficiency rate |
|---|---|---|---|---|---|
| The observation subgroup | 60 | 37 (61.67) | 18 (30.00) | 5 (8.33) | 55 (91.67) |
| The control subgroup | 60 | 20 (33.33) | 26 (43.33) | 14 (23.33) | 46 (76.67) |
| χ2 | 5.065 | ||||
| p | 0.024 |
Table 2: Comparison of Clinical Efficacy, n (%)
Before remedy, there was no distinction in serum FT3, FT4 and TSH concentrations (p>0.05) between the two subgroups. But after remedy, the concentrations of serum FT3 and FT4 in the two sub groups declined (p<0.05), and the concentrations of TSH boosted (p<0.05); compared with the control subgroup, the changes of serum FT3, FT4 and TSH concentrations in the observation subgroup after remedy were greater (p<0.05) as shown in Table 3 and fig. 3.
| Subgroups | n | FT3 (pg/ml) | FT4 (ng/dl) | TSH (mU/l) | |||
|---|---|---|---|---|---|---|---|
| Before remedy | After remedy | Before remedy | After remedy | Before remedy | After remedy | ||
| Observation subgroup | 60 | 12.58±3.12 | 3.61±0.95* | 7.16±1.23 | 1.20±0.65* | 0.24±0.06 | 2.63±0.75* |
| Control subgroup | 60 | 12.64±3.45 | 5.24±1.17* | 7.07±1.38 | 2.36±0.74* | 0.23±0.07 | 1.87±0.50* |
| t | 0.1 | 8.378 | 0.377 | 9.123 | 0.84 | 6.531 | |
| p | 0.621 | <0.001 | 0.707 | <0.001 | 0.403 | <0.001 | |
Note: *p<0.05, comparison of observation subgroup vs. control subgroup after remedy
Table 3: Comparison of Thyroid Function Indices (x̄±s)
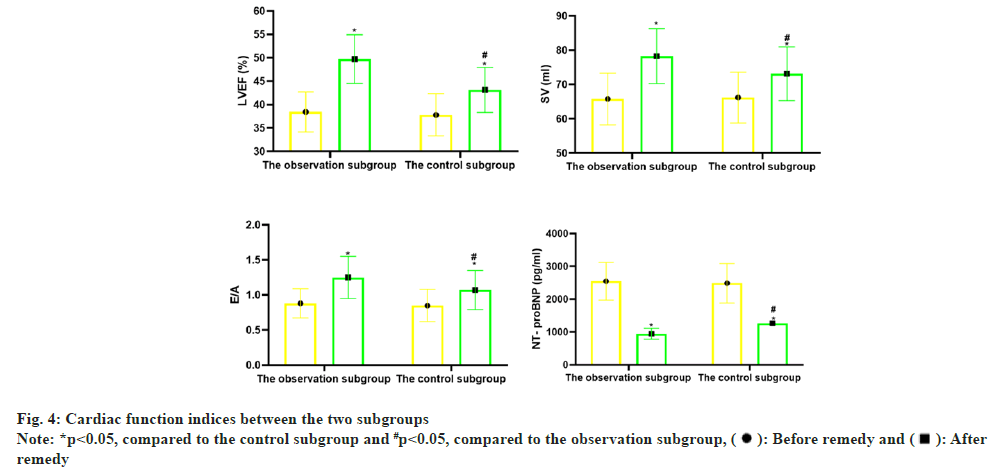
Before remedy, there was no notable distinction in LVEF, SV, E/A and NT proBNP between the two subgroups (p>0.05); vs. before remedy, LVEF, SV, E/A in the two subgroups increased notablely after remedy (p<0.05), and NT-proBNP declined notablely (p<0.05); vs. the control subgroup, the changes of the above indicators in the observation subgroup after remedy were greater (p<0.05) as shown in Table 4 and fig. 4.
| Subgroup | n | LVEF (%) | SV (ml) | E/A | NT- proBNP (pg/ml) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Before remedy | After remedy | Before remedy | After remedy | Before remedy | After remedy | Before remedy | After remedy | ||
| Observation subgroup | 60 | 38.45±4.26 | 49.74±5.20* | 65.81±7.55 | 78.29±8.01* | 0.88±0.21 | 1.25±0.30* | 2549.50±576.22 | 946.37±162.55* |
| Control subgroup | 60 | 37.81±4.50 | 43.16±4.79* | 66.23±7.40 | 73.14±7.85* | 0.85±0.23 | 1.07±0.28* | 2487.35±600.41 | 1268.00±210.25* |
| t | 0.800 | 7.209 | 0.308 | 3.557 | 0.746 | 3.398 | 0.578 | 9.374 | |
| p | 0.425 | <0.001 | 0.759 | 0.001 | 0.457 | 0.001 | 0.564 | <0.001 | |
Note: *p<0.05, comparison of observation subgroup vs. control subgroup after remedy
Table 4: Comparison of Cardiac Function Indices (x̄±s)
Before remedy, there was no notable distinction between the two subgroups in CD3+, CD4+, CD8+ and the ratio of CD4+/CD8+ (p>0.05); vs. before remedy, the ratios of CD3+, CD4+and CD4+/CD8+ in the two subgroups increased notablely after remedy (p<0.05), while the ratio of CD8+ declined (p<0.05); vs. the control subgroup, the changes of the above indicators in the observation subgroup after remedy were greater (p<0.05) as confirmed in Table 5.
| Subset | n | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Before remedy | After remedy | Before remedy | After remedy | Before remedy | After remedy | Before remedy | After remedy | ||
| Observation subgroup | 60 | 50.12±4.30 | 58.87±6.20 | 34.45±3.28 | 38.62±3.70 | 27.86±2.79 | 22.43±1.85 | 1.24±0.32 | 1.72±0.40 |
| Control subgroup | 60 | 50.07±4.18 | 54.76±5.57 | 34.01±3.14 | 36.52±3.46 | 27.60±2.55 | 25.13±1.54 | 1.23±0.35 | 1.45±0.38 |
| t | 0.065 | 3.82 | 0.751 | 3.211 | 0.533 | 5.689 | 0.163 | 3.791 | |
| p | 0.949 | <0.001 | 0.454 | 0.002 | 0.595 | <0.001 | 0.871 | <0.001 | |
Table 5: Comparison of Immune Function Indexes (x̄±s)
There were 2 patients having skin rash, 3 patients having damaged liver functioning, 1 patient with leukopenia in the observation subgroup, and the total adverse reaction was 10.00 % (6/60) vs. the control subgroup which had 2 patients having skin rash, 2 patients having damaged liver cell, and the total adverse reaction was 6.67 % (4/56). The distinction was not statistically notable (χ2=0.436, p=0.509).
According to the location and etiology, hyperthyroidism can be divided into two categories; primary hyperthyroidism and central hyperthyroidism, in which primary hyperthyroidism can be divided into autoimmune hyperthyroidism (Graves's disease), thyroid autonomic hyper functional adenoma, multinodular toxic goiter and iodine hyperthyroidism[13]. Of all types of hyperthyroidism, 80 % are autoimmune hyperthyroidism. Clinical studies have found that Thyroid Stimulating Antibody (TSAB) is the main pathogenic factor of autoimmune diseases, which can induce hyperthyroidism by activating TSH receptor, causing thyroid gland to synthesize and secrete a large amount of thyroid hormone to induce hyperthyroidism[14,15]. Some studies have found that too much thyroid hormone will have some effects on the heart[16-18], causing cardiomyopathy, arrhythmia, heart failure, etc. With the increase of age, the prevalence of cardiovascular diseases caused by hyperthyroidism has increased year by year, and the number of hyperthyroidism deaths has also increased notably[19]. Heart failure has become a major global public health problem[20], but little attention has been paid to people such as hyperthyroidism merged with heart failure, so it is of great significance to explore the medication scheme of hyperthyroidism merged with heart failure.
The results of this study showed that the total effective rate of the observation subgroup was notablely higher than that of the control subgroup, suggesting that tapazole integrated with anti-heart failure drugs can improve the therapeutic effect of hyperthyroidism merged with heart failure. Hyperthyroidism is mainly manifested by excessive thyroid hormone secretion, and the remedy principle is to regulate the thyroid hormone concentration[21]. Thiourea drugs (Including methimazole and propylthiouracil) are commonly used in clinical remedy of hyperthyroidism. Generally, imidazole is the first choice. Tabazol is the representative of imidazole drugs. The mechanism of action is to reduce the thyroid hormone concentration by reducing the activity of thyroid endoperoxide, preventing the further oxidation of iodide, and blocking the coupling of tyrosine to reduce thyroid hormone concentrations[22,23]. Heart failure refers to the dysfunction of cardiac systolic and diastolic functions, which leads to the reduction of ventricular pumping function, the aggravation of myocardial load and the reduction of cardiac output. ARNI+BB+MRA are the three cornerstones for clinical remedy of heart failure. Long term regular medication can reduce the heart load and improve the heart function of subjects[24]. SGLT2I daggligin is a new hypoglycemic drug, which can improve left ventricular diastolic function, delay myocardial fibrosis, and inhibit myocardial remodeling[25]. In recent years, studies have shown that daggligin can reduce the incidence of major cardiovascular events and the risk of hospitalization and death of subjects with heart failure[26], and it is recommended as a class of drugs in the new version of the heart failure guidelines. The combination of anti-heart failure drugs and tapazole can play a synergistic role in further improving the clinical symptoms of subjects and improving the therapeutic effect. In this study, the indexes of thyroid function and heart function in the observation subgroup were notablely better than those in the control subgroup, which also showed that the combination of drugs could enhance the therapeutic effect. Related studies also show that BB can not only inhibit the activity of sympathetic adrenoceptor[27,28], reduce the release of catechol, improve the symptoms such as fidgety mood, muscle tremor and tachycardia, but also inhibit the transformation between peripheral T4 and T3, and reduce the myocardial damage caused by thyroid hormone. Another study shows that hyperthyroidism is a high-risk factor for atrial fibrillation[29,30]. On the one hand, tapazole can maintain TSH in a normal range and reduce the occurrence of atrial fibrillation; on the other hand, it can also reduce the damage of thyroid hormone to myocardial cells and improve the myocardial hypertrophy caused by T3.
Researches have shown that humoral immunity and cellular immunity are involved in the pathogenesis of hyperthyroidism[31,32], and the immune function of subjects is obviously abnormal. In this study, the ratio of CD3+, CD4+ and CD4+/CD8+ in the observation subgroup was notablely higher than that in the control subgroup, while the concentration of CD8+ in the observation subgroup was notably lower than that in the control subgroup, indicating that methimazole integrated with anti-heart failure drugs can enhance the immune function of subjects. At present, the relevant evidence shows that tapazole can play an indirect immunomodulatory effect through thyroid cells, and the expression of First apoptosis signal Ligand (FasL) in thyroid cells interfered by tapazole is increased, which proves that tapazole can inhibit T lymphocyte apoptosis through FasL pathway[33]. The common adverse reactions of tapazole are liver function damage, peripheral blood leucopenia, allergic rash, etc.,[34]. vs. the control subgroup, the incidence of adverse reactions in the observation subgroup did not increase notablely, indicating that the combination of tapazole and anti-heart failure drugs is safe and controllable, and will not increase the side effects of drugs.
To sum up, tapazole integrated with anti-heart failure drugs has a notable effect in the remedy of hyperthyroidism with heart failure, which can effectively reduce the serum thyroid hormone concentration of subjects, improve cardiac function, enhance cellular immune function, and will not increase the side effects of drugs.
Conflict of interests:
The authors declared no conflict of interests.
References
- Li D, Hao G, Liu K, Woodward L, Ioannides D, Lu CJ, et al. A Novel role for B-type natriuretic peptide and phosphodiesterase 2A in cardiac sympathetic neurons from prehypertensive rats. Biophys J 2015;108(2):106a-7.
- Smith TJ, Hegedüs L. Graves’ disease. New Engl J Med 2016;375(16):1552-65.
- Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European thyroid association guideline for the management of Graves’ hyperthyroidism. Europ Thyroid J 2018;7(4):167-86.
[Crossref] [Google Scholar] [PubMed]
- Niculescu DA, Dusceac R, Galoiu SA, Capatina CA, Poiana C. Serial changes of liver function tests before and during methimazole remedy in thyrotoxic patients. Endocrine Pract 2016;22(8):974-9.
[Crossref] [Google Scholar] [PubMed]
- Cervilla-Muñoz E, Bringas-Beranek M, Demelo-Rodríguez P. Iatrogenic hyperthyroidism can be a triggering factor for takotsubo cardiomyopathy. Med Clin 2020;155(1):42-3.
[Crossref] [Google Scholar] [PubMed]
- McMurray JJ, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381(21):1995-2008.
[Crossref] [Google Scholar] [PubMed]
- Solomon SD, McMurray JJ, Anand IS, Ge J, Lam CS, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381(17):1609-20.
[Crossref] [Google Scholar] [PubMed]
- Cassis P, Locatelli M, Cerullo D, Corna D, Buelli S, Zanchi C, et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 2018;3(15):e98720.
[Crossref] [Google Scholar] [PubMed]
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017;27(3):315-89.
[Crossref] [Google Scholar] [PubMed]
- Ge JB, Xu YJ, Wang C. Internal Medicine. 9th ed. People's Health Publishing Society: Beijing; 2018. p. 680-94.
- Heart failure group of cardiology branch of Chinese medical association, heart failure professional committee of Chinese medical doctor association, and editorial committee of Chinese journal of cardiology Chinese guidelines for the diagnosis and remedy of heart failure 2018. Chin J Cardiovasc Dis 2018;46(10):760-89.
- Chen MY, Zhang H, Xu W. Efficacy of Shakuba valsartan integrated with furosemide in the remedy of chronic heart failure. Progress Mod Biomed 2019;19 (22):4295-8.
- Luo H, Xian X, Zou S. Study on the effect of diet nursing for patients with hyperthyroidism and diabetes mellitus. Contemporary Med Sympos 2019;17(7):274-6.
- Biondi B, Bartalena L, Cooper DS, Hegedus L, Laurberg P, Kahaly GJ. The 2015 European thyroid association guidelines on diagnosis and remedy of endogenous subclinical hyperthyroidism. Eur Thyroid J 2015;4(3):149-63.
[Crossref] [Google Scholar] [PubMed]
- Wongcharoen W, Lin YJ, Chang SL, Lo LW, Hu YF, Chung FP, et al. History of hyperthyroidism and long-term outcome of catheter ablation of drug-refractory atrial fibrillation. Heart Rhythm 2015;12(9):1956-62.
[Crossref] [Google Scholar] [PubMed]
- Klein I, Danzi S. Thyroid disease and the heart. Circulation 2007;116(15):1725-35.
[Crossref] [Google Scholar] [PubMed]
- Golub D, Rodack V. Antipsychotics in hyperthyroid-related psychosis: Case report and systematic review. Neuroendocrinol Lett 2018;39(1):65-74.
[Google Scholar] [PubMed]
- Li DW. Effect of β-blockers in treating hyperthyroid heart disease and heart failure. J China Presc Drug 2018;18(5):68-9.
- Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: A cross-sectional study in 10 cities. Thyroid 2016;26(8):1125-30.
[Crossref] [Google Scholar] [PubMed]
- Sheng M, Tu W. Trends and reflections on the construction of public health discipline in China under the new situation. Int J General Pract 2023;3(3):229-40.
- Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol 2017;14(1):39-55.
[Crossref] [Google Scholar] [PubMed]
- Burch HB, Cooper DS. Management of graves’ disease: A review. JAMA 2015;314(23):2544-54.
[Crossref] [Google Scholar] [PubMed]
- Vargas-Uricoechea H, Bonelo-Perdomo A. Thyroid dysfunction and heart failure: Mechanisms and associations. Curr Heart Fail Rep 2017;14:48-58.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zhang J, Butler J, Yang X, Xie P, Guo D, et al. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: Results from the China heart failure (China-HF) registry. J Cardiac Fail 2017;23(12):868-75.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Rong C, Yao W, Zhang Y, Bai W, Jia Y, et al. Novel advances in the mechanism of cardiovascular benefit of sodium-glucose co-transporter 2 inhibitors. Chin General Pract 2021;24(3):272.
- Jackson AM, Dewan P, Anand IS, Belohlavek J, Bengtsson O, de Boer RA, et al. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA-HF. Circulation 2020;142(11):1040-54.
[Crossref] [Google Scholar] [PubMed]
- Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GY, et al. Safety and efficacy of digoxin: Systematic review and meta-analysis of observational and controlled trial data. BMJ 2015;351:1-9.
[Crossref] [Google Scholar] [PubMed]
- Yucel OE, Eraydin B, Niyaz L, Terzi O. Incidence and risk factors for retinopathy of prematurity in premature, extremely low birth weight and extremely low gestational age infants. BMC Ophthalmol 2022;22(1):367.
[Crossref] [Google Scholar] [PubMed]
- Reddy V, Taha W, Kundumadam S, Khan M. Atrial fibrillation and hyperthyroidism: A literature review. Indian Heart J 2017;69(4):545-50.
[Crossref] [Google Scholar] [PubMed]
- Zheng JL, Dai HL, Zhang XJ. The study of the pathogenesis of hyperthyroidism-induced atrial fibrillation and atrial sympathetic nerve remodeling. China Med Herald 2019;16(5):9-13.
- Li JH, Xiang XY. Clinical study of compound hyperthyroidism decoction integrated with western medicine on hyperthyroidism and its effect on immune function. J Sichuan Tradit Chin Med 2022;40(2):115-8.
- Furniss GO, Panagopoulos D, Kanoun S, Davies EJ, Tomlinson DR, Haywood GA. The effect of atrial fibrillation ablation techniques on P wave duration and P wave dispersion. Heart Lung Circulation 2019;28(3):389-96.
[Crossref] [Google Scholar] [PubMed]
- Kawakami A, Matsuoka N, Tsuboi M. CD4+ T cell-mediated cyto-toxicity toward thyrocytes: The importance of Fas/FasL ligand interaction inducing apoptosis of thyrocytes and the inhibitory effect of thyroid stimulating hormone. Lab Invest 2000;80(5):471-84.
- Vyas AA, Vyas P, Fillipon NL, Vijayakrishnan R, Trivedi N. Successful remedy of thyroid storm with plasmapheresis in a patient with methimazole-induced agranulocytosis. Endocr Pract 2010;16(4):673-6.
[Crossref] [Google Scholar] [PubMed]
 : Observation subgroup and
: Observation subgroup and  : Control subgroup
: Control subgroup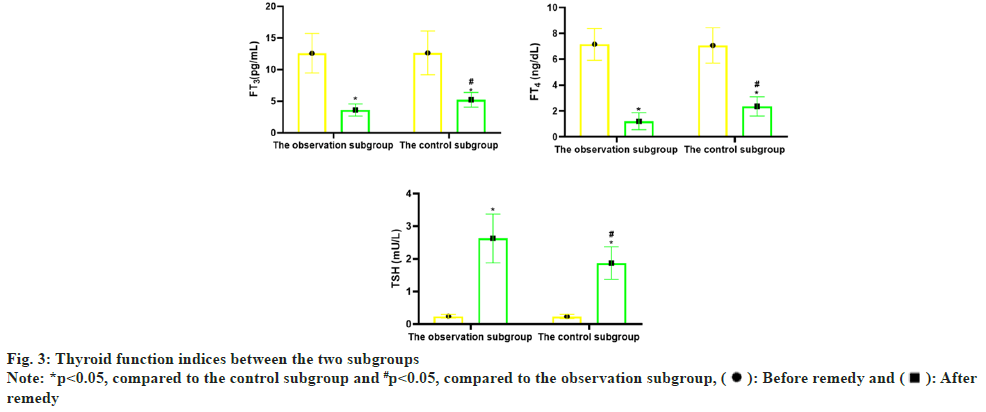
 : Before remedy and
: Before remedy and  : After
remedy
: After
remedy