- *Corresponding Author:
- Shijin Bu
Jiangsu Co-Innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou 225009, China
E-mail: yubonky@163.com
| This article was originally published in a special issue,“Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “392-402” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To determine the optimal dosage regimen for compound Kushen powder in treating white scour of piglets, a dose screening trial and field test were conducted, providing a scientific basis for its safe clinical application. In the dose screening trial, 100 piglets naturally affected by white scour were randomly divided into five groups. Compound Kushen powder was administered orally in three different dosage groups twice a day for five consecutive days, in addition to a positive drug control group, an infection control group, and a blank control group. The clinical efficacy of compound Kushen powder in treating white scour of piglets was evaluated by calculating the therapeutic index based on the score of symptoms. In the field efficacy test, 60 naturally affected piglets were randomly divided into two groups, with 30 animals in each group. Compound Kushen powder was orally administered twice a day at a dose of 0.5 g/kg bw/d for 5 d. The therapeutic index was calculated based on the clinical symptoms of white scour in the piglets, and the clinical efficacy of the compound Kushen powder was verified. The results showed that the cure rates of the high and medium dose groups of compound Kushen powder after 5 d of administration were 85.0 % and 80.0 %, which were significantly higher than those of the positive control group (Zhilisan) and the low dose group (p<0.05). There was no significant difference in the overall effectiveness rate among the high-dose group, the intermediate-dose group, and the positive drug control group (p>0.05). In the field efficacy test, the cure rate of the compound Kushen powder treatment was 80.0 %, and the overall effectiveness rate was 93.3 %, slightly surpassing the cure rate of 70.0 % and the overall effectiveness rate of 86.73 % in the positive drug control group. The average symptom scores in the compound Kushen powder treatment group were lower than those in the positive drug control group, but the difference was not significant (p>0.05). From the 4th d onwards, the symptom scores in the high and medium-dose groups were significantly lower than those in the positive drug control group (p<0.05). The results indicated that the compound Kushen powder, at the clinically recommended dose of 0.5 g/kg bw/d, administered twice daily for five consecutive days, had a better therapeutic effect on white scour of piglets, and was superior to the positive control drug in alleviating or eliminating the symptoms of white scour of piglets.
Keywords
White scour, antibiotics, Kushen powder, piglets, diarrhea, dose screening, field efficacy
White scour of piglets is a bacterial diarrhea epidemic disease of piglets aged 10 d to 30 d caused by pathogenic Escherichia coli (E. coli). It is clinically characterized by diarrhea and the discharge of grayish-white porridge feces[1-3]. Antibiotics and chemical antibacterial drugs are commonly used in the clinical treatment of white scour of piglets. However, with the extensive and irrational use of antibiotics, the number of drug-resistant strains of E. coli has gradually increased, leading to the emergence of multidrug-resistant strains[4-6]. In July 2020, anti-antibiotic measures or ban on antibiotics were implemented comprehensively in livestock and poultry feeds in China, banning the addition of antibiotics such as aureomycin to feed[7], it has become one of the research hotspots to develop Chinese veterinary medicine with controlled safety, effectiveness, and quality as a replacement for antibiotics[8,9].
The compound Kushen powder developed in this study is based on the theoretical formulations of traditional Chinese veterinary medicine, which uses the traditional Guizhou formula (Sophora flavescens (S. flavescens), pomegranate peel, and fraxini cortex) for treating porcine white scour. Our previous work has found that the optimal formula combination of S. flavescens, pomegranate peel, and fraxini cortex was 3:3:2, which could be produced by industrial techniques such as extraction, concentration, and spray drying[10]. Pharmacological tests showed that the high, medium, and low dose groups of compound Kushen powder effectively reduced diarrhea induced by castor oil in mice. Additionally, it increased the gastric residual rate and decreased the intestinal propulsive rate. Moreover, it notably inhibited the intestinal peristalsis induced by neostigmine, indicating strong acute anti-inflammatory activity[10]. The Lethal Dose 50 (LD50) for mice is 28379 mg/kg, the 30 d feeding test of compound Kushen powder showed that it has high safety and is a low-toxic and safe Chinese veterinary medicine preparation suitable for clinical application[11].
This study aims to identify the optimal dosage regimen for treating white scour of piglets with compound Kushen powder. Firstly, we performed dose screening experiments to determine the dosage regimen in field efficacy trials. Subsequently, the effectiveness of the compound preparation is further confirmed by field efficacy trials, providing a scientific basis for safe medication in the clinical setting
Materials and Methods
Test drugs and reagents:
Compound Kushen powder (batch #170802) was produced on a pilot scale by Chengdu Animal Pharmaceutical Co., Ltd., (Chengdu, China). The compound Kushen powder had a 1-fold formula amount of S. flavescens 750 g, pomegranate peel 750 g, and fraxini cortex 500 g. The commercial drug Zhilisan powder (batch #20170205) was purchased from Zhengzhou Hongsheng Biotechnology Co., Ltd., and served as a positive control drug group.
MacConkey agar and LB nutrient broth medium were purchased from Haibo Bio-technology Co., Ltd., (Qingdao, China). E. coli O antigen single factor serum was purchased from the China institute for veterinary drugs control. Monoclonal antibody against E. coli F4 pili was donated by Professor Gao from the college of veterinary medicine, Yangzhou university. Enterobacteriaceae microbiochemical fermentation tubes were purchased from Tianhe microbial reagent Co., Ltd., (Hangzhou, China). TRIzol reagent and SuperScript® III reverse transcriptase reagent kit were purchased from Invitrogen (Beijing, China). Deoxyribonucleic Acid (DNA) Marker DL2000, 2×Taq Polymerase Chain Reaction (PCR) mix, was purchased from Tiangen Biotech Co., Ltd., (Beijing, China). The pig Tumor Necrosis Factor-Alpha (TNF-α), Interleukin-1 Beta (IL-1β), and IL-10 Enzyme-Linked Immunosorbent Assay (ELISA) antibody detection kits were acquired from enzyme-linked Biotechnology Co., Ltd., (Shanghai, China). All other conventional reagents used were of domestic analytical grade.
Animals:
Equal numbers of male and female, clean-grade Institute of Cancer Research (ICR) mice, weighing (18-22) g, were purchased from the Experimental Animal Center of Yangzhou University. Duroc- Landrace-Yorkshire crossed lean-type piglets (15- 21) d old, (with white scour but no infection with other diarrhea pathogens), weighing 3.0-5.0 kg, were selected for the dose selection experiment. Healthy piglets of the same age, equally divided between males and females, weighing 3.5-6.0 kg, were included as the control. All piglets were provided by the Jiangsu Tai’an Pig Farm (Taizhou, Jiangsu).
Duroc-Landrace-Yorkshire crossed lean-type piglets naturally affected with white scour were selected for the field efficacy trials. As the range of onset age of white scour of piglets is wide, usually 10 d-30 d old, the selection of piglets for field efficacy trials was divided into groups based on the age of piglets; (15- 21) d old group and (22-30) d old group. Each group has 15 piglets naturally affected with white scour (no infection with other diarrhea pathogens), weighing 3.8-7.0 kg, provided by Tai’an Pig Industry Co., Ltd., (Taizhou, Jiangsu).
Synthesis of primers:
Primers were designed and synthesized based on the Transmissible Gastroenteritis Virus (TGEV), Porcine Epidemic Diarrhea Virus (PEDV), and PRoV specific gene sequences in GenBank. The primers were synthesized by Takara Bio (Dalian) Co., Ltd. The complementary DNA (cDNA) of TGEV, PEDV, and PRoV viruses were stored at Guizhou livestock and poultry major disease monitoring and prevention laboratory (Guiyang, China).
Reverse Transcription-PCR (RT-PCR):
Select the piglets with typical white scour symptoms, and choose three per litter. Collect rectal fecal samples from sick piglets, extract total Ribonucleic Acid (RNA) using TRIzol, and perform TGEV, PEDV, and PRoV virus RT-PCR detection.
Isolation and identification of E. coli from natural cases of white scour of piglets:
Sterile cotton swabs were used to collect fecal samples from the rectum of the sick piglets, which were inoculated onto MacConkey medium and incubated at 37° for (18-24) h. E. coli was isolated and identified using gram staining and biochemical fermentation tubes for Enterobacteriaceae microorganisms. The agglutination test was performed using E. coli O antigen single factor diagnostic serum, and the pilus identification was conducted using F4 pilus monoclonal antibody.
After pure culture, each identified E. coli was diluted to 5×109 cfu/ml. 0.2 ml of each mouse was used for the abdominal cavity, and the dead mice were isolated and identified for E. coli.
Grouping of dose screening trial:
A total of 100 naturally infected white scour piglets that met the inclusion criteria were selected. The piglets in the same litter were randomly divided into five groups with 20 animals in each group; the high-dose group (1.0 g/kg bw/d), medium-dose group (0.5 g/kg bw/d), low-dose group (0.25 g/kg bw/d), positive drug control group (Zhili powder), and infection control group were set up. Meanwhile, 20 healthy piglets of similar age from the same farm were selected as a blank control group. Group information was kept undisclosed to researchers involved in processes of observation and evaluation to prevent bias during scoring throughout the whole experiment. Researchers who were not blinded were not allowed to discuss the group assignments with those conducting daily observations, clinical assessments, or autopsies.
Efficacy evaluation criteria of dose screening trial:
According to the literature[12], observation was made on the mental and appetite status of the piglets, as well as the fecal morphology and color. Additionally, any recurrence of diarrhea symptoms in the piglets was also noted and the symptoms were accumulated for analysis. Statistical calculations were conducted to determine the efficacy index, rates of inefficiency, efficiency, significant efficiency, and rates of recovery for each group of piglets.
Therapeutic index=(Symptom score before treatment-symptom score after treatment)/symptom score before treatment×100 %
Inefficiency=(Number of failures/total number of animals)×100 %
Efficiency=(Number of successes/total number of animals)×100 %
Marked efficiency=(Number of animals showing marked improvement/total number of animals)×100 %
Recovery rate=(Number of recovered animals/total number of animals)×100 %
And the dead animals are considered as invalid animals, and the efficiency+effectiveness+significant efficiency+recovery rate=100 %.
Changes in body weight gain rate of dose screening trial:
Body weight was measured before drug administration, at the end of drug administration, and the end of the observation period. The weight gain rates between each group were calculated.
Weight gain rate=(Weight at the end of experimentweight before experiment)/weight before experiment×100 %
Levels of serum cytokines of dose screening trial:
After the end of the treatment trial, blood was collected from the anterior vena cava of each experimental group to isolate serum. Then, the levels of TNF-α, IL-1β, and IL-10 in the serum were measured using ELISA.
Pathological observation of dose screening trial:
After 24 h of the end of the 5 d treatment, two piglets were randomly culled from each group and were evaluated based on the macroscopic pathological changes of the intestines and tissue samples.
Grouping and treatment selection of field efficacy trial:
After being numbered and weighed, 60 naturally infected piglets with white scour were randomly divided into two groups, the compound Kushen powder group and the Zhili powder group (positive drug control), with 30 animals in each group. All dose groups were administered twice daily for five consecutive days.
Clinical observation indicators and efficacy score of field efficacy trial:
The efficacy evaluation criteria and weight gain rate used in the field efficacy trial are the same as those described in the dose screening trials above.
Statistical analysis:
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 25.0. The data are presented as the mean±standard deviation of three replicate measurements for each sample. The significance of the test results was determined through independent sample t-tests and Chi-square (χ2) tests. Significant differences were determined at p<0.05, and no significant differences were determined at p>0.05.
Results and Discussion
The 160 piglets from 36 litters showed obvious clinical symptoms of white scour. All the sick piglets presented diarrhea with milk-white or gray-white feces was found in all the sick piglets, some of which displayed symptoms of depression, slow movements, and decreased appetite (fig. 1).
Out of the 36 litters of piglets, 108 piglets underwent RT-PCR tests to detect the presence of TGEV, PEDV, and PRoV. As a result, none of the tested piglets from the diarrheic litters were infected with these three viruses.
Sterile cotton swabs were used to collect fecal samples from the rectum of three sick piglets per litter from a total of 36 l. 108 strains of E. coli were isolated, as shown in Table 1.
| Detection project | Separate bacteria (strain) | Gram negative (strain) | Positive biochemical test (strain) | Negative biochemical test (strain) | O8 serotype (strain) | O138 serotype (strain) | F4 pilus (strain) |
|---|---|---|---|---|---|---|---|
| Acid and gas production, lactose and glucose production, β-galactosidase, methyl red, indole | Acetyl methyl methanol, hydrogen sulfide, citrate, decomposed urea, potassium cyanide | ||||||
| Detection result | 108 | 108 | 108 | 108 | 71 | 37 | 108 |
Table 1: Results of Bacterial Isolation and Identification
The selected isolated O8 and O138 serotypes of E. coli were inoculated into ICR mice and subsequently died within 30 h. E. coli with the same serotype as the challenge strain was isolated from the livers of the dead mice, while the negative control group showed no abnormalities. The clinically selected cases were confirmed to be caused by pathogenic E. coli (O8:F4, O138:F4).
As shown in Table 2, the cure rates of the highdose and medium-dose groups of compound Kushen powder after 5 d of administration were 85.0 % and 80.0 %, respectively. There was no significant difference between the two groups (p>0.05), and both were significantly higher than the positive drug control group and low-dose group (p<0.05). The total effective rate was significantly higher in the highdose group, intermediate-dose group, and positive drug control group than that in the low-dose group (p<0.05).
| Group | Number of cured heads | Cure rat (%) | Total effective heads | Total apparent efficiency (%) | Total effective headers | Total effective rate (%) | Number of invalid headers | Inefficiency (%) |
|---|---|---|---|---|---|---|---|---|
| High dose | 17 | 85a | 19 | 95a | 19 | 95a | 1 | 5c |
| Medium dose | 16 | 80a | 19 | 95a | 19 | 95a | 1 | 5.0c |
| Low dose | 6 | 30b | 8 | 40c | 14 | 70b | 6 | 30b |
| Positive drug control | 8 | 40b | 15 | 75b | 20 | 100a | 0 | 0c |
| Infection control | 0 | 0c | 0 | 0c | 3 | 15c | 17 | 80a |
| Blank control | / | / | / | / | / | / | / | / |
Note: ap>0.05; bp<0.05 and cp>0.05
Table 2: Results of Cure Rate and Effective Rate in Dose Screening Test (N=20)
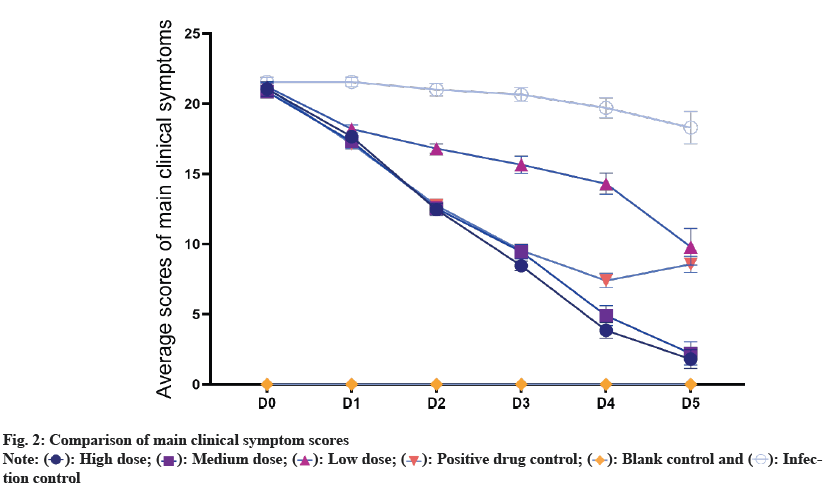
Statistical analysis of the daily symptom scores after medication (fig. 2) revealed that the high- and intermediate-dose groups of compound Kushen powder, as well as the positive drug control group, demonstrated significantly lower symptom scores from 2 d onwards compared to both the low-dose group of compound Kushen powder and the infection control group (p<0.05). From the 2nd d of treatment, the symptom scores of the high-dose group were lower than those of the intermediate-dose group, but this difference was not statistically significant (p>0.05). From 4 d, the symptom scores of both the high-dose and intermediate-dose groups were found to be significantly lower than those of the positive drug control group (p<0.05).
At the end of 5 d of treatment, the weight gain rates in the high and intermediate dose groups of compound Kushen powder and the positive drug control group were significantly higher when compared to the infection control group and low dose group (p<0.05). There was no significant difference in weight gain rate between the high dose group and the blank control group (p>0.05). The weight gain rate of the medium dose group was significantly lower than that of the blank control group (p<0.05). After the treatment, a follow-up observation was conducted for 7 d. The weight gain rates in the high-dose group, intermediate-dose group, and the positive drug control group were significantly higher compared to the infection control group and low-dose group (p<0.05). Moreover, the weight gain rates of these three groups were significantly lower than that of the blank control group (p<0.05). There was no significant difference in the weight gain rate among the high-dose group, intermediate-dose group, and the positive drug control group (p>0.05), as shown in Table 3.
| Group | Pre-medication weight (kg) | At the end of 5 d of medication | Observe for another 7 d | ||
|---|---|---|---|---|---|
| Weight (kg) | Weight gain rate (%) | Weight (kg) | Weight gain rate (%) | ||
| High dose | 3.75±0.87 | 4.53±0.88 | 21.84±6.29ab | 5.69±0.98 | 54.09±16.26b |
| Medium dose | 3.81±0.82 | 4.57±0.92 | 20.40±4.62b | 5.69±0.91 | 51.34±10.50b |
| Low dose | 3.63±0.85 | 3.98±0.92 | 9.77±7.79c | 4.97±1.01 | 38.09±11.03c |
| Positive drug control | 4.03±0.90 | 4.76±1.04 | 18.45±3.21b | 5.96±1.08 | 49.84±10.78b |
| Infection control | 3.80±0.66 | 3.85±0.75 | 1.20±8.14d | 1.20±0.59 | 32.82±16.47c |
| Blank control | 4.10±0.37 | 5.13±0.46 | 25.21±9.87a | 6.93±0.58 | 69.02±13.31a |
Note: ap>0.05; bp<0.05; cp>0.05 and dp<0.05
Table 3: Body Weight Changes of Piglets in the Dose Screening Experiment (N=20)
Fig. 3 demonstrates that the levels of TNF-α and IL- 1β in the serum of the high and medium dose groups, as well as the positive drug control group, were significantly lower than those in the infection control group (p<0.05), but higher than those of the blank control group (p<0.05). The levels of IL-10 in the serum of the high-dose group, medium-dose group, and positive drug control group were significantly higher compared to the infection control group (p<0.05). Furthermore, there was no significant difference in the levels of IL-10 in the serum of the high dose group and the positive drug control group compared to the blank control group (p>0.05). Similarly, no significant difference in the levels of TNF-α, IL-1β, and IL-10 in the serum between the low dose group and the infection control group was observed (p>0.05).
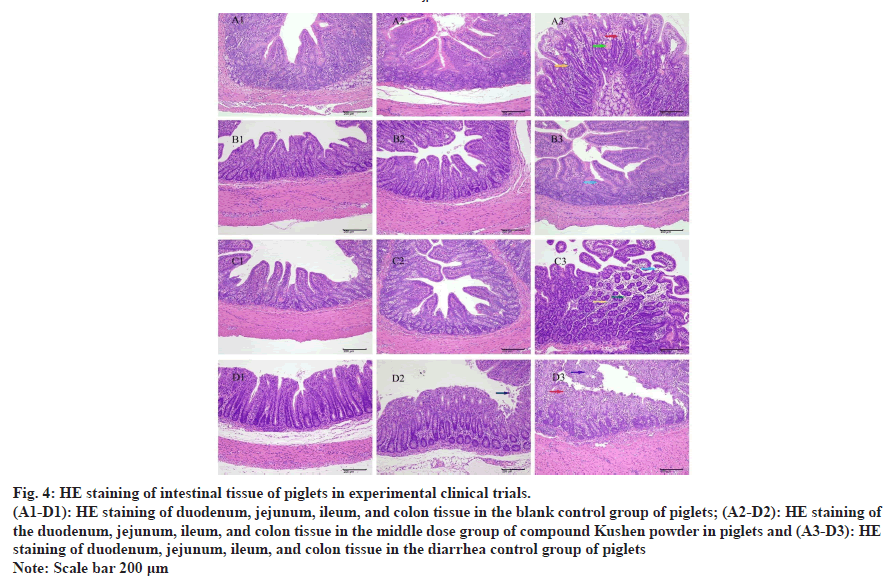
HE staining pathological sections, which were obtained from the duodenum, jejunum, ileum, and colon tissues of the piglets in the middle dose group, the blank control group, and the infection control group, were examined under a microscope as shown in fig. 4 and Table 4.
| Sample | Blank control group | Compound Kushen powder treatment test group (medium dose) | Infection control group |
|---|---|---|---|
| Duodenum | The mucosa, submucosa, muscularis, and adventitia are arranged neatly | Duodenal structure was clear without characteristic lesions | Duodenal mucosal lamina propria edema, lymphocytosis, vascular congestion and expansion |
| Jejunum | The villi were neatly arranged and the submucosa structure was clear | The villus structure was relatively intact without characteristic lesions | The villus structure was relatively intact, and the number of goblet cells was reduced |
| Ileum | Epithelial cells were arranged neatly without swelling and bleeding | The villus structure is relatively complete, without characteristic lesions | Intestinal villi were broken and shed, mucosal lamina propria edema, and lymphocytosis |
| Colon | Complete organizational structure and orderly arrangement | The intestinal tissue was relatively intact, and a small amount of mucosal epithelial cells fell off | Mucosal epithelial cells shed, and cells in the lamina propria degenerated and necrotic |
Table 4: Pathological Observation Results Of Integral Tissue In Dose Screening Test
A total of 60 piglets from 12 litters with obvious clinical symptoms of white scour were observed to have milky white or grayish-white paste-like feces with stench smells, which adhered to the ventral side of the tail root and around the anus. Some of the piglets showed signs of depression, decreased appetite, arched back, cold intolerance, and slow movement. Moreover, their coats became rough, disheveled, and lackluster.
As shown in Table 5, the cure rate and the total effective rate of compound Kushen powder were 80.0 % and 93.3 % after 5 d of administration, respectively, which were slightly higher than the cure rate of 70.0 % and the total effective rate of 86.7 % in the positive drug control group (p>0.05).
| Group | Total number of heads | Cure number of heads | Cure rate (%) | Total effective number of heads | Total effective rate (%) | Invalid number of heads | Inefficiency (%) |
|---|---|---|---|---|---|---|---|
| Compound Kushen powder | 30 | 24 | 80a | 28 | 93.3a | 2 | 6.67 |
| Zhili powder | 30 | 21 | 70a | 26 | 86.7a | 4 | 13.33 |
Note:
Table 5: Cure Rate And Effective Rate In The Field Efficiency Trials
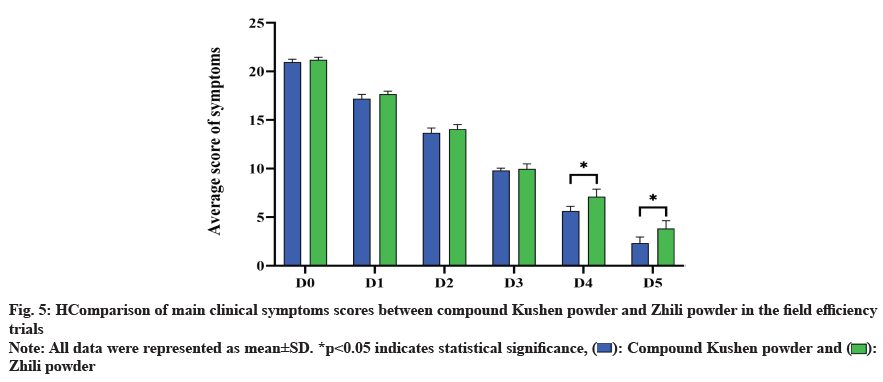
The results of fig. 4 showed that the average symptom scores of the compound Kushen powder and the positive drug control group were slightly lower than those of the positive drug control group during the first 3 d of treatment, but the difference was not significant (p>0.05). However, on d 4 and d 5, the average scores of symptoms were significantly lower than those of the positive drug control group (p<0.05).
Fig. 4: HE staining of intestinal tissue of piglets in experimental clinical trials.
(A1-D1): HE staining of duodenum, jejunum, ileum, and colon tissue in the blank control group of piglets; (A2-D2): HE staining of
the duodenum, jejunum, ileum, and colon tissue in the middle dose group of compound Kushen powder in piglets and (A3-D3): HE
staining of duodenum, jejunum, ileum, and colon tissue in the diarrhea control group of piglets Note: Scale bar 200 μm
As shown in fig. 5, comparison of main clinical symptoms scores between compound Kushen powder and Zhili powder in the field efficiency trials. All data were represented as means±Standard Deviation (SD) (*p<0.05).
Statistical analysis was conducted on the weight gain rate after 5 d of medication treatment and 7 d of observation (Table 6). As a result, at the end of the 5 d treatment, the weight gain rate of compound Kushen powder was significantly different from that of the positive drug control group (p<0.05). After 7 d of observation, the difference in weight gain between compound Kushen powder and the positive drug control group was not significant (p>0.05).
| Group | Pre-medication | At the end of 5 d of medication | Weight gain rate (%) | At the end of 7 d of observation | Weight gain rate (%) |
|---|---|---|---|---|---|
| Weight (kg) | Weight (kg) | Weight (kg) | |||
| Compound Kushen | 5.26±1.60 | 6.34±1.85 | 21.19±4.60a | 7.68±2.08 | 48.29±15.51a |
| Positive drug control | 5.43±1.38 | 6.30±1.51 | 16.81±5.54b | 7.88±1.86 | 46.13±15.16a |
Note: ap>0.05 and bp<0.05
Table 6: Weight Gain Rate Of Each Experimental Group (N=30)
Due to the presence of various active ingredients such as alkaloids, flavonoids, and tannins, Chinese herbal medicine has emerged as a promising research area in the context of combating antibiotic resistance and developing alternative products due to their potential to achieve multi- target therapeutic effects[13]. Currently, the research and development of traditional Chinese veterinary medicine formulations for treating white scour of piglets mainly focuses on clinical efficacy[14-16].
According to the compilation of technical guidance principles for veterinary drug research[17], either artificial diarrhea models or clinical cases are suitable for dose screening tests (experimental clinical trials). However, the selection of these clinical cases requires a combination of symptoms, bacterial isolation and identification, and antigen-serotype identification of the bacteria. When setting the case exclusion criteria, it is also necessary to exclude cases of piglet diarrhea that may be caused by TGEV, PEDV, and PRoV virus infections. However, it is challenging to make a differential diagnosis based solely on clinical symptoms, as mixed infections and overlapping symptoms may occur. Therefore, when selecting cases for clinical trials, it is necessary to exclude piglets that may be infected with TGEV, PEDV, and PRoV through laboratory pathogenic diagnosis. An selected piglets naturally infected with E. coli and excluded cases caused by TGEV, PEDV, and PRoV virus infections[18]. The effects of high, medium, and low dose groups of Kubaishi granules on diarrhea were studied. The results showed that the cure rate of pathogenic E. coli diarrhea in the high and medium dose groups was 85 %, and the cure rate in the low dose group was 80 %, which are all higher than the control drug Zhili granules that had 75 % of cure rate.
The findings of this study indicated that the administration of high and medium doses of compound Kushen powder had good therapeutic effects on white scour of piglets, featured with a shorter period of reduction or elimination of diarrhea symptoms, and a significantly better recovery compared to the positive control drug. The pathological observation of intestinal tissue suggested that compound Kushen powder effectively promoted the repair of the intestinal mucosa of diseased pigs by reducing the breakage and shedding of intestinal villi, minimizing the edema and cell degeneration and necrosis of the lamina propria, and decreasing vascular congestion and dilation.
Moreover, the results of the dose screening test showed that compound Kushen powder can effectively treat white scour of piglets when administered at high and medium doses twice daily for five consecutive days. However, the cure rate and overall efficacy between the high-dose group and the intermediate-dose group were similar (p>0.05). Under the principle of achieving therapeutic effects while reducing costs, the recommended medication regimen for treating white scour of piglets with compound Kushen powder is to administer a medium dose (0.5 g/kg bw/d) by gavage twice daily for five consecutive days. Additionally, this dose of compound Kushen powder was verified to have good safety and no adverse effect in our previous study[19].
During the onset of diarrhea, the intestinal immune system is activated. As a pro-inflammatory cytokine, TNF-α participates in the initiation and amplification of the inflammatory response[20]. In various experimental models of diarrhea, an up-regulation of TNF-α expression has been observed, which can lead to the destruction of the intestinal barrier and the induction of apoptosis in intestinal epithelial cells[21,22]. In addition, as inflammatory cytokines, the levels of IL-1β and TNF-α are elevated during the early stages of inflammation, accompanied with the occurrence of diarrhea. On the other hand, IL-10 is an anti-inflammatory cytokine that prevents excessive inflammation and tissue damage[23]. In this study, the effect of compound Kushen powder on inflammation in piglets was evaluated by detecting the levels of inflammatory cytokines TNF-α, IL-1β, and IL-10 in serum. The results suggested that the compound could downregulate the pro-inflammatory cytokines TNF-α and IL-1β in the serum of piglets, and increase the levels of anti-inflammatory factors, thereby reducing the occurrence of intestinal inflammation. The effective components of its action may be related to the compounds matrine, oxymatrine, esculin, aesculetin, and ellagic acid (tannins) contained in the compound Kushen powder, all of which have notable anti-inflammatory effects. Piglets orally inoculated with E. coli O149:F4 (1.0×109 CFU/ ml) were used for challenge tests to investigate the effects of a mixture of pomegranate peel and tea extract on piglets. The results demonstrated that the main components of the mixture of pomegranate peel and tea extract were ellagic acid and polyphenols, which significantly improved the intestinal health of weaned piglets and reduced the effects of E. coli challenge[24]. Zhang et al.[25] demonstrated that matrine and oxymatrine can suppress the production of TNF-α in mouse macrophages. Additionally, matrine can alleviate colonic injury and intestinal inflammation by improving the composition of the intestinal microbiota, reducing intestinal bleeding and diarrhea, and downregulating the expression of pro-inflammatory cytokines such as IL-1β and TNF-α[26]. Lu et al.[27] showed that the addition of ellagic acid to the diet of weaned piglets can effectively influence the intestinal immune response, reduce the occurrence of inflammatory reactions, and decrease the expression of the inflammatory cytokine TNF-α. Esculin and aesculetin from fraxini cortex have been displayed inhibitory effects on intestinal E. coli in animals, with aesculetin being the most effective against E. coli O157 strain[28]. However, due to the complex composition of compound Kushen powder, it is very challenging to clarify the pharmacological mechanism underlying the effects of compound Kushen powder against diarrhea. Further investigation is required to determine which specific component in the compound Kushen powder plays the key role in the therapeutic effects on white scour of piglets.
The results of the field efficacy test demonstrated that compound Kushen powder has the potential to rapidly improve the clinical symptoms of piglets and ultimately achieve a complete recovery. It further confirmed that the recommended clinical dose of compound Kushen powder is 0.5 g/kg bw/d, administered twice daily for five consecutive days, which has a favorable therapeutic effect on white scour of piglets.
Through dose screening experiments and field efficacy trials in the present study, it was demonstrated that the compound Kushen powder could effectively improve the clinical symptoms of white scour of piglets, probably by modulating the levels of inflammatory cytokines such as TNF-α, IL- 1β, and IL-10. In summary, these results established that the optimal dosage regimen for preventing and treating white scour of piglets was 0.5 g/kg bw/d, administered twice daily for five consecutive days, which exhibited considerable clinical efficacy in treating this condition.
Author’s contributions:
Conceptualization by Bo Yu and Shijin Bu; methodology by Sixuan Zhou and Bo Yu; investigation by Lingling Jiang and Bo Yu; resources done by Kaizhi Shi and Shijin Bu; data curation by Bo Yu and Yuanfeng Zhao; visualization by Yuanfeng Zhao; writing-original draft preparation by Bo Yu and Yuanfeng Zhao; writing-review and editing by Bo Yu, Yuanfeng Zhao and Shijin Bu and supervision by Shijin Bu. All authors have read and agreed to the published version of the manuscript. Bo Yu and Yuanfeng Zhao have contributed equally to this work.
Funding:
This research was funded by the project of Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions. And construction project of livestock and poultry germplasm resources innovation and basic science and technology platform of Guizhou Academy of Agricultural Sciences, Qiannongke Germplasm Resources (2023) No: 04.
Acknowledgments:
We thank all of the staff at the Jiangsu Co-Innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou, who were involved in the response to this work but are not listed as authors.
Conflict of interests:
The authors declared no conflict of interests.
References
- Martins RP, da Silva MC, Dutra V, Nakazato L, da Silva Leite D. Preliminary virulence genotyping and phylogeny of Escherichia coli from the gut of pigs at slaughtering stage in Brazil. Meat Sci 2013;93(3):437-40.
- Post KW, Bosworth BT, Knoth JL. Frequency of virulence factors in Escherichia coli isolated from pigs with postweaning diarrhea and edema disease in North Carolina. J Swine Health Production 2000;8(3):119-20.
- Fairbrother JM, Nadeau É, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Animal Health Res Rev 2005;6(1):17-39.
[Crossref] [Google Scholar] [PubMed]
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 2010;74(3):417-33.
[Crossref] [Google Scholar] [PubMed]
- Rozwandowicz M, Brouwer MS, Fischer J, Wagenaar JA, Gonzalez-Zorn B, Guerra B, et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother 2018;73(5):1121-37.
[Crossref] [Google Scholar] [PubMed]
- Arbab S, Ullah H, Wang W, Zhang J. Antimicrobial drug resistance against Escherichia coli and its harmful effect on animal health. Veterinary Med Sci 2022;8(4):1780-6.
[Crossref] [Google Scholar] [PubMed]
- Chang M, Li M, Li M, Xie Y, Li Y, Yang W, et al. Changes of gut microbiota in pregnant sows induced by 5-aminolevulinic acid. Res Veterinary Sci 2021;136:57-65.
[Crossref] [Google Scholar] [PubMed]
- Pi G, Wang J, Song W, Li Y, Yang H. Effects of isomalto-oligosaccharides and herbal extracts on growth performance, serum biochemical profiles and intestinal bacterial populations in early-weaned piglets. J Anim Physiol Anim Nutr 2022;106(3):671-81.
[Crossref] [Google Scholar] [PubMed]
- Sayed S, Alotaibi SS, El-Shehawi AM, Hassan MM, Shukry M, Alkafafy M, et al. The anti-inflammatory, anti-apoptotic, and antioxidant effects of a pomegranate-peel extract against acrylamide-induced hepatotoxicity in rats. Life 2022;12(2):224.
[Crossref] [Google Scholar] [PubMed]
- Yu B, Yu J, Jiang L, Chen X, Song X, Zhou S, et al. Antibacterial, antidiarrheal, anti-inflammatory and analgesic activities of compound Shikuqin powder. Pak J Pharm Sci 2019;32(3):1333-42.
[Google Scholar] [PubMed]
- Yu B, Chen X, Jiang L, Shang Y, Song X, Zhou S, et al. Acute and subchronic toxicity and the evaluation of safety pharmacology of Chinese herbal compound preparation" Shikuqin". Pak J Pharm Sci 2018;31(6SI):2855-63.
[Google Scholar] [PubMed]
- Zheng, H, Zhu MX, Zhang YF, Cao G. Study on the preventive effect of Lianmeizhili oral solution on hot and humid diarrhea in piglets. J Tradit Chin Veterinary Med Pharm 2015;34:27-30.
- Lin JH, Panzer R. Use of Chinese herbal medicine in veterinary science: History and perspectives. Rev Sci Tech 1994;13(2):425-32.
[Crossref] [Google Scholar] [PubMed]
- Guowang L, Zhiguo M, Junjie C. Observation on the efficacy of compound Zhili powder in treating white diarrhea in piglets. Heilongjiang Animal Husbandry Veterinary Med 2011.
- Liu N, Wu Y, Lu M, Deng F. Observation on the effect of Ma Zhen granules in treating yellow and white dysentery in piglets. China Anim Health Care 2020;22:61-2.
- Fang C, Zhang L. Study on the effects of compound preparations of traditional Chinese veterinary medicine on the production performance and diarrhea of weaned piglets. J Tradit Chin Veterinary Med 2021:3-5.
- Agriculture, collection of technical guidance principles for veterinary drug research. Veterinary drug evaluation center of the ministry of agriculture; 2012.
- An H, Xie X, Chen Z, Zhang Y, Yang J, Shi J, et al. Experimental clinical treatment trial of white scour of piglets by Kubaishi granules. Prog Veterinary Med 2020;41:131-6.
- Bo Yu, Yanan Z, Jinge Xu, Sixuan Z, Shijin Bu. Safety test of compound Kushen powder on target animal piglets. Heilongjiang Anim Sci Veterinary Med 2021.
- Silva I, Mendes P, Guerra S, Pinto R, Mateus V. Anti-inflammatory effect of topiramate in a chronic model of TNBS-induced colitis. Int J Mol Sci 2022;23(16):9127.
[Crossref] [Google Scholar] [PubMed]
- Watson AJ, Hughes KR. TNF-α induced intestinal epithelial cell shedding: Implications for intestinal barrier function. Ann N Y Acad Sci 2012;1258(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Woznicki JA, Saini N, Flood P, Rajaram S, Lee CM, Stamou P, et al. TNF-α synergises with IFN-γ to induce caspase-8-JAK1/2-STAT1-dependent death of intestinal epithelial cells. Cell Death Dis 2021;12(10):864.
[Crossref] [Google Scholar] [PubMed]
- Saraiva M, O'garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010;10(3):170-81.
[Crossref] [Google Scholar] [PubMed]
- Bontempo V, Jiang XR, Cheli F, Verso LL, Mantovani G, Vitari F, et al. Administration of a novel plant extract product via drinking water to post-weaning piglets: Effects on performance and gut health. Animal 2014;8(5):721-30.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Wang S, Li Y, Xiao Z, Hu Z, Zhang J. Sophocarpine and matrine inhibit the production of TNF-α and IL-6 in murine macrophages and prevent cachexia-related symptoms induced by colon26 adenocarcinoma in mice. Int Immunopharmacol 2008;8(13):1767-72.
[Crossref] [Google Scholar] [PubMed]
- Li P, Lei J, Hu G, Chen X, Liu Z, Yang J. Matrine mediates inflammatory response via gut microbiota in TNBS-induced murine colitis. Front Physiol 2019;10:28.
[Crossref] [Google Scholar] [PubMed]
- Lu Y, Zhao M, Mo J, Lan G, Liang J. Dietary supplementation ellagic acid on the growth, intestinal immune response, microbiota, and inflammation in weaned piglets. Front Veterinary Sci 2022;9:980271.
[Crossref] [Google Scholar] [PubMed]
- Duncan SH, Flint HJ, Stewart CS. Inhibitory activity of gut bacteria against Escherichia coli O157 mediated by dietary plant metabolites. FEMS Microbiol Lett 1998;164(2):283-8.
[Crossref] [Google Scholar] [PubMed]

 Infection
control
Infection
control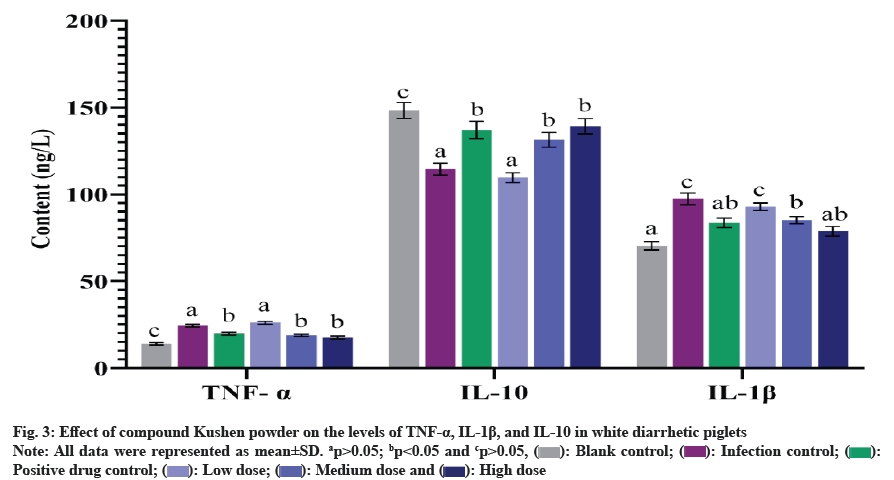

 : Compound Kushen powder and
: Compound Kushen powder and  :
Zhili powder
:
Zhili powder



