- *Corresponding Author:
- Chaoxiang Ren
Department of Clinical Medicine, DongYang People's Hospital, Dongyang 322100, Zhejiang Province, China
E-mail: ren_chaoxiang@163.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “1-7” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This paper analyzed the clinical efficacy of cisplatin intrapleural perfusion with pemetrexed disodium in the treatment of lung adenocarcinoma complicated with malignant pleural effusion and its influence on carcinoembryonic antigen levels in pleural effusion. 105 patients having lung adenocarcinoma with malignant pleural effusion who were admitted between July 2018 and July 2022 were selected and divided into research (n=55) and control group (n=50). Patients of the research group received cisplatin intrapleural perfusion with pemetrexed disodium and the control group patients were treated with carboplatin intrapleural perfusion along with pemetrexed disodium was given. Data of clinical efficacy, adverse reactions like fatigue, fever, gastrointestinal discomfort, decreased white blood cell count and reduced neutrophil count were analyzed. Similarly pleural effusion volume, pleural effusion tumor markers like carcinoembryonic antigen, neuron specific enolase and pulmonary function were comparatively analyzed. Further, maximal voluntary ventilation and forced expiratory volume in 1 s/forced vital capacity) were collected for comparative analysis. Results showed higher total effective rate of treatment with markedly lower incidence of adverse reactions and smaller pleural effusion volume in research group compared with control group. Besides, evidently reduced levels of pleural effusion tumor markers and elevated pleural effusion indices in research group vs. control group were determined after treatment. It is suggested that cisplatin intrapleural infusion with pemetrexed disodium is effective in the treatment of lung adenocarcinoma with malignant pleural effusion, which can significantly inhibit carcinoembryonic antigen levels, reduce pleural effusion volume and improve pulmonary function while ensuring treatment safety.
Keywords
Cisplatin, pemetrexed disodium, lung adenocarcinoma, pleural effusion, carcinoembryonic antigen
Malignant Pleural Effusion (MPE) is produced when metastatic cancer cells infiltrate thoracic lymph nodes and pleura, leading to fluid accumulation and reaction of tumors with immune cells, stroma and soluble factors, thus promoting malignant processes such as tumor proliferation and Epithelial-Mesenchymal Transition (EMT)[1,2]. MPE is common in patients with Lung Adenocarcinoma (LUAC), with a risk of up to 40 %, resulting in symptoms such as pain, chest tightness, shortness of breath, palpitation and inability to lie flat, which negatively affects the quality of life of patients resulting in adverse outcomes[3,4]. Common treatment strategies for patients with LUAC complicated with MPE include systemic chemotherapy, immunotherapy, molecular targeted therapy and intrathoracic infusion of chemotherapy drugs, but all with unsatisfactory curative effects, warranting treatment optimization[5-8]. Therefore, this study intends to explore new treatment options for LUAC+MPE patients, in the hope of contributing to the management optimization of this disease.
As an anti-tumor angiogenesis drug, pemetrexed disodium can be used to treat Non-Small Cell Lung Cancer (NSCLC) and Malignant Pleural Mesothelioma (MPM), and its inhibitory effect on tumor growth is related to the inhibition of cellular replication by disrupting intracellular folate-dependent metabolic processes[9,10]. Intravenous chemotherapy with pemetrexed disodium has also shown its effectiveness in controlling Pleural Effusions (PE) accumulation[11]. In addition, evidence has demonstrated the significant inhibitory action of intrapleural chemotherapy drugs against PE formation, which not only has favorable curative effects and safety, but also has conducive effect thereby helping to improve the patients’ quality of life[12,13]. Therefore, clinically, intravenous chemotherapy and pleural perfusion chemotherapy are often combined to treat LUAC with MPE. Cisplatin (DDP) has a good effect on tumor cells and can significantly reduce the risk of pleurisy and PE leakage[14]. Carboplatin (CBP) is a chemotherapeutic drug with good pharmacokinetic advantages when administered intrapleurally and belongs to the platinum chemotherapy drug similarly like DDP, which has certain advantages in tolerability[15].
This study intends to compare and analyze the clinical effect of CBP or DDP intrapleural perfusion+pemetrexed disodium in the treatment of LUAC complicated with MPE, in an attempt to provide an optimized scheme for the treatment of such a patient population.
Materials and Methods
General information:
In this study, 105 LUAC+MPE individuals admitted consecutively to our hospital between July 2018 and July 2022 were selected as the research participants strictly following the inclusion and exclusion criteria as described in this study. All the patients were divided into research group and control group, with 55 and 50 patients respectively. The patients of research group received DDP intrapleural perfusion+pemetrexed disodium while the patients of control group received CBP intrapleural perfusion+pemetrexed disodium. Research and control groups showed no notable difference in baseline data (p>0.05) and were clinically comparable. This study was approved by the Ethics Committee of DongYang People's hospital. Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Inclusion criteria:
Patients who were diagnosed as LUAC complicated with MPE by pathology; patients with chest Computerized Tomography (CT) and PE cytology; patients having intact medical records with an expected survival time of >3 mo and patients who did not undergo chemotherapy, targeted therapy or any other anti-tumor treatments in the recent month were included in the study.
Exclusion criteria:
Patients with serious cardiovascular, lung, brain and kidney dysfunction; patients who were allergic to the medication used in this study; patients who previously had the history of drug injection for PE; pregnant and lactating women and patients with MPE caused by non-lung cancer were excluded from the study.
Treatment method:
Patients in both the groups were given intravenous chemotherapy with pemetrexed disodium, as per the instructions manual of the drug. Dexamethasone tablets, folic acid tablets and vitamin B12 tablets were used for pre-treatment. During intravenous chemotherapy, close attention to the patient's body temperature, blood pressure, breathing, heart rate, pulse and other vital signs, and the occurrence of any adverse reactions in real time were monitored. Once there were any abnormalities, the chemotherapy was terminated and corresponding measures were adopted for symptomatic supportive treatment. Following accurate positioning of the PE in both the groups, a pleural drainage tube was placed for intermittent drainage, which was carried out after full drainage of the PE.
The control group was given 300 mg of CBP which was dissolved in 20 ml of normal saline and was injected into the chest cavity. Similarly, the research group received 50 mg/m² DDP for fractional intrapleural perfusion. The drainage tube was clamped immediately after the primary perfusion and was re-opened after 24 h for secondary DDP perfusion.
Endpoints:
Clinical efficacy: The clinical efficacy of drug is categorized into three levels namely, marked effectiveness, effectiveness and ineffectiveness. Marked effectiveness means that the patient's PE completely disappeared, the clinical symptoms such as chest pain, shortness of breath, dyspnea and fatigue were completely resolved, and all physiological indices returned to normal after treatment. Similarly, effectiveness refers to obviously reduced PE, ameliorated clinical symptoms and gradual return of various physiological indices to normal after treatment. While, ineffectiveness corresponds to little reduction or increase of PE after treatment, with persistent or even worsened clinical symptoms and no obvious changes in various physiological indices.
Total effective rate=(marked effectiveness cases+effectiveness cases)/total cases×100 %
Adverse Reactions (ARs): We mainly observed and recorded the patients with adverse reactions such as fatigue, fever, gastrointestinal discomfort, decreased White Blood Cell (WBC) count, and reduced neutrophil count. Subsequently, we calculated the rate of incidence of Ars between both the groups.
PE volume: PE volume in all the patients of the two groups was measured before and after treatment using B-ultrasonography.
PE tumor markers: 6 ml of blood sample was collected from all the patients for measurement of tumor markers. Then tumor markers such as Carcinoembryonic Antigen (CEA) and Neuron Enolase (NSE) were detected using electrochemiluminescence (ECL) immunoassay. The ECL immunoanalyzer (Cobas 8000) and kits were all supplied by Roche diagnostics.
Pulmonary Function (PF): PF was evaluated by comparative analysis between both the groups. PF refers to the ratio of Forced Expiratory Volume in 1 s (FEV1) to Forced Vital Capacity (FVC) (FEV1/FVC). Similarly, Maximal Voluntary Ventilation (MVV) was also measured using a lung function tester.
Statistical analysis:
Mean±Standard Error of Mean (SEM) was used for analyzing the statistical analysis and measurement of the data. Inter- and intra-group comparisons were carried out using independent sample t-test and paired t-test, respectively. Count data was represented by the ratio (percentage), and the comparison between the two groups was studied using Chi-square (χ²) test. The collected experimental data was analyzed using Statistical Package of Social Sciences (SPSS) version 24.0, where p<0.05 was considered to be statistically significant.
Results and Discussion
Baseline data such as age, gender, scores of Eastern Cooperative Oncology Group (ECOG) which is used for determining the patient's level of functioning, pathological site, smoking history and alcohol abuse history of the research and control groups was compared. It was observed that no marked differences were found (p>0.05) (Table 1).
| Factors | Research group (n=55) | Control group (n=50) | χ²/t | p |
|---|---|---|---|---|
| Age (y) | 61.00±6.64 | 59.56±6.79 | 1.098 | 0.275 |
| Gender (male/female) | 29/26 | 28/22 | 0.113 | 0.737 |
| ECOG (points) | 2.55±0.63 | 2.36±0.66 | 1.509 | 0.134 |
| Pathological site (left lung/right lung) | 20/35 | 23/27 | 1.006 | 0.316 |
| History of smoking (yes/no) | 15/40 | 20/30 | 1.909 | 0.167 |
| History of alcohol abuse (with/without) | 18/37 | 14/36 | 0.276 | 0.599 |
Table 1: Baseline information of patients.
The clinical effectiveness between the two groups was compared and the total effective rate of the research group was found to be 89.09 % which was significantly >70.00 % of the control group (p<0.05) (Table 2).
| Factors | Research group (n=55) | Control group (n=50) | χ²/t | p |
|---|---|---|---|---|
| Marked effectiveness | 29 (52.73) | 22 (44.00) | ||
| Effectiveness | 20 (36.36) | 13 (26.00) | ||
| Ineffectiveness | 6 (10.91) | 15 (30.00) | ||
| Total effectiveness | 49 (89.09) | 35 (70.00) | 5.966 | 0.015 |
Table 2: Clinical effectiveness of patients in two groups.
ARs observed during the treatment process between both the groups was analyzed comparatively. The analysis of the incidence of fatigue, fever, gastrointestinal discomfort, decreased WBC and reduced neutrophil count revealed an obviously lower total incidence in research group compared with the control group (18.18 % vs. 36.00 %), with statistical significance (p<0.05) (Table 3).
| Factors | Research group (n=55) | Control group (n=50) | χ²/t | p |
|---|---|---|---|---|
| Fatigue | 2 (3.64) | 3 (6.00) | ||
| Fever | 3 (5.45) | 5 (10.00) | ||
| Gastrointestinal discomfort | 2 (3.64) | 5 (10.00) | ||
| Decreased WBC count | 1 (1.82) | 2 (4.00) | ||
| Reduced neutrophil count | 2 (3.64) | 3 (6.00) | ||
| Total | 10 (18.18) | 18 (36.00) | 4.252 | 0.039 |
Table 3: Adverse reactions of patients in two groups.
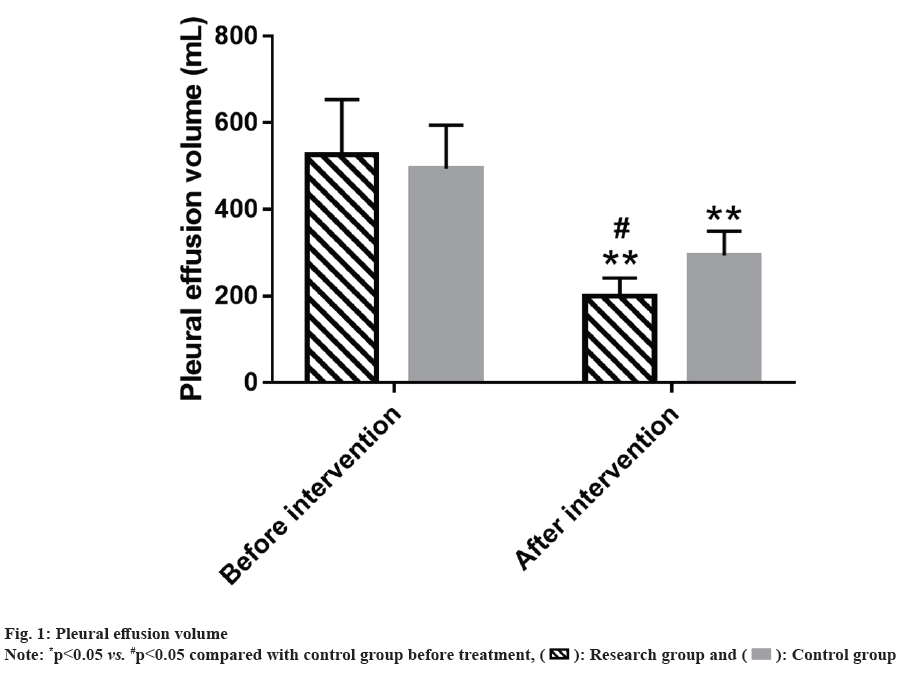
Further, PE volume of the research and control group was compared. Two groups had a similar amount of PE before intervention (p>0.05); PE volume in both the groups decreased significantly after intervention (p<0.05), with an even lower volume in the research group (p<0.05). (fig. 1).
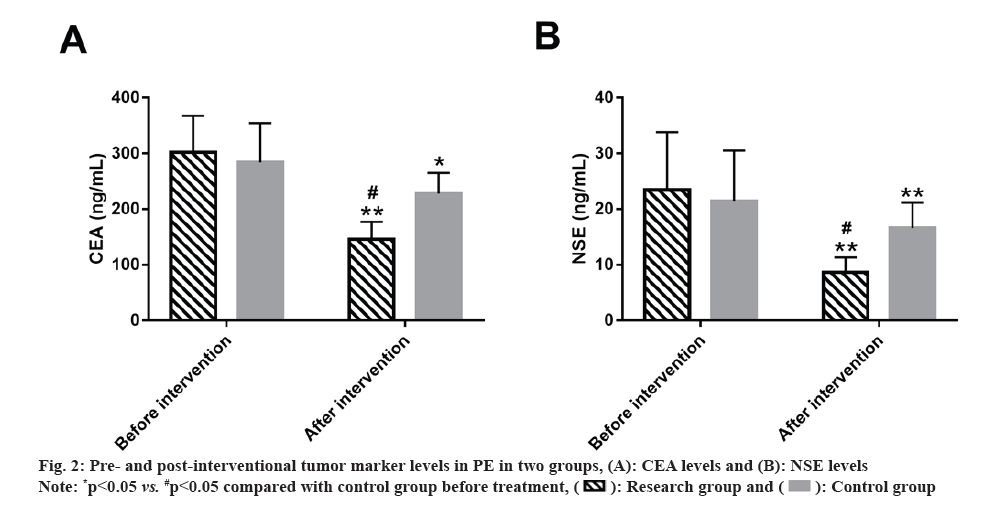
Tumor marker levels in PE patients were compared between the research and control groups. The levels of tumor markers such as CEA and NSE in PE patients of both the groups were detected. No statistical differences were identified in these tumor makers between research and control groups before intervention (p>0.05). However, the indices of both the groups decreased to varying degrees after intervention (p<0.05), with lower CEA and NSE levels in research group vs. control group (p<0.05). (fig. 2).
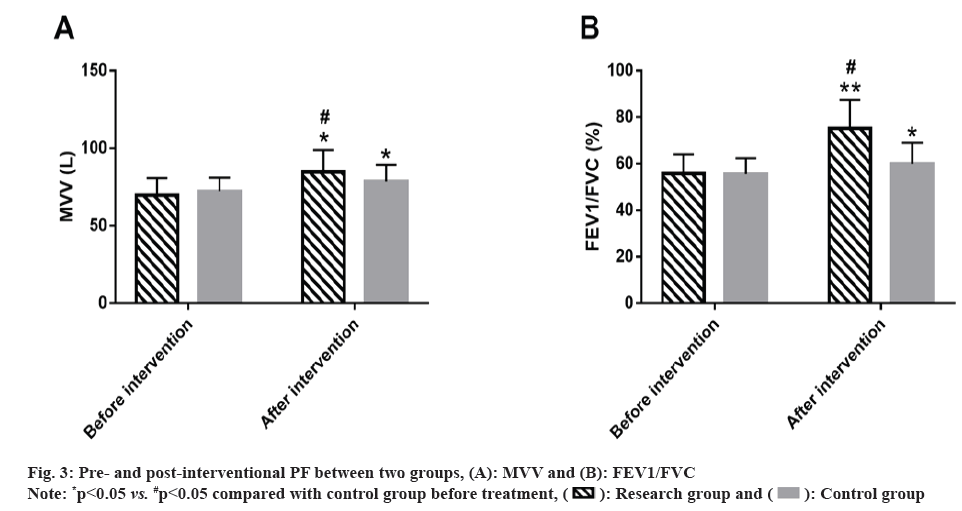
PF indices before and after intervention of the two groups were detected. MVV and FEV1/FVC showed no significant differences between groups before intervention (p>0.05); the indices of both the groups increased significantly after intervention (p<0.05), with high post-interventional MVV and FEV1/FVC in research group vs. control group (p<0.05) (fig. 3).
MPE, which is a common complication caused by systemic diseases such as tumor, infection, or inflammation, mostly originates from pleural metastasis of lung or breast tumors, with LUAC being the most common medical complication[16,17]; presence of MPE usually indicates late or advanced stage of the disease. LUAC patients with MPE tend to have shorter overall survival and a higher risk of Epidermal Growth Factor Receptor (EGFR) mutations, which further increases the risk of its recurrence[18,19]. An effective treatment strategy is therefore urgently needed to prevent the progression of LUAC+MPE, which is of great significance to improve the clinical outcomes of such patients.
At present, there is still limited analysis comparing the clinical application of pemetrexed disodium+CBP or DDP for LUAC+MPE. This study carries out relevant analysis in this condition and reports it in detail. In this study, the total effective rate was statistically higher in research group than in the control group (89.09 % vs. 70.00 %), indicating superior clinical effectiveness of DDP+pemetrexed disodium than CBP+pemetrexed disodium, similar to the results reported by Rodríguez-Abreu et al.[20].
CBP is known to be a cell cycle non-specific chemotherapy drug with a concentration-dependent therapeutic effect, which can directly kill tumor cells without liver activation and can help to promote the absorption of effusion[21,22]. Intrapleural administration of DDP controls Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2)-dependent endothelial cell proliferation associated with the pathophysiological process of MPE[23]. While pemetrexed disodium inhibits purine and pyrimidine synthesis by negatively regulating dihydrofolate reductase, thymidylate synthase and other folate-dependent metabolic pathways, thus playing an anti-tumor therapeutic role[24,25]. The total incidence of ARs (e.g., fatigue, fever, gastrointestinal discomfort, decreased WBC and reduced neutrophil count) was found to be significantly lower in research group vs. control group (18.18 % vs. 36.00 %), suggesting that DDP intrapleural perfusion+pemetrexed disodium has good safety in the treatment of LUAC+MPE. In the study of Dong et al.[26], pemetrexed disodium+DDP intrapleural perfusion also showed good efficacy and clinical safety in the treatment of advanced non-squamous NSCLC patients complicated by MPE, which is similar to our research results. In addition, research group had significantly reduced PE volume after intervention, lower than the pre-interventional level and control group, demonstrating the outstanding effect of DDP intrapleural perfusion+pemetrexed disodium on reducing PE in LUAC+MPE patients. As reported by Chen et al.[27], DDP+pemetrexed disodium has definite efficacy in patients with MPM-mediated MPE, significantly reducing PE, alleviating symptoms, improving patients' quality of life and prolonging their survival. The PE CEA and NSE levels of research group reduced notably after intervention and were greatly lower compared with control group, suggesting that the treatment of DDP intrapleural perfusion+pemetrexed disodium has a significant down-regulation effect on the abnormal levels of tumor markers in PE in LUAC+MPE patients. Moreover, MVV and FEV1/FVC of research group were also significantly reduced after intervention, lower than those of control group, which indicates that DDP intrapleural infusion+pemetrexed disodium has a positive effect on improving PF in LUAC+MPE patients.
Conclusively, DDP intrapleural infusion+pemetrexed disodium can significantly enhance clinical efficacy in LUAC patients complicated with MPE with good drug tolerance, which can effectively reduce the PE volume, inhibit CEA and NSE levels in PE, and improve patients’ PF, thereby providing new treatment directions and cognition for LUAC complicated with MPE.
Funding:
This study was supported by the 2021 public welfare Jinhua Science and Technology Research Plan Project (Project name: Exploration of a new mode of DOPS in the teaching of pleural and ascites cell morphology diagnosis and operation skills) with Project No: 2021-4-136.
Conflict of interests:
The authors declared no conflict of interests.
References
- He J, Xu WF, Chen NS. Differential diagnosis of tuberculous pleurisy and malignant pleural effusion by combined detection of TNF-α and IL-6 in pleural effusion. Chin J Clin Healthcare 2023;26(5):671-3.
- Hu J, You QH, Sun GY. Diagnostic value of combined measurement of metastasis-associated in colon cancer 1 and mesenchymal-epithelial transition factor in lung adenocarcinoma patients with malignant pleural effusion. Chin J Lung Dis 2016;9(2):120-4.
- Thomas R, Jenkins S, Eastwood PR, Lee YC, Singh B. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med 2015;21(4):338-45.
[Crossref] [Google Scholar] [PubMed]
- Iyer NP, Reddy CB, Wahidi MM, Lewis SZ, Diekemper RL, Feller-Kopman D, et al. Indwelling pleural catheter vs. pleurodesis for malignant pleural effusions. A systematic review and meta-analysis. Ann Am Thorac Soc 2019;16(1):124-31.
[Crossref] [Google Scholar] [PubMed]
- Marquez-Medina D, Popat S. Closing faucets: The role of anti-angiogenic therapies in malignant pleural diseases. Clin Transl Oncol 2016;18(8):760-8.
[Crossref] [Google Scholar] [PubMed]
- Bruschini S, Pallocca M, Sperandio E, D'Ambrosio L, Ascenzi F, De vitis C, et al. Deconvolution of malignant pleural effusions immune landscape unravels a novel macrophage signature associated with worse clinical outcome in lung adenocarcinoma patients. J Immunother Cancer 2022;10(5):1-13.
[Crossref] [Google Scholar] [PubMed]
- Cao D, Zhang J, Cheng YW. Effect of intrapleural perfusion hyperthermic chemotherapy on patients with non-small cell lung cancer complicated with pleural effusion. Oncol Prog 2023;21(1):49-52.
- Jones DR, Taylor MD, Petroni GR, Shu J, Burks SG, Daniel TM, et al. Phase I trial of intrapleural docetaxel administered through an implantable catheter in subjects with a malignant pleural effusion. J Thorac Oncol. 2010;5(1):75-81.
[Crossref] [Google Scholar] [PubMed]
- Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397(10272):375-86.
[Crossref] [Google Scholar] [PubMed]
- Bagley SJ, Vitale S, Zhang S, Aggarwal C, Evans TL, Alley EW, et al. Pretreatment red blood cell total folate concentration is associated with response to pemetrexed in stage IV nonsquamous non-small-cell lung cancer. Clin Lung Cancer 2017;18(2):143-9.
[Crossref] [Google Scholar] [PubMed]
- Wang XZ. Effect of aidi and cisplatin combined with intravenous pemetrexed disodium in the treatment of lung adenocarcinoma complicated with malignant pleural effusion. China Med 2022;20(1):110-12.
- Ma L, Wu Y, Zeng YL. Short-term efficacy and safety of cisplatin combined with pleural perfusion in the treatment of lung cancer with malignant pleural effusion: A mesh meta-analysis. Pract Clin J Integr Tradit Chin West Med 2023;3:13-6.
- Zhan F, Jiang GR, Wang ZT. Clinical efficacy of hyperthermia treatment combined with intrathoracic perfusion chemotherapy in the treatment of malignant pleural effusion. Jian Kang Zhong Gao 2020;10:133-6.
- Xu GJ, Shi Y, Fu JZ. Analysis of the effect of hemocoagulase and recombinant human endostatin combined with cisplatin thoracic perfusion in the treatment of malignant pleural effusion and the effect on serum VEGF level. Med Innov China 2023;20(12):51-5.
- Yang ZJ. Analysis of the effectiveness of carboplatin injection in the treatment of malignant pleural effusion. Mod Dig Interv 2022;27(2):233-6.
- Zhang HY, Fan Q. A clinical study of intrapleural infusion of bevacizumab or recombinant human vascular endostatin combined with cisplatin in treatment of malignant pleural effusion of lung adenocarcinoma. China Sci Tech J Med Health 2022;51(2):33-6.
- Asciak R, Rahman NM. Malignant pleural effusion: From diagnostics to therapeutics. Clin Chest Med 2018;39(1):181-93.
[Crossref] [Google Scholar] [PubMed]
- Wu SG, Yu CJ, Tsai MF, Liao WY, Yang CH, Jan IS, et al. Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur Respir J 2013;41(6):1409-18.
[Crossref] [Google Scholar] [PubMed]
- Liang YP, Li WM, Xu L. Characterization of immune cells in malignant pleural effusion of EGFR-mutant lung adenocarcinoma. Chin Clin Oncol 2023;28(7):627-31.
- Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann Oncol 2021;32(7):881-95.
[Crossref] [Google Scholar] [PubMed]
- Hu ZY, Zhou YP, Li SW. 1,25-dihydroxyvitamin D3 enhances killing effect of carboplatin on growth in lung cancer cell A549. Cancer Res Prev Treat 2013;40(2):125-30.
- He RF, Xie XP. Observation of the efficacy of compound Kushen injection combined with cisplatin in treatment of malignant pleural effusion. Shandong Med J 2010;50(33):27-32.
- Chrn GP, Wang YF, Wang G. Effect of Fufeijian combined with DDP on growth of Lewis lung carcinoma and VEGFA and VEGFR-2. Chin Arch of Tradit Chin Med 2016;34(5):1172-5.
- Li T, Huang JB, Lu JG, Li R, Wang Y, Shi XR, et al. Plasma thymidylate synthase and dihydrofolate reductase mRNA levels as potential predictive biomarkers of pemetrexed sensitivity in patients with advanced non-small cell lung cancer. J Thorac Dis 2020;12(12):7313-9.
[Crossref] [Google Scholar] [PubMed]
- Shimizu T, Nakanishi Y, Nakagawa Y, Tsujino I, Takahashi N, Nemoto N, et al. Association between expression of thymidylate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyltransferase and efficacy of pemetrexed in advanced non-small cell lung cancer. Anticancer Res 2012;32(10):4589-96.
[Google Scholar] [PubMed]
- Dong X, Huang Y, Yi T, Hu C, Gao Q, Chen Y, et al. Intrapleural infusion of tumor cell-derived microparticles packaging methotrexate or saline combined with pemetrexed-cisplatin chemotherapy for the treatment of malignant pleural effusion in advanced non-squamous non-small cell lung cancer: A double-blind, randomized, placebo-controlled study. Front Immunol 2022;13:1-9.
[Crossref] [Google Scholar] [PubMed]
- Chen D, Li X, Zhao H, Fu Y, Yao F, Hu J, et al. The efficacy of pemetrexed and bevacizumab intrapleural injection for malignant pleural mesothelioma-mediated malignant pleural effusion. Indian J Cancer 2014;51:82-5.
[Crossref] [Google Scholar] [PubMed]
 .
.