- *Corresponding Author:
- Ping Zhao
Department of Endocrinology, Chongqing Seventh People’s Hospital, Banan, Chongqing 400054, China
E-mail: ppl0629@163.com
| Date of Received | 29 March 2021 |
| Date of Revision | 16 October 2021 |
| Date of Acceptance | 04 August 2022 |
| Indian J Pharm Sci 2022;84(4):812-820 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the clinical worth of glucagon-like peptide-1 receptor agonist (liraglutide) combined with sodium glucose cotransporter-2 inhibitor (dapagliflozin) in the treatment of overweight or obesity patients with type 2 diabetes mellitus and to explore whether the beneficial effect of the combination can persist during a 1 y follow-up. Eighty overweight or obesity type 2 diabetes mellitus patients admitted to a hospital from January 2019 to January 2020 were randomly divided into a control group and an observation group, with 40 cases in each group. The control group was treated with liraglutide and the experimental group was treated with basic dapagliflozin in the control group. All patients were continuously treated for 12 w. The measurement results of parameters before and after treatment, the changes in glucose metabolism parameters, blood lipid metabolism parameters and the occurrence of adverse reactions during treatment were recorded. After that, 74 treated patients were followed up for 1 y (6 refused) and measurements of various parameters were recorded. The differences in body mass index, waist circumference, hemoglobin A1c, fasting plasma glucose, triglyceride, total cholesterol, high-density lipoprotein cholesterol, lowdensity lipoprotein cholesterol, homeostatic model assessment-insulin resistance, homeostatic model assessment-beta and insulin sensitivity index before and after treatment in the experimental group were higher than those in the control group (p<0.05). Weight loss during treatment was sustained over 1 y in both groups, with more significant changes in the experimental group. The combination of glucagon-like peptide-1 agonist and sodium glucose cotransporter-2 inhibitor can improve diabetes in overweight or obesity patients with type 2 diabetes mellitus and effectively reduce blood glucose and body mass index, which is of great significance for reducing body weight and improving efficacy. Therefore, using glucagonlike peptide-1 agonists and sodium glucose cotransporter-2 inhibitors in overweight, diabetic patients is a promising option.
Keywords
Diabetes mellitus, glucagon-like peptide-1 agonists, sodium glucose cotransporter-2 inhibitors, liraglutide, dapagliflozin
The term "obesity" has been cast-off to define the pathophysiological connection between Type 2 Diabetes Mellitus (T2DM) and obesity/overweight, most often since the middle of the 20th century[1]. Type 2 diabetes prevalence has rapidly grown during the last several decades as obesity increases, with an estimated 422 million individuals afflicted globally lately[2]. In many situations, bariatric surgery may considerably decrease weight and diabetes despite just recently being a viable choice for treating obesity[3-5]. However, many patients are unwilling to undergo invasive procedures or pay relatively high prices. Additionally, there might be both short-term and long-term negative belongings from bariatric surgery. A metabolic disorder called T2DM is characterized by ongoing hyperglycemia. Visceral adipose tissue, liver, skeletal muscle and excess cardiac tissue fat storage are characteristics of type 2 diabetes[6]. Persistent hyperglycemia in the T2DM affected role leads to chronic damage to tissues and organs such as the retina, heart and kidney, as well as glucose metabolism abnormalities, which pose a major danger to the patient's life and health[7]. Due to its anabolic effects, insulin treatment often causes weight gain, raising insulin resistance[8,9].
Consequently, dialectologists treat overweight T2DM patients with very little time spent on these medications. Strengthening body weight management while reducing blood glucose should be a priority for treating overweight and obese T2DM patients[10]. This calls for creating novel type 2 diabetes treatments that don’t cause weight gain. Doctors often combine several additional antidiuretic medications to treat diabetes when metformin alone is unsuccessful. Because they are linked to weight reduction, Glucagon-Like Peptide-1 (GLP-1) agonists and Sodium Glucose Cotransporter-2 (SGLT-2) inhibitors are chosen medications. SGLT-2 and GLP-1 analog medically favorable preliminary findings have been seen in diabetic combination treatment with inhibitors[11,12]. In these investigations, more individuals receiving combination treatment showed improvement in their modest weight loss and glycosylated hemoglobin levels (Hemoglobin A1c (HbA1c)). Liraglutide is a GLP- 1 Receptor Agonist (GLP-1RA) that is often prescribed to persons through sort 2 diabetes for glycemic management. Liraglutide consumes several actions, enhancing repletion and stimulating glucose-dependent insulin release from beta-cells, lowering calorie intake and promoting weight reduction.
Liraglutide primarily targets subcutaneous fat; visceral fat is not affected[13,14]. As an alternative, liraglutide causes a dose-dependent, fast weight loss lasting up to 2 y[15,16]. While decreasing food consumption may account for some of the causes of weight reduction, yes GLP-1 analogues have been found to impact energy metabolism[17]. In animal studies, GLP-1 analogues enhanced the heat production in Brown Adipose Tissue (BAT) and brown adipocyte degeneration, indicating that GLP-1 analogues may at least partially contribute to weight reduction via activating BAT[18]. In contrast, some investigations[19, 20] have not been able to show an increase in energy expenditure after GLP-1 analogue therapy. Innovative therapy for diabetes with insulin resistance is dapagliflozin. It accomplishes the goal of reducing blood glucose and provides weight reduction and blood pressure regulation benefits by blocking SGLT2 in renal tubular epithelium. Here, we present the findings of a study on patients who were administered a GLP-1 agonist in conjunction with SGLT-2 inhibitors. 1 y of followup research, further exploration of the combination use of SGLT-2 inhibitors and GLP-1 agonists treatment of together temporary besides permanent consequences.
Materials and Methods
General information:
Eighty overweight T2DM affected one, which was initially pickled within a hospital from January 2019 to January 2020 selected and altogether patients met the diagnostic criteria for T2DM. They remained haphazardly separated as control group and then the observation group, with 40 cases in each group. In the control group, there remained 21 men and 19 women; the mean age was (52.12±10.2) y, and the mean Body Mass Index (BMI) was (27.31±5.7) kg/m2. In the observation group, there were 23 men and 17 women; the mean age was (51.23±10.4) y, and the mean BMI was (27.82±5.6) kg/m2.
Exclusion criteria: Pregnant or lactating women; complicated with severe heart, lung, liver and kidney dysfunction and diagnosed with severe osteoporosis and provocative fracture. The education was accepted through the clinic morals board, and affected role besides their relatives and informed agreement form. The difference in overall statistics among the 2 groups obligated no statistical significance (p>0.05) with comparability.
Study methods:
Following admission, all patients received advice from a dietitian about their diabetic diets, medical professionals monitored their blood pressure and fingertip blood glucose levels and different parameter values were accurately measured and documented. Improve the detection of negative patient responses, therapy and workout advice. Liraglutide was injected subcutaneously into the abdomen of the control group once daily, just before breakfast. After a week of therapy, the dosage was proportionally raised to 1.8 mg/d from the original 0.6 mg/d. On the same schedule as the control group, dapagliflozin was given orally to the experimental group once a day, just before breakfast, at a dosage of 10 mg/ time. All patients received continuous care in the hospital for 12 w, after which they had a year of follow-up.
Outcome measures:
All patients had their body weight and waist circumference measured before and after treatment, and their BMI was calculated. Additionally, their Triglyceride (TG), Total Cholesterol (TC), High-Density Lipoprotein Cholesterol (HDL-C), and Low-Density Lipoprotein Cholesterol (LDL-C) levels, Fasting Plasma Glucose (FPG), Diastolic Blood Pressure (DBP) and Systolic Blood Pressure (SBP) levels were noted. Calculations were made for the Homeostatic Model Assessment- Beta (HOMA-β), the Insulin Sensitivity Index (ISI) and the HOMA-Insulin Resistance (HOMA-IR). Adverse responses that occurred during therapy were noted. The major outcomes of the follow-up survey were the changes in body weight, combined BMI and glycosylated hemoglobin. Among the secondary outcomes, there were alterations in insulin dosage, renal function and blood pressure.
Statistical methods:
Statistical software called Statistical Package for Social Sciences (SPSS) 23.0 stood cast-off aimed at statistics analysis. Enumeration statistics stood uttered as rate (%), associated with Chi-square (χ²) test and p>0.05, considered statistically significant. Dimension statistics were uttered by way of unkind typical eccentricity (x±s), associated with t-test and follow-up results were analyzed by one-way analysis of variance to examine outcome of combined treatment. Any independent variables influencing the main or secondary positive outcomes were investigated using linear regression.
Results and Discussion
There was no noteworthy alteration in the baseline statistics before treatment between the two groups, with comparability as shown in Table 1.
| Item | Control group | Experimental group | T/χ2 | p value |
|---|---|---|---|---|
| Age (years) | 52.12±10.2 | 51.23±10.4 | 0.1 | 0.93 |
| Gender (M/F) | 21/19 | 23/17 | 0.2 | 0.91 |
| Disease duration (years) | 3.53±1.8 | 3.41±1.7 | 0.28 | 0.78 |
| Weight (kg) | 84.44±10.2 | 83.58±10.6 | 0.13 | 0.9 |
| BMI (kg/m2) | 27.31±5.7 | 27.82±5.6 | 0.08 | 0.94 |
| HbA1c (%) | 7.41±0.8 | 7.32±0.9 | 0.01 | 0.98 |
Table 1: Comparison of Baseline Data before Treatment between Two Groups
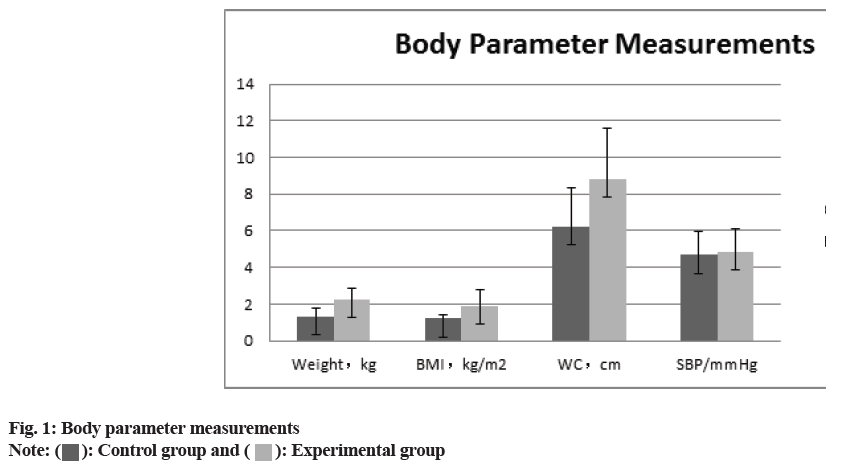
The comparison of the anthropometric measurements (body weight, BMI, waist circumference, SBP), the glucose metabolism measurements HbA1c, FPG, HOMA-IR, HOMA, ISI), and the blood lipid measurements (total serum cholesterol, TG, HDL-C and LDL-C) between two groups revealed that all of the experimental groups measurements were higher than those of control group. (p>0.05) the difference was statistically significant as shown in fig. 1-fig. 3 and Table 2.
| Variable | Control group | Experimental group | T value | p value |
|---|---|---|---|---|
| Body parameter measurements | ||||
| Weight (kg) | 1.32±0.45 | 2.25±0.64 | 6.147 | 0.01 |
| BMI (kg/m2) | 1.21±0.23 | 1.91±0.87 | 7.732 | 0.01 |
| Waist circumference (cm) | 6.24±2.07 | 8.82±2.74 | 9.183 | 0.02 |
| SBP (mmHg) | 4.67±1.27 | 4.88±1.22 | -0.26 | 0.96 |
| Glucose metabolism parameters | ||||
| HbA1c (%) | 2.11±0.72 | 3.38±1.05 | 9.188 | 0.01 |
| FPG (mol/l) | 1.60±0.37 | 2.55±0.89 | 7.703 | 0.01 |
| HOMA-IR | 1.67±0.62 | 2.48±0.95 | 4.601 | 0.01 |
| HOMA-β | 11.58±2.27 | 15.36±2.16 | 11.708 | 0.01 |
| ISI | 0.14±0.28 | 0.26±0.32 | 8.723 | 0.01 |
| Lipid parameters | ||||
| Serum total cholesterol | 1.34±0.28 | 2.14±0.42 | 7.619 | 0.02 |
| Triglyceride | 0.46±0.13 | 0.76±0.18 | 8.793 | 0.01 |
| HDL-C (mol/l) | 0.52±0.08 | 0.77±0.12 | 8.243 | 0.01 |
| LDL-C (mol/l) | 0.78±0.11 | 1.21±0.19 | 3.089 | 0.03 |
Table 2: Differences in Monitoring Indicators before and after Treatment between the Two Groups
The contrary replies occurred in two groups during the treatment recorded and it was observed that the frequency of gastrointestinal reactions in the experimental cluster stood slightly higher than that in the control group, and there was no noteworthy alteration in the incidence of other adverse reactions as shown in Table 3.
| Results | Control group | Experimental group |
|---|---|---|
| Hypoglycemia | 6 | 8 |
| Dizziness | 2 | 1 |
| Infection | 2 | 3 |
| Gastrointestinal reactions (nausea and vomiting) | 4 | 7 |
| Diabetic ketoacidosis | 1 | 1 |
Table 3: Number of Cases of Adverse Reactions during Treatment in the Two Groups
Out of the 80 patients, 74 were followed up for a year and 6 declined to take part; 4 in the control group and 2 in the experimental group. Survey findings revealed that the patient’s metabolic parameters had greatly improved, with an average weight loss of 3.27 kg, a reduction in glycosylated hemoglobin of 2.88 % and a reduction in BMI of 2.33 kg/m2. The mixture considerably decreased the need for insulin (mean 6.8 units). Although Estimated Glomerular Filtration Rate (EGFR) fell by 2.88 ml/min and mean creatinine rose by 1.67 mol/l, there was a general decline in renal function that was not statistically noteworthy. Baseline HbA1c was as an independent predictor of decreased glycated hemoglobin, according to multivariate analysis using linear regression as shown in Table 4.
| Variable | Mean difference | 95 % confidence interval | p value |
|---|---|---|---|
| Weight | 3.27 | 1.981-4.789 | 0 |
| HbA1c | 2.88 | 0.88-1.62 | 0 |
| BMI | 2.33 | 0.74-1.77 | 0 |
| SBP | 1.081 | 7.19-8.26 | 0.867 |
| Creatinine | -1.67 | 6.24-2.49 | 0.651 |
| EGFR | -2.884 | 2.34-6.89 | 0.384 |
Table 4: Study Outcomes at 6-12 Mo for Patients Receiving Combination Therapy
The study’s GLP-1 agonist SGLT-2 inhibitor combination treatment protocol was administered to overweight patients with T2DM. T2DM and obesity it is connected, causes insulin resistance and results in T2DM. Seizure risk factors[21] being overweight and obesity when using insulin and some hypoglycemic medications, T2DM increased insulin resistance, weight gain and hypoglycemia are common in patients. A vicious loop between weight increase and dyslipidemia will make the loss in pancreatic-cell function worse[22]. Consequently, while treating T2DM caused by obesity and excess weight, when choosing a course of therapy for patients, numerous factors should be taken into account in addition to efficiently lowering blood sugar. In order to successfully decrease the pace of gastric emptying and raise the patient’s satiety, liraglutide may increase the number of cells in the arcuate nucleus of the hypothalamus Cocaine and Amphetamine-Regulated Transcript (CART), messenger Ribonucleic Acid (mRNA) levels. This creates a positive feedback loop in the body that lowers the patient’s BMI[23]. Contrarily, dapagliflozin is a very selective SGLT2 inhibitor, and its hypoglycemic action is rarely reliant on the activity of islet cells, which may decrease SGLT2 expression in the tubular epithelium. This reduces the reabsorption of urine glucose[24]. Dapagliflozin may efficiently remove glucose from patient’s blood, lower patient’s sugar consumption and restrict patient’s calorie intake, all of which are helpful for weight reduction and treating obesity. Dapagliflozin T2DM combined with liraglutide efficient cardiovascular disease prevention and maintenance treatment of overweight and obesity individuals was made possible by effective glycemic control with considerable insulin dosage reduction BMI[25].
In our study, combination treatment resulted in statistically noteworthy decreases in HbA1C, body mass and BMI. Affected role in the experimental group fared better than individuals in the control group; i.e., however, the combination had no statistically noteworthy impact on blood pressure management. Higher baseline HbA1c levels were exposed to stand a statistically noteworthy sovereign analyst of the grade of HbA1c decrease within additional treatments for T2DM in earlier studies[26, 27]. Rosen stock and others had a new randomized, double-blind experiment, which shows that when the SGLT-2 inhibitor dapagliflozin was combined with other antidiuretic medications, higher baseline levels of HbA1c predicted bigger decreases in HbA1c levels[28]. In our subsequent poll, the high difference in mean HbA1c levels at baseline may be the cause of the study’s mean drop in HbA1c, which was 2.88 and 1.33. This finding is consistent with prior reports on the present difference effect of combination therapy. This research also showed that the experimental group’s alterations in blood lipid metabolism levels were noticeably improved than persons in the control group. Liraglutide and dapagliflozin medication may alter blood lipid levels for several reasons, but the most crucial is that the patient’s blood glucose is under good control, followed by a decrease in the buildup of liver fat in the patient[29]. Alternately, it has been discovered that SGLT receptor inhibition is linked to the synthesis of adiponectin and that dapagliflozin’s hypoglycemia mechanism may impact processes related to fat metabolism[30]. Patients with T2DM who are overweight or obesity have persistent hyperglycemia and hyperlipidemia, which may harm pancreatic beta-cells, raise insulin resistance and lower the effectiveness of conventional hypoglycemic medications.
Hyperlipidemic conditions put extra strain on the liver, which is harmful to controlling lipid and glucose metabolism[31]. Therefore, patients must quickly restore islet function during the first stages of therapy and properly manage their blood sugar and cholesterol levels.
The findings of this research demonstrated that, before and after therapy, the islet function indicators of patients in the observation group were greater than those in the control group. The findings demonstrated that liraglutide combined with dapagliflozin effectively controlled blood sugar, decreased insulin resistance, improved insulin sensitivity and partially reversed the vicious cycle of islet function impairment[32,33]. This was beneficial for the management and prognosis of the patient’s condition.
The incidence of adverse responses did not significantly vary between the two groups in this investigation. These findings support earlier studies[34] and imply that the combination of liraglutide and dapagliflozin is safe and effective for treating T2DM. Then again, we have posed several research-related queries, particularly in light of recent clinical studies utilizing SGLT-2 inhibitors or GLP-1 agonists that have shown encouraging outcomes in cardiovascular and mortality in T2DM patients[35, 36]. From a clinical and pharmacological perspective, GLP-1 analogues and SGLT-2 inhibitors should make an appropriate and sensible combination since they have little interaction potential[37]. Besides antidiuretic benefits linked to various classes of these compounds, the potential for weight reduction is quite distinct and there could be a synergy in how they affect diabetes.
According to studies, the effectiveness of SGLT- 2 inhibitors progressively diminishes over time[35], probably because this class of medications causes a rise in pancreatic glucagon secretion, which is counteracted by GLP-1 agonists[38]. Utilizing combination regimens in clinical practice should follow another logical reasoning line. In well-planned randomized controlled studies, the potential synergistic impact of this combination treatment in managing obesity has to be further investigated. A key clinical characteristic related to diabetes, abdominal obesity, has been linked to the use of GLP-1 agonists in the obesity affected role, according to a recent systematic appraisal besides meta-analysis[39]. SGLT-2 inhibitors stand an unusual period of antidiuretic medications because of a wide range of non-glycemic positive effects they are linked to, including weight loss, blood pressure reduction, improved cardiovascular mortality and morbidity, renal protection and ease of administration as a verbal agent[40].
The use of these two medication types in combination to treat obesity has not yet been thoroughly investigated. Because both of the patients in our study are Chinese men residing in Asia, it is also necessary to do more research into the causes of the considerable difference in diabetes results between our study and earlier studies. There have been reports of cultural disparities in the effectiveness of GLP-1 agonists[41]. Various racial and ethnic groups may experience SGLT-2 inhibitor’s (moreover unaided or in the mixture within extra drugs) effects differently. Treatment and hyperglycemia in patients with T2DM were the subjects of an update to the American Diabetes Association/European Association for Study of Diabetes (ADA/EASD) policy report in 2015[42].
The amended statement still lists metformin as the preferred medication for immunotherapy, but additional medications are mentioned as a viable option for secondline treatment due to the recent fast accumulation of information on SGLT-2 inhibitors. As long as adequate data from large randomized placebo-controlled clinical studies are available, techniques like the mixture of SGLT are evolving 0.2 inhibitors and GLP-1 agonists might be safe and successful and may be comprised of our medical arsenal aimed at the conduct of diabetes. In assumption, combination conduct for diabetes with SGLT-2 inhibitors and GLP-1 agonist’s treatment was successful, resulting in moderate decreases in figure mass, BMI, HbA1c levels and insulin dosage, but the mixture had no observable impact on blood pressure.
Conflict of interests:
The authors declared no conflict of interests.
References
- Sims EA, Danforth Jr E, Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res 1973;29:457-96.
[Crossref] [Google Scholar] [PubMed]
- Collaboration NR. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based measurement studies with over 4.4 million participants. Lancet 2016;387(10027):1513-30.
[Crossref] [Google Scholar] [PubMed]
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery vs. intensive medical therapy for diabetes—3-y outcomes. N Eng J Med 2014;370(21):2002-13.
[Crossref] [Google Scholar] [PubMed]
- Mingrone G, Panunzi S, de Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric–metabolic surgery vs. conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. The Lancet 2015;386(9997):964-73.
[Crossref] [Google Scholar] [PubMed]
- Paulus GF, de Vaan LE, Verdam FJ, Bouvy ND, Ambergen TA, van Heurn LW. Bariatric surgery in morbidly obese adolescents: A systematic review and meta-analysis. Obes Surg 2015;25(5):860-78.
[Crossref] [Google Scholar] [PubMed]
- van der Meer RW, Lamb HJ, Smit JW, de Roos A. MR imaging evaluation of cardiovascular risk in metabolic syndrome. Radiology 2012;264(1):21-37.
[Crossref] [Google Scholar] [PubMed]
- Sheu WH, Chan SP, Matawaran BJ, Deerochanawong C, Mithal A, Chan J, et al. Use of SGLT-2 inhibitors in patients with type 2 diabetes mellitus and abdominal obesity: An Asian perspective and expert recommendations. Diabetes Metab J 2020;44(1):11-32.
[Google Scholar] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352(9131):837-53.
[Crossref] [Google Scholar] [PubMed]
- Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes–causes, effects and coping strategies. Diabetes ObesMetab 2007;9(6):799-812.
[Crossref] [Google Scholar] [PubMed]
- Shim K, Begum R, Yang C, Wang H. Complement activation in obesity, insulin resistance and type 2 diabetes mellitus. World J Diabetes 2020;11(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Saroka RM, Kane MP, Busch RS, Watsky J, Hamilton RA. SGLT-2 inhibitor therapy added to GLP-1 agonist therapy in the management of T2DM. Endocr Pract 2015;21(12):1315-22.
[Crossref] [Google Scholar] [PubMed]
- Kaku K, Maegawa H, Tanizawa Y, Kiyosue A, Ide Y, Tokudome T, et al. Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: An open-label study. Diabetes Ther 2014;5(2):415-33.
[Crossref] [Google Scholar] [PubMed]
- Harris S, Abrahamson MJ, Ceriello A, Charpentier G, Evans M, Lehmann R, et al. Clinical considerations when initiating and titrating insulin degludec/liraglutide (IDegLira) in people with type 2 diabetes. Drugs 2020;80(2):147-65.
[Crossref] [Google Scholar] [PubMed]
- Bizino MB, Jazet IM, de Heer P, van Eyk HJ, Dekkers IA, Rensen PC, et al. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: A pre-specified secondary study on ectopic fat accumulation. Diabetologia 2020;63(1):65-74.
[Crossref] [Google Scholar] [PubMed]
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: An overview of the LEAD 1–5 studies. Diabetes Obes Metab 2009;11:26-34.
[Crossref] [Google Scholar] [PubMed]
- Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: The SCALE diabetes randomized clinical trial. JAMA 2015;314(7):687-99.
[Crossref] [Google Scholar] [PubMed]
- Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014;63(10):3346-58.
[Crossref] [Google Scholar] [PubMed]
- Kooijman S, Wang Y, Parlevliet ET, Boon MR, Edelschaap D, Snaterse G, et al. Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 2015;58(11):2637-46.
[Crossref] [Google Scholar] [PubMed]
- Van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes 2014;38(6):784-93.
[Crossref] [Google Scholar] [PubMed]
- Harder H, Nielsen L, Thi TD, Astrup A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care 2004;27(8):1915-21.
[Crossref] [Google Scholar] [PubMed]
- Malone JI, Hansen BC. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr Diabetes 2019;20(1):5-9.
[Crossref] [Google Scholar] [PubMed]
- Volaco A, Cavalcanti AM, Précoma DB. Socioeconomic status: The missing link between obesity and diabetes mellitus? Curr Diabetes Rev 2018;14(4):321-6.
[Crossref] [Google Scholar] [PubMed]
- Gorgojo-Martínez JJ, Feo-Ortega G, Serrano-Moreno C. Effectiveness and tolerability of liraglutide in patients with type 2 diabetes mellitus and obesity after bariatric surgery. Surg Obes Relat Dis 2016;12(10):1856-63.
[Crossref] [Google Scholar] [PubMed]
- Chilton RJ. Effects of sodium-glucose cotransporter-2 inhibitors on the cardiovascular and renal complications of type 2 diabetes. Diabetes Obes Metab 2020;22(1):16-29.
[Crossref] [Google Scholar] [PubMed]
- Sharma A, Verma S. Mechanisms by which glucagon-like-peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors reduce cardiovascular risk in adults with type 2 diabetes mellitus. Can J Diabetes 2020;44(1):93-102.
[Crossref] [Google Scholar] [PubMed]
- Esposito K, Chiodini P, Capuano A, Maiorino MI, Bellastella G, Giugliano D. Baseline glycemic parameters predict the hemoglobin A1c response to DPP-4 inhibitors. Endocrine 2014;46(1):43-51.
[Crossref] [Google Scholar] [PubMed]
- Henry RR, Buse JB, Sesti G, Davies MJ, Jensen KH, Brett J, et al. Efficacy of anti-hyperglycemic therapies and the influence of baseline hemoglobin A1C: A meta-analysis of the liraglutide development program. Endocr Pract 2011;17(6):906-13.
[Crossref] [Google Scholar] [PubMed]
- Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: A randomized double-blind trial of saxagliptin plus dapagliflozin addition vs. single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2015;38(3):376-83.
[Crossref] [Google Scholar] [PubMed]
- Terauchi Y, Fujiwara H, Kurihara Y, Suganami H, Tamura M, Senda M, et al. Long-term safety and efficacy of the sodium–glucose cotransporter 2 inhibitor, tofogliflozin, added on glucagon-like peptide-1 receptor agonist in Japanese patients with type 2 diabetes mellitus: A 52-week open-label, multicenter, post-marketing clinical study. J Diabetes Investig 2019;10(6):1518-26.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Gao P, Sun F, Li Q, Chen J, Yu H, et al. Sodium intake regulates glucose homeostasis through the PPARδ/adiponectin-mediated SGLT2 pathway. Cell Metab 2016;23(4):699-711.
[Crossref] [Google Scholar] [PubMed]
- Tajik S, Mirzababaei A, Ghaedi E, Kord-Varkaneh H, Mirzaei K. Risk of type 2 diabetes in metabolically healthy people in different categories of body mass index: An updated network meta-analysis of prospective cohort studies. J Cardiovasc Thorac Res 2019;11(4):254-63.
[Crossref] [Google Scholar] [PubMed]
- Lajara R. Combination therapy with SGLT-2 inhibitors and GLP-1 receptor agonists as complementary agents that address multi-organ defects in type 2 diabetes. Postgrad Med 2019;131(8):555-65.
- Gómez JC, Lorido JC, Huelgas RG, de Lucas DG, Polo LM, Aguilar JM, et al. Combination therapy with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors in older patients with type 2 diabetes: A real-world evidence study. Can J Diabetes 2019;43(3):186-92.
[Crossref] [Google Scholar] [PubMed]
- Consoli A, Formoso G, Baldassarre MP, Febo F. A comparative safety review between GLP-1 receptor agonists and SGLT2 inhibitors for diabetes treatment. Expert Opin Drug Saf 2018;17(3):293-302.
[Crossref] [Google Scholar] [PubMed]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Eng J Med 2015;373(22):2117-28.
[Crossref] [Google Scholar] [PubMed]
- Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Eng J Med 2016;375(4):323-34.
[Crossref] [Google Scholar] [PubMed]
- May M, Schindler C. Clinically and pharmacologically relevant interactions of antidiabetic drugs. Ther Adv Endocrinol Metab 2016;7(2):69-83.
[Crossref] [Google Scholar] [PubMed]
- Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Cahen DL, van Raalte DH. Gastrointestinal actions of glucagon-like peptide-1-based therapies: Glycaemic control beyond the pancreas. Diabetes Obes Metab 2016;18(3):224-35.
[Crossref] [Google Scholar] [PubMed]
- Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, et al. Effect of GLP-1 receptor agonists on waist circumference among type 2 diabetes patients: A systematic review and network meta-analysis. Endocrine 2015;48(3):794-803.
[Crossref] [Google Scholar] [PubMed]
- Monica Reddy RP, Inzucchi SE. SGLT2 inhibitors in the management of type 2 diabetes. Endocrine 2016;53(2):364-72.
[Crossref] [Google Scholar] [PubMed]
- Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: A systematic review and meta-analysis. Diabetes Obes Metab 2014;16(10):900-9.
[Crossref] [Google Scholar] [PubMed]
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care 2015;38(1):140-9.
[Crossref] [Google Scholar] [PubMed]
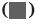 : Control group and
: Control group and  : Experimental group
: Experimental group
 : Control group and
: Control group and  : Experimental group
: Experimental group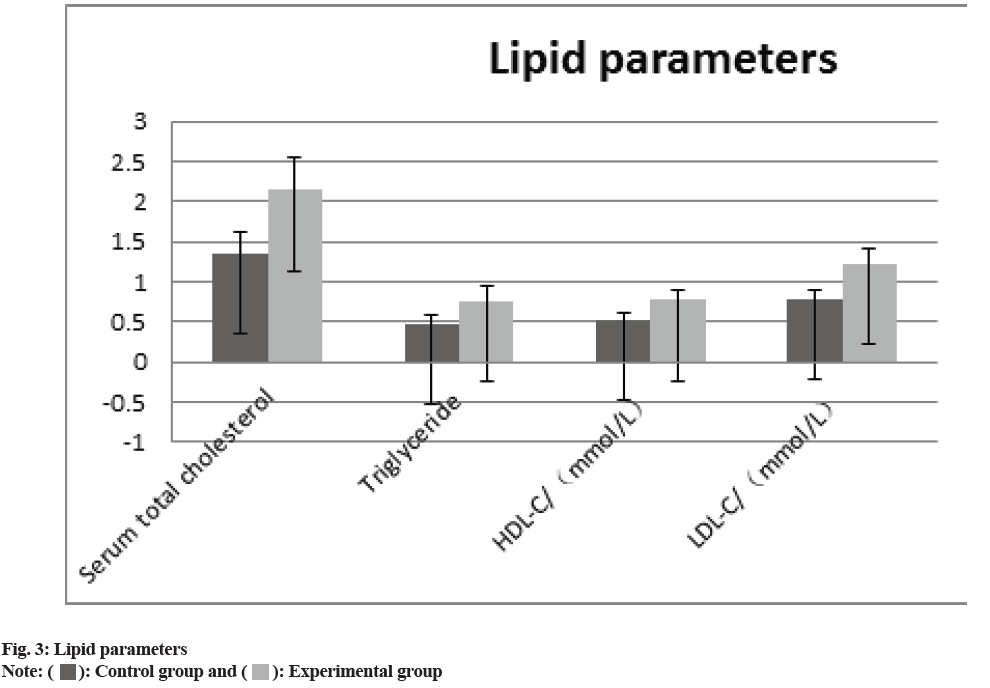
 : Control group and
: Control group and  : Experimental group
: Experimental group



