- *Corresponding Author:
- L. Chen
Department of Cardiology, Xiamen Hospital of Traditional Chinese Medicine, Siming, Xiamen 361009, China
E-mail: clf7008@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “178-185” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present research sets out to elucidate the clinical effectiveness and safety of olprinone combined with qiliqiangxin capsule in the treatment of severe pulmonary arterial hypertension complicated with right heart failure. 55 severe pulmonary arterial hypertension and right heart failure individuals admitted between April 2020 and April 2023 were selected and were assigned as control group (n=25) receiving olprinone treatment while the research group (n=30) received olprinone combined with qiliqiangxin capsules. The clinical effectiveness, safety (rash, chest pain, nausea, vomiting, dizziness, hypotension and tachycardia), echocardiography systolic pulmonary artery pressure, tricuspid annulus plane systolic excursion, right ventricular fractional area change, inferior vena cava collapse index, 6 min walking test and serum N-terminal pro-brain B-type natriuretic peptide were comparatively analyzed. The results showed an evidently higher overall effective rate and a comparable safety profile of the research group compared with the control group. The research group showed markedly reduced systolic pulmonary artery pressure and N-terminal pro-brain B-type natriuretic peptide levels, elevated tricuspid annulus plane systolic excursion, fractional area change, inferior vena cava collapse index and 6 min walking test after treatment, than the control group. Therefore, the combination of olprinone and qiliqiangxin capsule is effective and safe in the treatment of severe pulmonary arterial hypertension+right heart failure patients, which can significantly improve echocardiography results and patients’ exercise ability while exerting a significant inhibitory effect on serum N-terminal pro-brain B-type natriuretic peptide.
Keywords
Pulmonary arterial hypertension, olprinone, qiliqiangxin capsule, tachycardia, echocardiography
Pulmonary Arterial Hypertension (PAH) is a heterogeneous disease closely associated with abnormalities of the pulmonary vascular system. Its etiology is related to dynamic obstruction caused by abnormal pulmonary vasoconstriction, structural obstruction induced by abnormal vascular remodeling, and pathological abnormalities due to vascular fibrosis and stiffness[1,2]. The disease is characterized by an increase in mean Pulmonary Artery Pressure (mPAP) to at least 25 mmHg, which is usually significantly linked to a higher risk of morbidity and mortality and can complicate the condition of patients with connective tissue disease[3]. According to epidemiological data, PAH tends to occur in the elderly and developing countries, accounting for 1 % of the global population, and heart failure patients with preserved left ventricular ejection fraction have an 80 % risk of developing PAH[4,5]. Although PAH remains difficult to cure and the overall prognosis is still poor, patient median survival has been significantly increased from (2-3) y to >6-10 y[6-8]. Currently, there is no consensus on the most effective and safest drug treatment scheme for PAH, and comparative studies are still needed to explore therapeutic strategies with both efficacy and safety, which is of great significance for improving the curative effect and drug safety of PAH patients.
Olprinone (OLP), which is a Phosphodiesterase III (PDE III) inhibitor can strengthen cardiac contraction and dilate peripheral blood vessels by inhibiting PDE III, blocking cyclic Adenosine Monophosphate (cAMP) degradation and accelerating calcium ion influx into cardiomyocytes, which has been used to treat cardiovascular diseases such as postoperative acute cardiac insufficiency and acute heart failure[9,10]. In the study of Han et al.[11], OLP can also be used to alleviate Ischemia-Reperfusion (IR) related heart injury, mainly through the regulation of apoptosis and autophagy signaling pathway to play a therapeutic role. According to a PAH model experiment in an adult beagles, OLP has a certain therapeutic potential for PAH by acting as a vasodilator[12]. Qiliqiangxin capsule is a kind of Chinese patent medicine composed of Radix Astragali, ginseng rootlet and root, Cucumis hystrix chakrav, Polygonum cuspidatum, Cinnamomi cortex, Flos Carthami, Radix Salvia miltiorrhiza and pericarp of Citrus reticulata, etc., which can mediate cell apoptosis and autophagy by regulating the Reactive Oxygen Species (ROS) /Adenosine Monophosphate-activated Protein Kinase (AMPK)/mammalian Target of Rapamycin (mTOR) axis, thus realizing cardiac protection[13,14]. A meta-analysis has demonstrated its good clinical efficacy and safety in congestive heart failure[15]. Han et al.[16] also pointed out that qiliqiangxin capsule can be used to treat PAH and its therapeutic mechanism is related to its activation of the Phosphoinositide-3-Kinase/Protein kinase B (PI3K/Akt) axis.
At present, there are few studies on the effect of OLP combined with qiliqiangxin capsule on the clinical effectiveness and safety of patients with PAH. This study attempts to conduct relevant analysis to verify the clinical advantages of this therapy and is hereby reported.
Materials and Methods
Right Heart Failure (RHF) individuals admitted to our hospital between April 2020 and April 2023 were screened out according to the inclusion and exclusion criteria. Among them, 25 individuals in the control group received routine treatment, based on which 30 individuals in the research group received OLP+qiliqiangxin capsule therapy. The hospital's ethics committee approved the study protocol without reservations. No statistical intergroup difference was identified in general data (p>0.05), which was clinically comparable.
Inclusion criteria:
Patients with mPAP ≥45 mmHg (1 mmHg=0.133 kPa) indicated by right cardiac catheterization; patients having complications with chronic respiratory diseases; New York Heart Association (NYHA) cardiac function classification III/ IV; presence of clinical manifestations of right ventricular dysfunction such as lower limb edema and hepatomegaly and patients having complications with RHF were included in this study.
Exclusion criteria:
Patients with acute and chronic left ventricular dysfunction, acute myocardial infarction, acute coronary syndrome, acute pulmonary embolism and idiopathic pulmonary hypertension or congenital heart disease; those with allergic reactions to the medication used in the study; presence of a medical complication such as malignancy, which may affect the assessment of the condition; patients with serious diseases of lung, liver, kidney and other vital organs; autoimmune defects, or coagulation dysfunction and patients with mental illness or cognitive dysfunction were excluded in the study. In this study, 55 severe PAH patients depicted complication with RHF.
Treatment methods:
Patients in both the groups received routine treatment such as diuretic, cardiotonic and vasodilation therapies, with the treatment plan adjusted according to the patient's heart rate, blood pressure, electrolytes, hepatorenal function and adverse reactions. The control group was given oral, 20 mg of OLP tablets alone, 3 times per day. On the basis of the control group, the research group was treated with OLP hydrochloride injection, which was dripped at 10 μg/kg for 10 min and then at 0.2 μg/kg via intravenous pumping for 250 min. At the same time, 20 mg of qiliqiangxin capsule was administered orally, 4 capsules each time, 3 times per day. The course of treatment lasted for 12 w.
Observation indicators:
Clinical effectiveness: Marked effectiveness corresponds to the reduction of clinical symptoms by >70 % and systolic (s) PAP <50 mmHg; effectiveness is defined as a >30 % reduction in clinical symptoms and reduced sPAP that is still >50 mmHg; ineffectiveness refers to no improvement or even aggravation of clinical symptoms and no reduction or even increase in sPAP.
Overall effective rate=sum of marked effectiveness+effective cases/total number of cases×100
Safety: We observed and recorded the number of cases of side effects such as rash, chest pain, nausea and vomiting, dizziness, hypotension and tachycardia in both groups after treatment, and calculated the total incidence.
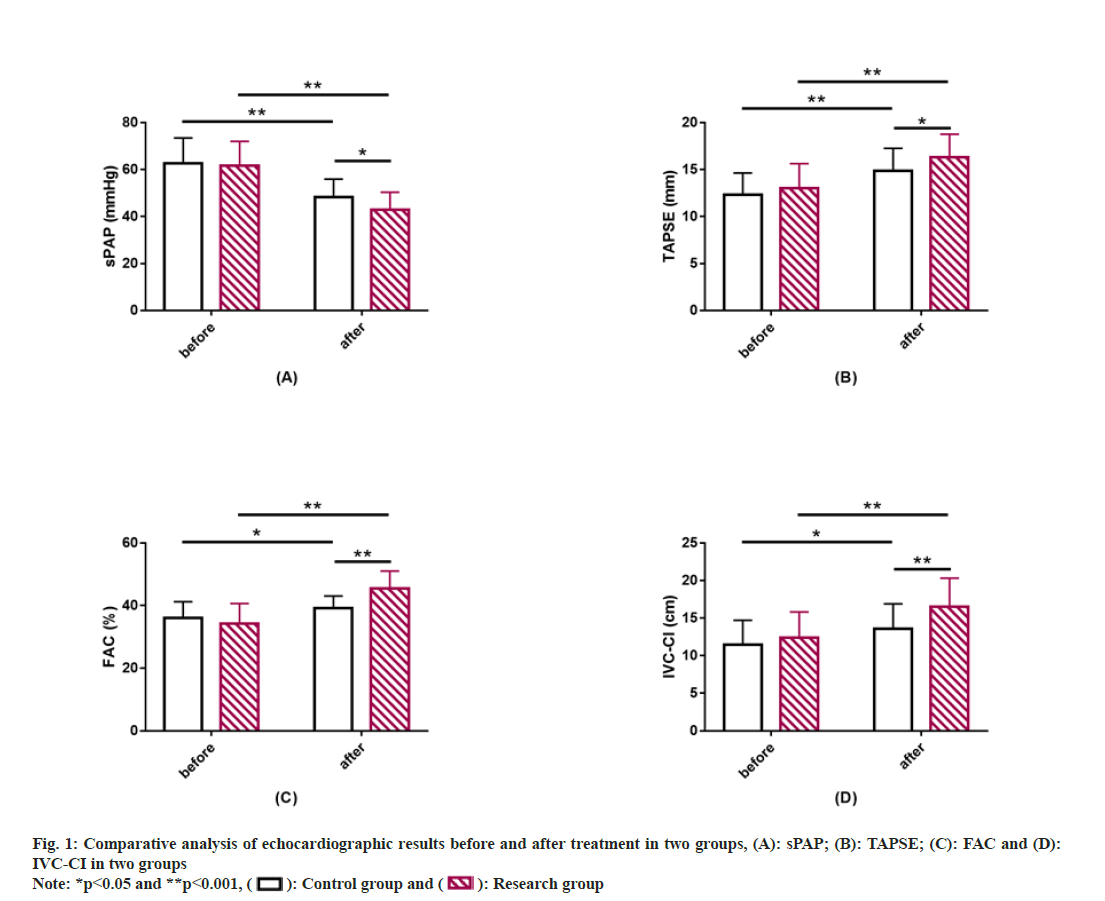
Echocardiography: Echocardiographic parameters such as sPAP, Tricuspid Annulus Plane Systolic Excursion (TAPSE), right ventricular Fractional Area Change (FAC), and Inferior Vena Cava Collapse Index (IVC-CI) were recorded before and after treatment.
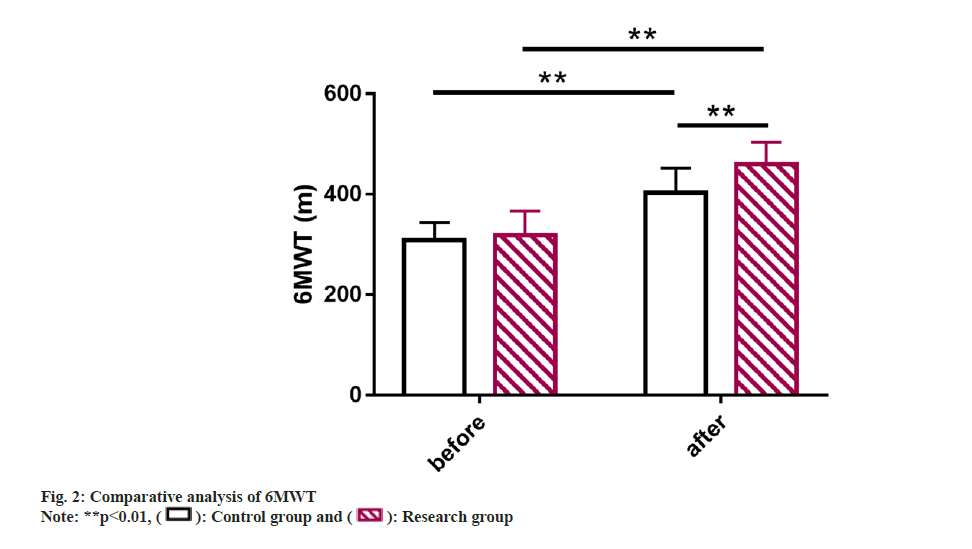
Further, 6 Min Walking Test (6MWT) was analyzed and statistically compared to assess the functional capacity of patients with heart failure.
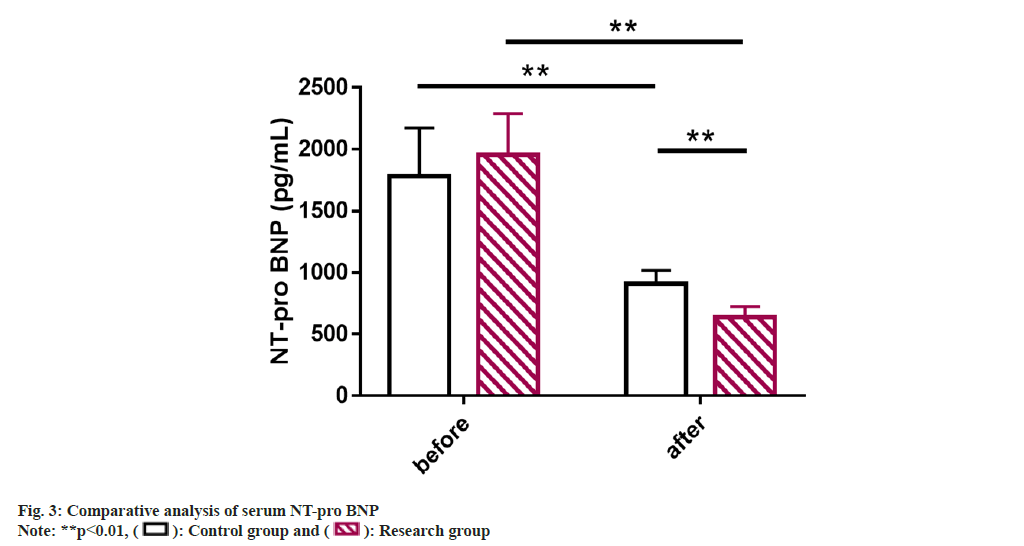
Serum N-Terminal pro-brain B-type Natriuretic Peptide (NT-pro BNP): Blood specimens were collected from all the patients before and after treatment for detection. 5 ml of fasting elbow venous blood was collected from morning on the test day, and the supernatant was collected after centrifugation and stored in the refrigerator at -30° for testing. An Enzyme-Linked Immunosorbent Assay (ELISA) test was performed to determine serum NT-pro BNP.
Statistical analysis:
Continuous variables were statistically described by mean±Standard Error of Mean (SEM), and the inter-group comparison was performed by the independent sample t-test. Categorical variables were expressed by the rate (%), and the intragroup comparison was made by the Chi-square (χ2) test. Statistical Package for Social Sciences (SPSS) software version 19.0 was adopted for data analysis where p<0.05 was considered as a statistical difference.
Results and Discussion
The base-line data such as mean age, gender, disease course, weight, pulmonary artery pressure and cardiac function between the research and control groups were compared (p>0.05) which showed clinical comparability (Table 1).
| Factors | Control group (n=25) | Research group (n=30) | t/χ2 | p |
|---|---|---|---|---|
| Age (y) | 59.32±6.43 | 60.17±8.24 | 0.420 | 0.676 |
| Sex (male/female) | 13/12 | 17/13 | 0.120 | 0.729 |
| Course of disease (y) | 4.56±0.82 | 4.63±1.10 | 0.263 | 0.794 |
| Weight (kg) | 64.24±8.47 | 62.53±10.17 | 0.670 | 0.506 |
| mPAP (mmHg) | 71.80±7.43 | 69.93±5.87 | 1.043 | 0.302 |
| NYHA classification (grade III/IV) | 18/7 | 20/10 | 0.182 | 0.670 |
Table 1: Baseline Information of Patients in the Two Groups
Clinical effectiveness of the two groups was comparatively evaluated. Comparative analysis of clinical effectiveness showed that the total number of effective patients in control and research groups was 17 (68.00 %) and 27 (90.00 %), respectively, indicating significantly more overall number of effective cases in the research group compared with the control group (p<0.05) (Table 2).
| Factors | Control group (n=25) | Research group (n=30) | χ2 | p |
|---|---|---|---|---|
| Markedly effective | 7 (28.00) | 12 (40.00) | ||
| Effective | 10 (40.00) | 15 (50.00) | ||
| Ineffective | 8 (32.00) | 3 (10.00) | ||
| Total effectiveness | 17 (68.00) | 27 (90.00) | 4.125 | 0.042 |
Table 2: Comparative Analysis of Clinical Effectiveness between Two Groups
Safety and the adverse effects of the treatment between the two groups were evaluated. We observed and recorded the incidence of rash, chest pain, nausea, vomiting, dizziness, hypotension and tachycardia in the two groups, and found a similar incidence of adverse events between the research and control groups (p>0.05) (Table 3).
| Factors | Control group (n=25) | Research group (n=30) | χ2 | p |
|---|---|---|---|---|
| Rash | 1 (4.00) | 0 (0.00) | ||
| Chest pain | 1 (4.00) | 0 (0.00) | ||
| Nausea and vomiting | 2 (8.00) | 2 (6.67) | ||
| Dizziness | 1 (4.00) | 2 (6.67) | ||
| Hypotension | 2 (8.00) | 1 (3.33) | ||
| Tachycardia | 1 (4.00) | 2 (6.67) | ||
| Total | 8 (32.00) | 7 (23.33) | 0.516 | 0.472 |
Table 3: Comparative Analysis of Treatment Safety between Two Groups
Similarly echocardiographic results between the two groups were also compared. sPAP, TAPSE, FAC, and IVC-CI did not differ much between groups prior to treatment (p>0.05). After treatment, sPAP decreased while TAPSE, FAC and IVC-CI increased in both groups, to all varying degrees (p<0.05). Moreover, the post-treatment sPAP was lower while TAPSE, FAC and IVC-CI were higher in the research group compared with the control group, with statistical significance (p<0.05) (fig. 1).
6MWT was also compared where it was found to be similar between group before the intervention (p>0.05). However, it elevated markedly and was higher in the research group vs. control group after the intervention (p<0.05) (fig. 2).
Serum NT-pro BNP of the patients of two groups were studied. Detection of serum NT-pro BNP by ELISA showed no significant inter-group difference before treatment (p>0.05); but the NT-pro BNP level in both groups decreased significantly after treatment, with an even lower level in the research group (p<0.05) (fig. 3).
PAH is a progressive fatal lung disease with clinical symptoms such as limited exercise, RHF and progressive dyspnea[17]. However, the early symptoms of PAH patients are usually nonspecific, which may lead to delayed diagnosis and untimely treatment[18]. In addition, although the treatment strategies for PAH have played a good role in improving patients’ clinical outcomes in recent years, the curative effects still vary from person to person and the effective treatment strategy has not yet reached a consensus[19]. The purpose of this study is to verify the clinical effectiveness and safety of OLP+qiliqiangxin capsule in sPAH+RHF so as to provide new options for the management of PAH patients.
In this study, the research group had an obviously higher overall effective rate than the control group (90.00 % vs. 68.00 %), suggesting that the intervention of OLP+qiliqiangxin capsule for sPAH+RHF patients renders significant benefits in improving curative effects. From the perspective of traditional Chinese medicine, the treatment of PAH mainly aims at activating qi circulation, promoting blood circulation, hydrating and expelling phlegm[20]. Qiliqiangxin capsule has been clinically applied in the treatment of PAH, and its components which include Radix Astragali, Radix Salvia miltiorrhiza and rhizome and extracts of Radix Salvia miltiorrhiza, have been confirmed to be powerful active ingredients for the prevention and treatment of PAH[21]. The underlying mechanism of this therapy is related to the repair of mitochondrial structure and the inhibition of mitochondrial-dependent apoptosis pathway that reverses right ventricular remodeling[22]. In addition, the incidence of adverse events such as rash, chest pain, nausea, vomiting, dizziness, hypotension and tachycardia was statistically found to be 23.33 % in the research group and was lower with 8.67 % in the control group, suggesting that OLP+qiliqiangxin capsule is safe for sPAH+RHF patients. In the report of Sun et al.[23], qiliqiangxin capsule showed high clinical effectiveness and safety in patients with ischemic heart failure, similar to our research results. Detection of echocardiographic parameters showed that compared with the baseline and the control group, the sPAP in the research group was significantly lower while TAPSE, FAC and IVC-CI were significantly higher after treatment, indicating that the intervention of OLP+qiliqiangxin capsule has a certain protective effect on cardiac function in PAH+RHF patients. The data of 6MWT showed that the 6MWT of the research group increased significantly after intervention than that of the control group, suggesting that the intervention of OLP+qiliqiangxin capsule in patients with sPAH+RHF has a significant enhancement effect on exercise performance and tolerance.
NT-pro BNP is a biologically inactive fragment of BNP released by stimulation such as myocardial tension and increased intravascular volume[24]. It can be used as a biomarker for screening diseases such as PAH, heart failure and systemic sclerosis and its abnormal increase is related to increased cardiac load and myocardial remodeling[25-28]. Previous studies have also demonstrated that the 4-level model of evaluation in which NT-pro BNP participates (the other 3 are based on functional category, 6MW Distance (6MWD) and BNP respectively) is more sensitive to the identification of PAH disease risk and patient prognosis[29]. In addition, NT-pro BNP levels can also reflect the risk of death or lung transplantation in PAH patients to a certain extent[30]. Subsequently, ELISA results showed significantly inhibited serum NT-pro BNP levels in the research group after treatment than the control group, suggesting that OLP+qiliqiangxin capsule can significantly reduce cardiac load and improve ventricular remodeling in sPAH+RHF patients. In the report of Li et al.[31], the application of qiliqiangxin capsule in patients with chronic heart failure not only helps to improve the quality of life and prolong 6MWT, but also effectively inhibits the level of NT-proBNP, similar to our findings.
This study still shows several limitations that need further investigation and analysis. First, the sample size included in this study is relatively small and an increase in the sample size is needed in the future to ensure the accuracy of the research results. Second, due to the lack of prognostic analysis, it is necessary to supplement long-term follow-up to understand the potential long-term efficacy of the combination therapy. Finally, basic research has not been conducted and relevant research needs to be supplemented in the future to further understand the underlying mechanisms of this treatment regimen. The above limitations will be addressed in future research.
To sum up, OLP combined with qiliqiangxin capsule contributes to favorable clinical responses in sPAH patients complicated with RHF (overall effective rate is 90.00 %), which can reduce the risk of side effects to a certain extent, exert a certain cardioprotection effect, significantly promote the recovery of patients' physical exercise ability and effectively reduce the level of serum NT-pro BNP, with clinically better value.
Author’s contributions:
Jun Che and Na Xu are the co-first authors and both of them have contributed equally to this work.
Funding:
This study was supported by the clinical study on the therapeutic efficacy and quality of life effect of Qing Jin Hua Phlegm Tang in the treatment of arterial pulmonary hypertension with phlegm-heat-embedded lung evidence by Xiamen Municipal Science and Technology Bureau (No: 3502Z202242ZD1172).
Conflict of interests:
The authors declared no conflict of interests.
References
- Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018;360:1-27.
[Crossref] [Google Scholar] [PubMed]
- Haque A, Kiely DG, Kovacs G, Thompson AAR, Condliffe R. Pulmonary hypertension phenotypes in patients with systemic sclerosis. Eur Respir Rev 2021;30(161):1-18.
[Crossref] [Google Scholar] [PubMed]
- Mathai SC. Pulmonary hypertension associated with connective tissue disease. Cardiol Clin 2022;40(1):29-43.
[Crossref] [Google Scholar] [PubMed]
- Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa HK, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016;4(4):306-22.
[Crossref] [Google Scholar] [PubMed]
- Maron BA, Abman SH, Elliott CG, Frantz RP, Hopper RK, Horn EM, et al. Pulmonary arterial hypertension: Diagnosis, treatment, and novel advances. Am J Respir Crit Care Med 2021;203(12):1472-87.
[Crossref] [Google Scholar] [PubMed]
- Hassoun PM. Pulmonary arterial hypertension. N Engl J Med 2021;385(25):2361-76.
[Crossref] [Google Scholar] [PubMed]
- Galie N, Channick RN, Frantz RP, Grunig E, Jing ZC, Moiseeva O, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019;53(1):1801889.
[Crossref] [Google Scholar] [PubMed]
- Coons JC, Pogue K, Kolodziej AR, Hirsch GA, George MP. Pulmonary arterial hypertension: A pharmacotherapeutic update. Curr Cardiol Rep 2019;21(11):1-12.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Zhang G, Lu S. Research status of olprinone in cardiovascular diseases. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2018;30(12):1209-12.
[Crossref] [Google Scholar] [PubMed]
- Tsubokawa T, Ishizuka S, Fukumoto K, Ueno K, Yamamoto K. Population pharmacokinetics of olprinone in patients undergoing cardiac surgery with cardiopulmonary bypass. J Anesth 2013;27(2):243-50.
[Crossref] [Google Scholar] [PubMed]
- Han MX, Xu XW, Lu SQ, Zhang GX. Effect of olprinone on ischemia-reperfusion induced myocardial injury in rats. Biomed Pharmacother 2019;111:1005-12.
[Crossref] [Google Scholar] [PubMed]
- Kakura H, Miyahara K, Amitani S, Sohara H, Koga M, Sakamoto H, et al. Hemodynamic effects of intravenous administration of olprinone hydrochloride on experimental pulmonary hypertension. Arzneimittelforschung 2000;50(6):515-9.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Ouyang Y, Li S, Liu MY, Qiao L, Zhao SH. Determination of twelve active compounds in qili qiangxin capsules by UPLC-MS. Zhongguo Zhong Yao Za Zhi 2014;39(10):1822-5.
[Google Scholar] [PubMed]
- Fan CL, Cai WJ, Ye MN, Chen M, Dai Y. Qili qiangxin, a compound herbal medicine formula, alleviates hypoxia-reoxygenation-induced apoptotic and autophagic cell death via suppression of ROS/AMPK/mTOR pathway in vitro. J Integr Med 2022;20(4):365-75.
[Crossref] [Google Scholar] [PubMed]
- Xing X, Guo J, Mo J, Li H, Zhang H, Shao B, et al. Qili qiangxin capsules for chronic heart failure: A GRADE-assessed clinical evidence and preclinical mechanism. Front Cardiovasc Med 2023;9:1-14.
[Crossref] [Google Scholar] [PubMed]
- Han X, Li C, Yang P, Jiang T. Potential mechanisms of qili qiangxin capsule to prevent pulmonary arterial hypertension based on network pharmacology analysis in a rat model. Ann Transl Med 2022;10(8):453.
[Crossref] [Google Scholar] [PubMed]
- Luna-Lopez R, Martin AR, Subias PE. Pulmonary arterial hypertension. Med Clin 2022;158(12):622-9.
[Crossref] [Google Scholar] [PubMed]
- Ruopp NF, Cockrill BA. Diagnosis and treatment of pulmonary arterial hypertension: A Review. JAMA 2022;327(14):1379-91.
[Crossref] [Google Scholar] [PubMed]
- Pitre T, Su J, Cui S, Scanlan R, Chiang C, Husnudinov R, et al. Medications for the treatment of pulmonary arterial hypertension: A systematic review and network meta-analysis. Eur Respir Rev 2022;31(165):1-16.
[Crossref] [Google Scholar] [PubMed]
- Zhang JR, Ouyang X, Hou C, Yang QF, Wu Y, Lu WJ, et al. Natural ingredients from Chinese materia medica for pulmonary hypertension. Chin J Nat Med 2021;19(11):801-14.
[Crossref] [Google Scholar] [PubMed]
- Xue Z, Li Y, Zhou M, Liu Z, Fan G, Wang X, et al. Traditional herbal medicine discovery for the treatment and prevention of pulmonary arterial hypertension. Front Pharmacol 2021;12:1-27.
[Crossref] [Google Scholar] [PubMed]
- Lu Y, Wu J, Sun Y, Xin L, Jiang Z, Lin H, et al. Qiliqiangxin prevents right ventricular remodeling by inhibiting apoptosis and improving metabolism reprogramming with pulmonary arterial hypertension. Am J Transl Res 2020;12(9):5655-69.
[Google Scholar] [PubMed]
- Sun YL, Ruan XF, Li YP, Wang XL. Comparative analysis of clinical effects according to syndrome differentiation of qiliqiangxin capsules on ischemic heart failure: Meta-analysis. Zhongguo Zhong Yao Za Zhi 2019;44(22):4975-84.
[Crossref] [Google Scholar] [PubMed]
- Edwards KD, Tighe MP. How to use N-Terminal pro-Brain Natriuretic Peptide (NT-proBNP) in assessing disease severity in bronchiolitis. Arch Dis Child Educ Pract Ed 2020;105(5):282-8.
[Crossref] [Google Scholar] [PubMed]
- Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37(1):67-119.
[Crossref] [Google Scholar] [PubMed]
- Fu S, Ping P, Wang F, Luo L. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J Biol Eng 2018;12:1-21.
[Crossref] [Google Scholar] [PubMed]
- Coghlan JG, Denton CP, Grunig E, Bonderman D, Distler O, Khanna D, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: The DETECT study. Ann Rheum Dis 2014;73(7):1340-9.
[Crossref] [Google Scholar] [PubMed]
- Friedman KG, Sleeper LA, Fichorova RN, Weilnau T, Tworetzky W, Wilkins HLE. Myocardial injury in fetal aortic stenosis: Insights from amniotic fluid analysis. Prenat Diagn 2018;38(3):190-5.
[Crossref] [Google Scholar] [PubMed]
- Hoeper MM, Pausch C, Olsson KM, Huscher D, Pittrow D, Grünig E, et al. COMPERA 2.0: A refined four-stratum risk assessment model for pulmonary arterial hypertension. Eur Respir J 2022;60(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Hendriks PM, van de Groep LD, Veen KM, van Thor MCJ, Meertens S, Boersma E, et al. Prognostic value of brain natriuretic peptides in patients with pulmonary arterial hypertension: A systematic review and meta-analysis. Am Heart J 2022;250:34-44.
[Crossref] [Google Scholar] [PubMed]
- Li X, Zhang J, Huang J, Ma A, Yang J, Li W, et al. A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of qiliqiangxin capsules in patients with chronic heart failure. J Am Coll Cardiol 2013;62(12):1065-72.
[Crossref] [Google Scholar] [PubMed]
 ): Control group and (
): Control group and ( ): Research group
): Research group