- *Corresponding Author:
- N. K. Jain
Pharmaceutics Research Laboratory, Department of Pharmaceutical Sciences, Dr. H. S. Gour University, Sagar-470 003, India.
E-mail: jnarendr@yahoo.co.in
| Date of Submission | 16 May 2005 |
| Date of Revision | 18 November 2005 |
| Date of Acceptance | 24 July 2006 |
| Indian J Pharm Sci,2006, 68 (4): 479-484 |
Abstract
The aim of the present work is to prepare and evaluate the oral mucoadhesive sustained release nanoparticles of clarithromycin in order to improve patient compliance by simplifing its administration, improving its therapeutic effect and reducing its dose related side effect. Clarithromycin containing gliadin nanoparticles were prepared by desolvation method using pluronic F-68® as a stabilizing agent. The results showed that this method is reproducible, very easy and led to the efficient entrapment of drug as well as formation of spherical particles ranging from 250-500 nm. Some process variables like effect of gliadin concentration and effect of surfactant were also evaluated with respect to their % drug entrapment and % yields. The maximum % drug entrapment and % yield were about 73 and 88%, respectively. The sustained release behavior of gliadin nanoparticles were evaluated both in phosphate buffer saline (pH 7.4) and simulated gastric fluid (pH 1.2), respectively, at 37±1°. Their mucoadhesive properties were determined by in vitro and in vivo methods. The shelf life of prepared nanoparticles was determined by storage at various temperatures while assessed in simulated gastric fluid (pH 1.2) with and without enzyme.
Introduction
Clarithromycin is a broad spectrum macrolide antibacterial agent that is effective both in vitro and in vivo against major pathogens responsible for peptic ulcer by Helicobacter pylori [1] and other respiratory tract infections of Clamydia pneumoniae, Mycoplasma pneumoniae, [2-5] Streptococcus pneumoniae and Haemophilus influenzae. Clarithromycin is well absorbed from the GI tract, but its systemic bioavailability (55%) is relatively low due to first- pass metabolism. It undergoes rapid biodegradation to produce the microbiologically active 14-hydroxy metabolite.
Oral drug administration represents the most convenient and common route of drug delivery. However, the bioavailability of a large number of drugs after oral administration is very low due to various reasons; too short gastric residence times, drug instability in the GI tract or lack of intestinal permeation of the drug. One possibility to improve the gastrointestinal uptake of perorally poorly absorbed drugs by their binding to colloidal particles, is nanoparticles. Nanoparticles can protect labile molecules from degradation in the GI tract and might be able to transport non-absorbable molecules into the systemic circulation.
These nanoparticulate carriers have the ability for both controlling the release and protecting the loaded drug against its degradations. Moreover, the small particle size allows them to penetrate in mucus layer and thus bind to the underlying epithelium and /or adhere directly to mucus network [6]. These adhesive-interactions of the particles with the boundary layer may improve the drug bioavailability by a number of different mechanisms. Nanoparticles may enhance the drug absorption rate by reducing the diffusion barriers between the pharmaceutical dosage from and site of action or absorption [7]. Similarly, they may prolong the residence time. Gliadin nanoparticles have a strong adhesive capacity with GI mucosa, may be due to gliadin composition. Neutral amino acids of gliadin can promote hydrogen-bonding interactions with the mucosa whereas its lipophilic components can interact with the biologic tissue by hydrophobic interactions [8]. The bioadhesive capacity of gliadin nanoparticles also has been evaluated when administered by the oral route to animals [9]. Gliadin nanoparticles show a great tropism for the upper gastrointestinal regions and their presence in other intestinal regions is very low [10].
Materials and Methods
Clarithromycin was procured as gift from Sun Pharma Advanced Research Centre (Vadodara) India. Gliadin was purchased from Sigma (USA). Pluronic F 68? was obtained from Himedia, India. All other reagents were of analytical grade.
Preparation of gliadin nanoparticles
Gliadin nanoparticles were prepared by a desolvation procedure described previously [8]. Briefly, gliadin and Clarithromycin were dissolved in 20 ml of an ethanol: water phase (7:3 v/v) and this solution was poured into a stirred physiological saline phase (NaCl 0.9% w/v in water), containing 0.5% Pluronic F-68® as a stabilizer. Then ethanol was eliminated by evaporation under reduced pressure (Buchi R-140, Switzerland) and the resulting nanoparticles were purified by centrifugation at 15,000 rpm for 1 h (Eltech centrifuge, India). The supernatant was removed and the pellets were resuspended in water. The suspension was passed through a 0.45 μm pore size membrane filter and the filtrate was centrifuged again and finally, the nanoparticles were freeze-dried using 5% glucose solution as a cryoprotector. Nanoparticles were hardened by the addition of 2 mg glutaraldehyde per mg nanoparticles and stirred for 2 h at room temperature before purification and freeze-drying (Table 1).
| Drug:gliadin | Formulation | Volume (ml) of inner ethanol:water | Volume of outer phase |
|---|---|---|---|
| (ratio) | code(s) | phase (7:3 ratio) | (ml, saline) |
| 1:.05 | GNP1 | 20 | 100 |
| 1:1 | GNP2 | 20 | 100 |
| 1:2 | GNP3 | 20 | 100 |
| 1:3 | GNP4 | 20 | 100 |
GNP1 is gliadin nanoparticles (drug:gliadin ratio,1:0.5), GNP2 is gliadin nanoparticles (drug:gliadin ratio, 1:1), GNP3 is gliadin nanoparticles (drug:gliadin ratio, 1:2) and GNP4 is gliadin nanoparticles (drug:gliadin ratio, 1:3).
Table 1: Formulae For Preparation Of Gliadin Nanoparticles
Particle size and morphology
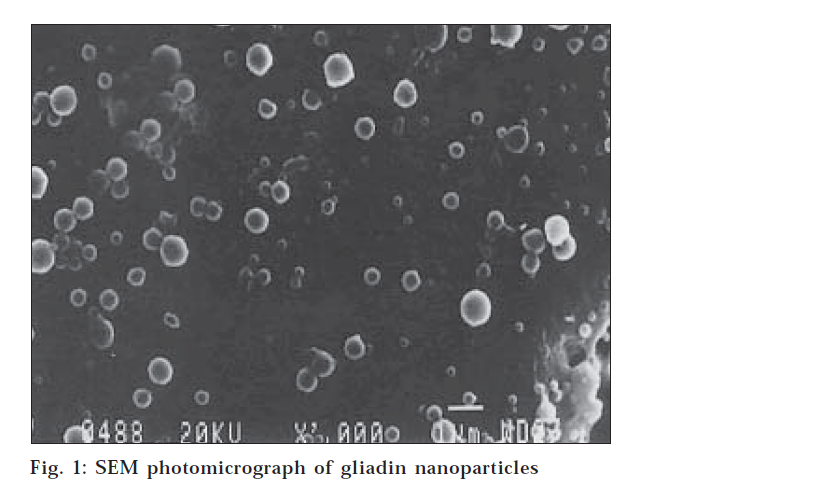
The particle size, size distribution and zeta potential of gliadin nanoparticles were measured in a Zetasizer 3000 HS (Malvern Instrument Ltd., UK). Surface morphology and internal structure of gliadin nanoparticles were determined by scanning electron microscopy (SEM). A thin film of aqueous dispersion of nanoparticles was applied on double stick tape over an aluminum stub and air dried to get uniform layer of particles. These particles were coated with gold to a thickness of about 450 Å using Sputter gold coater. (fig.1)
Percentage drug entrapment and percentage recovery
% Drug entrapment = Mass of drug in nanoparticles /Mass of drug used in the formulation x 100 % Nanoparticles recovery (% yield) = Concentration of drug in nanoparticles / Concentration of nanoparticles recovered x 100
The percentage drug entrapment and percentage recovery were determined using following equations [11]. Appropriate amount of freeze-dried gliadin nanoparticles were digested with minimum amount of ethanolic solution (water:ethanol in 7:3 ratios). The digested homogenates were centrifuged at 15,000 rpm for 30 min and supernatant was analyzed for drug entrapment. The clarithromycin entrapment was measured at 760 nm using Shimadzu 1601 UV/Vis. using spectrophotometer (Table 2).
| Formulation code(s) | % drug entrapment | % yield |
|---|---|---|
| GNP 1 | 43.7±2.3 | 63.6±1.7 |
| GNP 2 | 55.6±3.4 | 65.2±2.3 |
| GNP 3 | 73.1±1.3 | 88.7±2.8 |
| GNP 4 | 68.6±2.1 | 76.1±1.9 |
GNP1 is gliadin nanoparticles (drug:gliadin ratio,1:0.5), GNP2 is gliadin nanoparticles (drug:gliadin ratio, 1:1), GNP3 is gliadin nanoparticles (drug:gliadin ratio, 1:2) and GNP4 is gliadin nanoparticles (drug:gliadin ratio, 1:3).
Table 2: Percentage Drug Entrapment And% Recovery
In vitro drug release studies
Various formulations, respectively, CGNP1.CGNP4 were selected for the in vitro drug release studies [12]. In vitro drug release of clarithromycin from gliadin nanoparticles were estimated by the dialysis cell membrane method in two different media, respectively phosphate buffer saline (PBS) (pH 7.4) (Table 3), simulated gastric fluid (SGF) (pH 1.2) (Table 4) at 37±1° for 24 h under sink condition.
| Time (h) | % cumulative drug released | |||
|---|---|---|---|---|
| CGNP1 | CGNP 2 | CGNP 3 | CGNP 4 | |
| 1 | 8.3±1.2 | 9.1±2.3 | 5.2±2.1 | 5.0±2.2 |
| 2 | 12.9±3.2 | 9.9±2.4 | 5.7±2.3 | 6.8±2.3 |
| 3 | 13.4±2.2 | 11.6±3.0 | 6.1±1.8 | 8.1±2.6 |
| 4 | 15.2±1.3 | 13.9±1.6 | 6.9±2.3 | 11.7±1.3 |
| 5 | 18.7±3.0 | 16.7±1.9 | 8.3±2.6 | 14.2±2.9 |
| 6 | 21.2±2.9 | 17.2±2.4 | 10.5±1.6 | 17.6±1.5 |
| 7 | 25.8±2.3 | 19.1±3.3 | 19.7±2.4 | 18.5±2.4 |
| 8 | 26.2±1.8 | 19.9±2.1 | 21.8±3.2 | 20.7±3.6 |
| 24 | 65.7±2.3 | 58.2±2.6 | 63.4±1.6 | 49.7±2.3 |
GNP 1 is gliadin nanoparticles (drug:gliadin ratio,1:0.5), GNP2 is gliadin nanoparticles (drug:gliadin ratio, 1:1), GNP3 is gliadin nanoparticles (drug:gliadin ratio, 1:2) and GNP4 is gliadin nanoparticles (drug:gliadin ratio, 1:3).
Table 3: In Vitro Drug Release Profile In Pbs (Ph 7.4) At 37±1°
| Time(h) | % cumulative drug released | |||
|---|---|---|---|---|
| CGNP 1 | CGNP 2 | CGNP 3 | CGNP 4 | |
| 1 | 6.0±2.3 | 6.3±3.1 | 3.2±1.4 | 6.4±0.9 |
| 2 | 9.3±2.6 | 7.8±2.2 | 10.5±2.1 | 8.2±1.2 |
| 3 | 10.9±1.9 | 11.6±1.3 | 20.2±1.9 | 12.9±3.1 |
| 4 | 17.2±1.6 | 24.9±2.0 | 29.8±2.3 | 16.2±2.1 |
| 5 | 24.2±3.2 | 27.8±3.2 | 35.1±2.6 | 19.3±1.6 |
| 6 | 36.4±2.7 | 38.4±1.2 | 40.1±1.6 | 23.1±2.1 |
| 7 | 41.2±3.4 | 43.2±1.7 | 42.2±2.3 | 25.7±3.1 |
| 8 | 54.3±1.8 | 49.8±3.1 | 53.9±1.9 | 38.9±2.1 |
| 24 | 73.2±2.7 | 68.2±1.6 | 62.3±2.5 | 52.6±1.9 |
| GNP 1 is gliadin nanoparticles (drug:gliadin ratio,1:0.5), GNP2 is gliadin nanoparticles (drug:gliadin ratio, 1:1), GNP3 is gliadin nanoparticles (drug:gliadin ratio, 1:2) and GNP4 is gliadin nanoparticles (drug:gliadin ratio, 1:3). | ||||
Table 4: In Vitro Drug Release Profile In Sgf (Ph 1.2) At 37±1°
Appropriate volume of gliadin nanoparticles dialysed was taken in dialysis tube against 100 ml of media, continuously stirred with magnetic stirrer at 37±1°. After appropriate time intervals (one h) one ml sample was withdrawn and analyzed for clarithromycin content at 760 nm, in a Shimadzu 1601 UV/Vis. spectrophotometer. Equal volume of fresh media preheated to 37° was added to replace the withdrawn sample.
In vitro evaluation of gastric mucoadhesion of nanoparticles
Male Sprague Dawley rats weighing 200-250 g were fasted overnight before the experiments, but allowed free access to water ad libitum. The stomach was excised under anesthesia and perfused with physiological saline to remove the contents of stomach. The cleaned stomach was used immediately after preparation. A 100 mg of nanoparticles sample that were suspended in SGF (pH 1.2) was filled into the cleaned stomach, ligated and then incubated in physiological saline at 37° for 30 min. The liquid content of stomach was then removed by injecting the air and the stomach was perfused with SGF (pH 1.2) for 30 min, at a flow rate of 1 ml per min. The stomach was cut to open and nanoparticles that remained in stomach were recovered with SGF (pH 1.2). The final volume of washing solution was mixed with 10 ml of ethanolic solution and kept for 2 h for complete digestion of gliadin nanoparticles. After filtration through 0.45 μm filter paper, absorbance was determined spectrophotometrically at 230.6 nm (gliadin) and gastric mucoadhesion was determined as the % of nanoparticles remaining in stomach after perfusion.
In vivoevaluation of gastric mucoadhesion of nanoparticles
Sprague Dawley rats (200-250 g) were fasted for 24 h before experiment but were allowed free access to water ad libitum. Nanoparticles (100 mg) that were filled in capsule were administrated to rats using a gastric sonde.Four hr post administration, the rats were sacrificed and stomach was removed and washed with SGF (pH 1.2) to recover the remaining nanoparticles. The amount of nanoparticles that remained in the stomach was determined using the same methods as described above under in vitro methods.
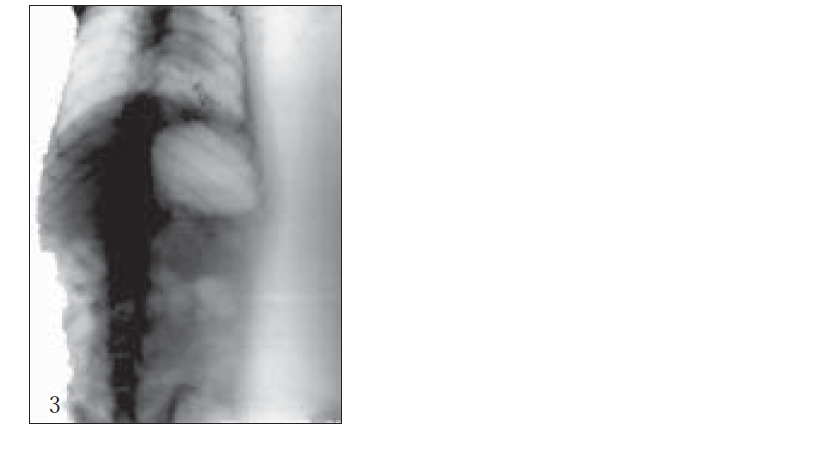
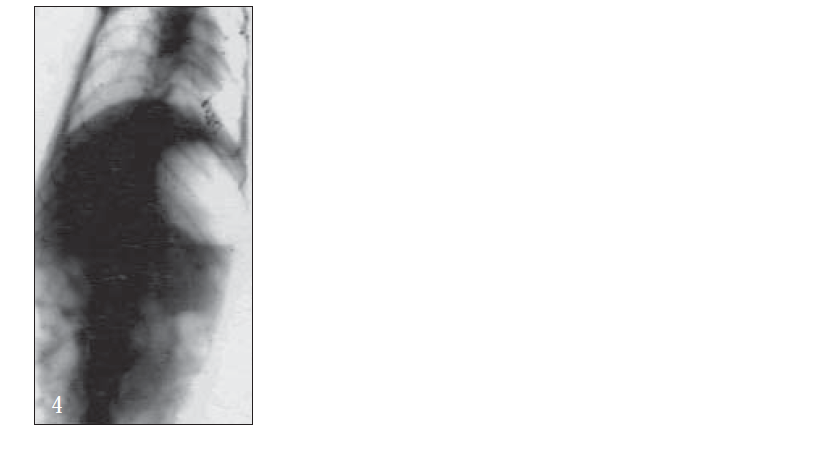
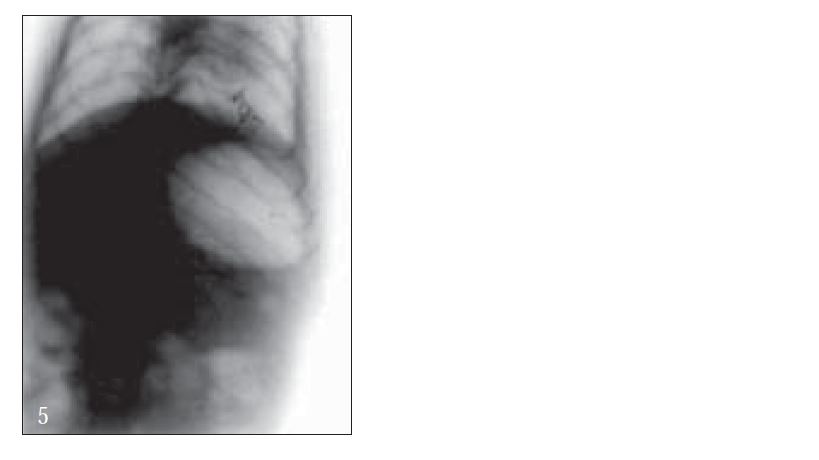
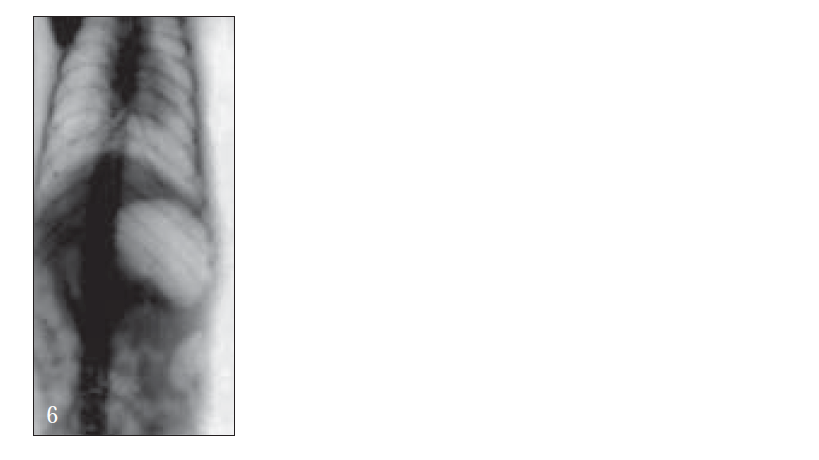
For X-ray studies, giadin nanoparticles were prepared containing barium sulfate as contrast agent for in vivo studies. The study was carried out with albino rats free of detectable gastrointestinal diseases or disorders, each animal, having fasted overnight. After 30 min, each subject was ingested with nanoparticles formulation together with 2 ml of water. The intragastric behavior of formulations after dosing was observed by taking a series of X-ray photographs at suitable time intervals (figs. 2-7).
Stability studies
The stability of gliadin nanoparticles was evaluated in PBS (pH 7.4) and in SGF (pH 1.2) with and without pepsin. Ten milligrams of formulations were incubated at 37±1° with 20 ml of PBS (pH 7.4) and SGF (pH 1.2) with and without pepsin, for a period of 2, 4, 8 and 24 h. After specified time intervals, the suspension was centrifuged at 15,000 rpm for 1 h, supernatant was removed and nanoparticles were dissolved in ethanolic solution (7:3 ratio). The gliadin content was determined by taking absorbance at 230.6 nm against blank (Table 6). The shelf life of all the formulations was determined by storage in amber colored vials, closed with rubber closers at room temperature, 0° and at 8° and % residual drug contents were determined after 10, 20 and 30 d (Table 7).
| Formulation code(s) | Average% gastric retention | |
|---|---|---|
| In vitro | In vivo | |
| GNP 1 | 74.9±2.7 | 62.4±2.9 |
| GNP 2 | 76.1±3.4 | 66.3±1.3 |
| GNP 3 | 81.5±2.3 | 69.7±2.4 |
| GNP 4 | 70.4±1.6 | 67.1±3.1 |
| GNP 1 is gliadin nanoparticles (drug:gliadin ratio,1:0.5), GNP2 is gliadin nanoparticles (drug:gliadin ratio, 1:1), GNP3 is gliadin nanoparticles (drug:gliadin ratio, 1:2) and GNP4 is gliadin nanoparticles (drug:gliadin ratio, 1:3). | ||
Table 5: % Gastric Retention Of Nanoparticles
| Media | % remaining of gliadin content | ||||
|---|---|---|---|---|---|
| 2h | 4h | 8h | 24h | ||
| PBS (pH 7.4) | 98.3±1.4 | 98.1±1.1 | 97.8±2.6 | 97.5±2.7 | |
| SGF (pH 1.2) | 98.4±1.8 | 97.6±2.3 | 97.4±1.5 | 93.2±1.3 | |
| SGF (pH 1.2)+ | 96.3±0.9 | 95.8±1.2 | 92.9±3.1 | 71.4±2.2 | |
| enzyme (pepsin) | |||||
| GNP 1 is gliadin nanoparticles (drug:gliadin ratio,1:0.5), GNP2 is gliadin nanoparticles (drug:gliadin ratio, 1:1), GNP3 is gliadin nanoparticles (drug:gliadin ratio, 1:2) and GNP4 is gliadin nanoparticles (drug:gliadin ratio, 1:3). PBS is phosphate buffer saline and SGF is simulated gastric fluid. | |||||
Table 6: Remaining % Of Gliadin Content In Different Media
| Formulation | Initial | % residual drug content | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| code (s) | 10 days | 20 days | 30 days | ||||||||||
| 0 | 8° | RT | 0 | 8o | RT | 0 | 8o | RT | 0 | 8o | RT | ||
| CGNP 3 | 100 | 100 | 100 | 97.9±2.1 | 98.7±1.9 | 98.6±2.2 | 93.9±2.9 | 94.3±3.6 | 91.8±1.3 | 83.4±1.5 | 89.2±43 | 88.1±1.6 | |
| CGNP 2 | 100 | 100 | 100 | 98.9±25 | 98.5±3.9 | 95.0±5.6 | 93.3±5.7 | 94.8±1.4 | 91.2±3.4 | 82.9±2.4, | 88.6±4.2 | 89.3±4.2 | |
| CGNP 3 | 100 | 100 | 100 | 99.6±5.9 | 98.1±4.9 | 97.4±2.1 | 91.7±5.2 | 91.4±1.8 | 90.1±3.2 | 84.6±5.7 | 85.8±7.1 | 88.9±5.7 | |
| CGNP 4 | 100 | 100 | 100 | 97.4±5.4 | 97.7±3.6 | 95.2±2.9 | 95.5±2.4 | 94.3±4.8 | 92.4±3.4 | 81.1±3.5 | 87.2±5.8 | 90.4±4.8 | |
| GNP 1 is gliadin nanoparticles (drug:gliadin ratio,1:0.5), GNP2 is gliadin nanoparticles (drug:gliadin ratio, 1:1), GNP3 is gliadin nanoparticles (drug:gliadin ratio, 1:2) and GNP4 is gliadin nanoparticles (drug:gliadin ratio, 1:3). | |||||||||||||
Table 7: Effect Of Storage On Residual Drug Content
Results and Discussion
The protein and polysaccharide from aqueous phase can be desolvated by pH change or change in temperature or by adding some appropriate counter ions or by any other desolvating agents like ethanol, isopropanol. Desolvation deaggregates the protein and turns the suspension colloidal and hence milky in appearance.
Gliadin nanoparticles were prepared by desolvation method for macromolecules, by adding a solvent phase of gliadin to a non-solvent phase, which has low viscosity and high mixing capacity in all proportions. Addition of a desolvating agent resulted in the desolvation of macromolecules. The process is commonly known as coaservation, a new phase, the coaservate phase when treated with a cross linking agent (glutraldehyde), produced nanoparticles of macromolecules.
The desolvation process can be regarded as a gradual removal of the solvent molecules from around the macromolecules and the molecules “rolling up’’ in the less friendly environment. The protein macromolecules would be a discrete, poorly solvated, rolled up molecules, thus able to produce colloidal particles.
The gliadin defines a group of polymorphic proteins extracted from gluten, which are soluble in ethanolic solution and show a remarkably low solubility in water except at extreme pH. Due to these physicochemical properties, gliadin nanoparticles can be prepared by desolvation method for macromolecules, using environmentally acceptable solvent such as ethanol and water. These macromolecules showed a high capacity for loading drug and were soluble without further chemical or physical cross linking treatment. In the present system, the diffusion of ethanol (good solvent for gliadin), from the gliadin solution in to the aqueous medium, drastically reduced the solubility of gliadin solution, forming nanoparticles in the solution. Gliadin nanoparticles have shown the average particles size range of 250-500 nm with positive zeta potential of 22.8 mV. The gliadin nanoparticles were spherical with smooth surface. (fig. 1.) The % drug entrapment of nanoparticles indicated that at different gliadin concentration, the % drug entrapment were 23.7±2.3, 41.6±3.4, 67.1±1.3 and 63.6±2.1, respectively.
The release of clarithromycin mainly depends upon the gliadin concentration. The burst release of clarithromycin from nanoparticles at initial stage resulted from the dissolution of drug crystals on the surface of nanoparticles; with increasing gliadin concentration, the release rate of clarithromycin from nanoparticles decreased drastically. The smaller nanoparticles released the drug more rapidly than larger ones in PBS and SGF at initial stage, as the smaller devices possess the larger surface area.
Mucoadhesion involves different kind of interaction forces between mucoadhesive materials and mucous surface, such as electrostatic attraction, hydrogen bonding, Van der Waals forces and mechanical interpenetration and entanglement.
Spectrophotometric method (λmax 230.6 nm) was used to measure in vitro mucoadhesive capacity of developed formulations. Table 5 shows the % gastric retention of gliadin nanoparticles in the rat gastrointestinal mucosa. The adhesion properties of nanoparticles increased with increasing concentration of gliadin among various gliadin concentrations, better mucoadhesion was observed for GNP4 formulations (3%).
The fact that disulfide bonds of GNP4 gliadin interact with the fucose and silialic acid groups, which are present in the gastric mucosa. The mucolytic activity of thiol, such as N-acetyl cysteine, disulfide exchange reactions take place between mucin glycoprotein in mucous and mucolytic agent. Due to exchange reaction intra as well as intermolecular disulfide bridge within the glycoprotein structure are cleaved leading to a breakdown of the mucous. Based on the observation that the mucolytic agent is thereby covalently bound to mucin glycoprotein in mucus, also other thiols bearing compounds in particular polymers with thiols group should be covalently bound to the mucus.
Further, bioadhesive behavior in vivo was confirmed by taking the X-ray photographs of nanoparticles containing barium sulfate in the stomach of rat (figs. 2-7). To deepen the radio-graphical contrast in vivo, sufficient barium sulfate should be enclosed in the nanoparticles. To meet this opposing requirement, barium sulfate nanoparticles having a particle density of 1.099/cm2 were prepared.
At the early stages, within 30 min, after dosing, the nanoparticles were found to adhere on the stomach (fig. 3). After 6 h, some nanoparticles still remained, since the present bioadhesive system, could delay their arrival at the pylorus. The prolonged residence time of nanoparticles in the stomach might be explained, by their mucoadhesive properties as well as by random emptying effect due to the presence of multiple unit system. Due to their wide spreading over the antrum, the subunits approached to the pylorus might pass through the stomach individually and release the drug at once in gut, leading to decreased variability of drug action among patients compared with that occurring in the case of single unit dosage from.
This study has suggested that the gliadin nanoparticles could serve as candidate novel delivery device to improve the bioavailability of clarithromycin and possibly other compounds, which are aimed to produce a local and specific effect in the stomach and are specifically observed through the upper region of the stomach.
In this study, we could prove that clarithromycin resided in the stomach for a longer period of time when it was administered in the form of the mucoadhesive nanoparticles than when administered as suspension or conventional system. The clarithromycin containing gliadin nanoparticles provided greater anti-bacterial activity than the plain drug formulations.
References
- Williams, M.P. and Pounder, R.E., Amer. J. Gastroenterol., 1991, 94, S11.
- Longtry, H.D. Brogden, R.N., Drugs, 1997, 53, 973.
- McCartry, J.M. Clin. Ther., 2000, 22, 281.
- Alvarez Elcoro, S. and Enzler, M.J. Mayo. Clin. Proc., 1999, 74, 613.
- Corbon, C. and Poole, M.D., J. Chemother., 1999, 11, 107.
- Durrer, C., Irache, J.M., Puisieux, F., Duchene, D. and Ponchel, G., Pharm. Res., 1994, 11, 680.
- Gupta, P.K., Leung, S.H. and Robinson, J.R., In; Bioadhesive drug delivery Systems. CRC Press, Boca Raton, FL, 1990, 65, 92.
- Arangoa, M.A., Ponchel, G., Orecchioni, A.M., Renedoi, M.J., Duchene, D. and Irache, J.M., Eur. J. Pharm. Sci., 2000, 11, 333.
- Arangoa, M.A. Campareso, M.A., Popineau, Y., Trache, J.M., Campareso, M.A, Popineau, Y. and Ireache, J.M., Chromatographia, 1999, 50, 43.
- Arangoa, M.A., Companero, M.A., Renedo, M.J., Ponchel, G. and Irache, J.M., Pharm. Res., 2001, 11, 1521.
- Goverder, T., Stolnc, S., Garnet, M.C.L. and Davises, Int. J. Pharm. 2000, 199, 95.
- Kreuter, J; Acta Pharm. Helv. 1983, 58, 196