- *Corresponding Author:
- Jiqin Tang
Shandong University of Traditional Chinese Medicine, China
E-mail: tangjiqin0312@163.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “301-313” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Post-stroke fatigue is one of the concomitant symptoms of stroke. Despite the existence of various non-pharmacological interventions, there are no specific medications for patients with post-stroke fatigue because of its unclear mechanisms. This systematic review and meta-analysis aimed to summarize the role of Chinese medicine decoctions in managing post-stroke fatigue based on randomized controlled trials. This study used the Cochrane risk of bias tool to assess randomized controlled trials quality. The meta-analysis was conducted using RevMan 5.3. Eighteen randomized controlled trials met the study criteria, including 1478 patients with stroke. The control and experimental groups comprised 747 and 731 cases, respectively. The meta-analysis demonstrated that the Chinese medicine group experienced a greater level of fatigue relief compared to the control group, with improved fatigue severity scale scores (mean difference=-6.40; 95 % confidence interval=-8.12-4.68; p<0.00001) and fatigue impact scale scores (mean difference=-19.64; 95 % confidence interval=-25-14.29; p<0.00001). The quality of life and energy improvement in the Chinese medicine group were higher than those in the non-Chinese-medicine group, with improved stroke-specific quality of life scale scores (mean difference=19.80; 95 % confidence interval=12.59-27.01; p<0.00001) and stroke-specific quality of life scale-energy scores (mean difference=2.32; 95 % confidence interval=1.32-3.32; p<0.0001). The daily living activities of the Chinese medicine group were higher than those of the non-Chinese-medicine group, with improved modified Barthel index scores (mean difference=6.34; 95 % confidence interval=3.19-9.49; p<0.0001). The overall quality of evidence evaluated by the Cochrane system was not high. Three studies reported no adverse reactions in the patients. The effectiveness and safety of utilizing Chinese medicine decoctions in treating post-stroke fatigue were demonstrated. However, more rigorous studies with superior quality and expansive data are needed to further validate these findings.
Keywords
Chinese medicine decoction, stroke, post-stroke fatigue, meta-analysis, ischemia
Stroke, an acute disorder of blood circulation in the brain, can be divided into ischemic and hemorrhagic stroke according to the broad lesion type. According to a report by the World Health Organization[1], stroke has emerged as the foremost cause of adult disability and ranks as the 2nd primary cause of mortality in humans, seriously jeopardizing human health. Stroke survivors usually experience cerebrovascular abnormalities and comorbidities that result in different types and degrees of structural dysfunction, severely affecting their daily living activities and ability to participate in society.
Post-Stroke Fatigue (PSF) is a pathological and persistent sensation of physical fatigue or lack of energy that occurs independently of previous activity levels and is characterized by a perception of inadequate energy during mental or physical tasks, as well as a lack of motivation or ability to sustain ongoing activities. It is usually not relieved by rest and often occurs during stroke[2]. The laziness, deficiency exhaustion and depression recorded in ancient Chinese medical tests are similar to the manifestations of PSF, including fatigue, weakness and lumbago, weakness of the legs, dizziness, and pulse weakness as the main clinical symptoms. This condition also embodies a fundamental imbalance between yin and yang energies, coupled with deficiencies in vital organs, specifically the liver, spleen, and kidneys[3]. Previous reports have shown that the incidence of PSF ranges from 23 % to 85 %[4]. PSF is common in the early and middle stages of stroke[5] and can last for >10 y[6]. It not only affects the neurological recovery of stroke survivors but also seriously reduces their quality of life. In recent years, research on this post-stroke symptom has gradually increased, and current rehabilitation treatments for PSF include respiratory training, noninvasive brain stimulation techniques, and cognitive-behavioral therapies[7]. Despite the existence of various nonpharmacological interventions, there are no specific medications for patients with PSF, and most medications are designed to relieve the effects of chronic pain and discomfort. Therefore, there is an urgent need for further research to recommend more effective medications in treating PSF[8].
The preventive and therapeutic approaches of Traditional Chinese Medicine (TCM) towards stroke and its associated complications have gained significant recognition globally[9]. It has been shown to be effective in the restoration of cortical function and functional reconstruction after brain injury[10] and can help with the restoration of the motor center and improvement of mood after stroke[11]. However, a comprehensive, objective and evidencebased examination summarizing the outcomes of Chinese medicine decoctions in treating PSF remains unexplored. Therefore, this systematic review and meta-analysis undertook an exhaustive search for Randomized Controlled Trials (RCTs) to rigorously assess the effectiveness and safety of these decoctions in managing PSF. The primary objective of this study is to provide a reliable reference for the clinical application of herbal medicines in PSF management, with the aspiration of laying a foundation for protocol development to enhance symptom relief and life quality after PSF.
Materials and Methods
Search methods:
The search period was from the time of the construction of each library to June 2023. This study was officially registered on the PROSPERO platform, bearing the Registration No: CRD42023433638. This study searched the platforms of China Knowledge Network, Wanfang Database, Wipu Database of Chinese Scientific and Technical Journals and China Biomedical Literature Database for the terms Chinese medicine, stroke, cerebrovascular accident, ischemic stroke, PSF, fatigue and other keywords or subject terms. Moreover, this study also relied on the Cochrane central register of controlled trials, PubMed, among others, to collect keywords or subject terms such as stroke, apoplexy, ischemic stroke, TCM, Chinese medicine, Chinese herbal medicine, fatigue and PSF. This study screened RCTs using a strategy with an logical term. The Chinese search logic was as follows; (stroke or cerebrovascular accident or ischemic stroke) and (fatigue or PSF) and (Chinese medicine). The English search logic was as follows; (apoplexy or cerebrovascular accident or ischemic stroke) and (fatigue or PSF) and (TCM or Chinese medicine). Additionally, the reference lists of the included studies were meticulously scanned to supplement the literature search and facilitate further in-depth research based on the previously reviewed references.
Inclusion criteria:
Every participant in the study conformed to the established diagnostic standards of either Chinese or Western medicine for stroke, as well as the criteria set for the diagnosis of PSF[12].
The experimental group received a combination of Chinese medicine decoction and non-Chinese medicine general therapies, whereas the control group received only general therapies, other non- Chinese medicine modalities or a placebo.
The main outcome measured was the level of fatigue, while secondary outcomes encompassed life quality, daily activity levels and any adverse events. Assessment tools use include the Fatigue Severity Scale (FSS), Fatigue Impact Scale (FIS), Stroke- Specific Quality of Life (SS-QOL) scale, SS-QOLEnergy (SS-QOL-E), and the Modified Barthel Index (MBI). The types of studies included in the literature were RCTs.
Exclusion criteria:
Literature retrieved as duplicates; conference papers, application guides, and review literature; studies of other appropriate Chinese medicine techniques, such as combined acupuncture and tuina; studies in which the intervention modality was TCM fumigation or other non-oral Chinese medicines, such as Chinese patent medicine and literature for which complete data were unavailable or for which there were obvious misrepresentations of the data were excluded from this study.
Data extraction and risk assessment:
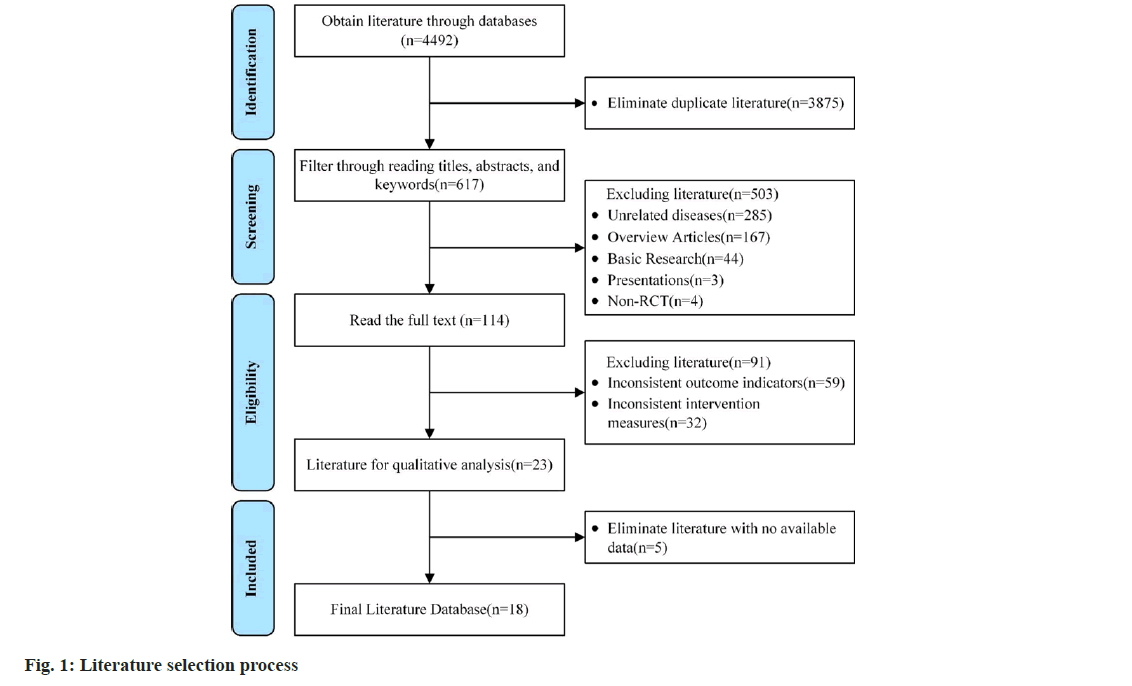
The literature was independently screened for compliance with the inclusion and exclusion criteria by two reviewers based on a search term strategy. Baseline and indicator data were entered into an information sheet. Disagreements were resolved by consensus or third-party means. The following data were extracted; authors, date of publication, study population and age, interventions (treatment and control), treatment duration, outcome indicators and adverse effects. The detailed process was illustrated in fig. 1.
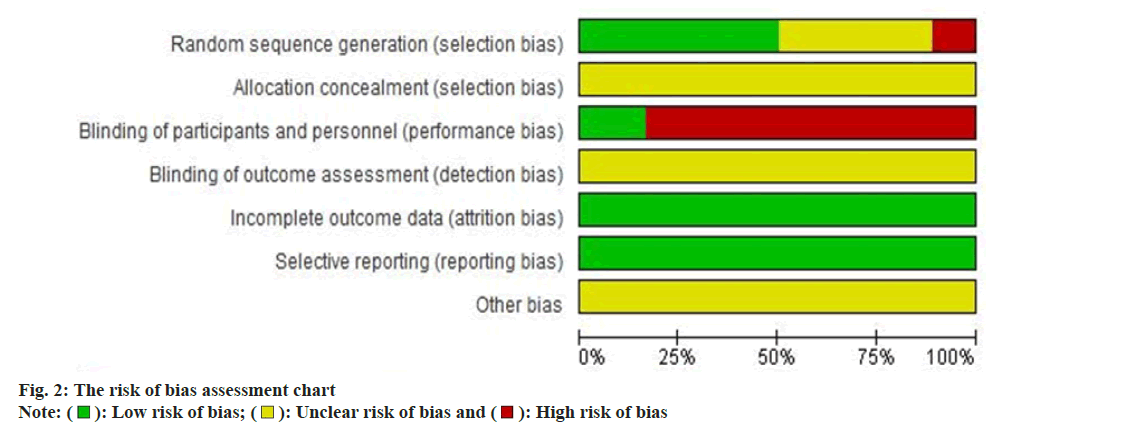
The risk of bias was assessed in the included literature according to the risk of bias tool recommended in the Cochrane Reviews handbook 5.1, including the generation of random sequences, execution of random concealment, implementation of blinding, implementation of blinding of outcome measures, incomplete information, selective reporting and other biases. Based on these criteria, the risk was classified as low, high or unclear. RevMan 5.3 software was used to summarize and graphically depict the risk of bias determination results.
Statistical analysis:
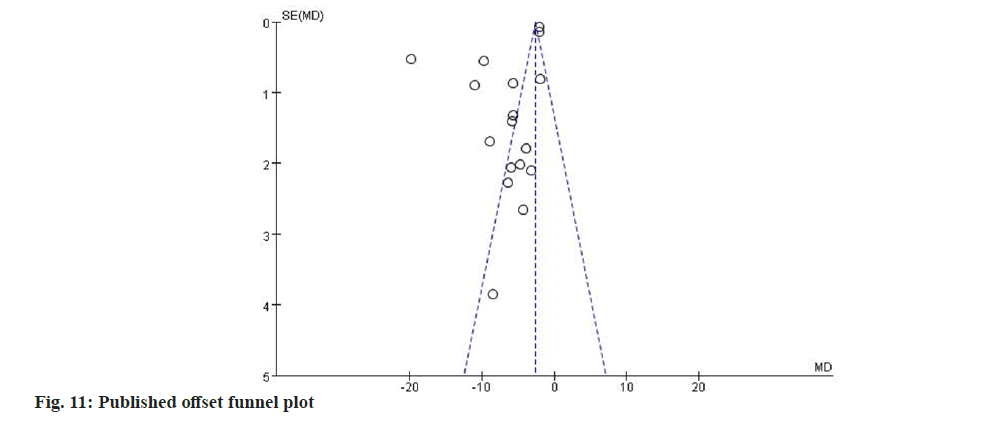
Statistical analyses were conducted utilizing the Revman 5.3 software. Each outcome indicator was a continuous variable (FSS, FIS, MBI, SS-QOL and SS-QOL-E), and the Mean Difference (MD) and 95 % Confidence Interval (CI) were calculated separately. Heterogeneity between the results of the included studies was analyzed using the Chi- Square (χ2) test (test level, Alpha (α)=0.1). The heterogeneity was then combined with the I2 statistic; mild (I2 0 %-50 %), moderate (I2 30 %-60 %), large (I2 50 %-90 %), or considerable (I2 75 %–100 %). When I2 was <50 %, the fixed effects model was used if there was literature homogeneity. When I2 was >50 %, the heterogeneity was higher, and this study chose a random effects model or subgroups to re-conduct meta-analysis and analyze the source of heterogeneity, which could exclude some anomalous results. If the heterogeneity was still high after creating subgroups, this study performed sensitivity analysis and removed lower-quality studies to obtain robust results. The assessment of potential publication bias was conducted through a funnel plot. Symmetrical graph distributions in the funnel plot indicated an absence of publication bias in the study, while asymmetry suggested possible publication bias. p<0.05 is regarded statistically significant.
Results and Discussion
Initially, 4492 relevant studies were identified through electronic search. Following a thorough review of article titles, abstracts, keywords and full texts, 18 papers met the inclusion and exclusion criteria and were selected for the systematic evaluation[13-30]. The total number of participants in these studies was 1478, with 731 in the experimental group and 747 in the control group.
Fourteen Chinese medicine decoctions were included; Peiyuan Huanwu Tang, Buyang Huanwu Tang, Buyang Huanwu Tang combined with Chaihu Shugan San, Buyang Huanwu Tang combined with Xiaoyao San, qi-tonifying fatigue-relieving decoction, modified middle-tonifying qi-replenishing decoction, formula for tonifying qi and reinforcing yang, formula for tonifying qi, minor life-prolonging decoction, self-formulated formula for cleansing phlegm and unblocking collaterals, Looseleaf Millettia fatigue-relieving decoction, major livertonifying decoction, three ingredients Rehmannia decoction, and compound Sini-wendang tang. The details of the studies included are summarized Table 1.
| Research | Number of cases (T/C) | T/C (M/F) | Age (T/C) | Interventions | Treatment of treatment group | Outcome indicators | |
|---|---|---|---|---|---|---|---|
| Treatment group | Control group | ||||||
| Han et al.[13] | 138 (69/69) | 97/41 | 65.39±2.39/65.42±2.42 | Qi-tonifying fatigue-relieving decoction | Conventional rehabilitation | One dose per day and 4 mo as a course of treatment | FSS and SS-QOL-E |
| Liu et al.[14] | 60 (30/30) | 30/30 | 54-84/49-84 | Qi-tonifying fatigue-relieving decoction | Conventional rehabilitation | One dose per day and 4 mo as a course of treatment | FSS and SS-QOL-E |
| Shen et al.[15] | 90 (45/45) | 49/41 | 61.5±8.9/60.1±8.7 | Three ingredients rehmannia decoction | Conventional rehabilitation | 150 ml each time, twice a day and 4 mo as a course of treatment | FSS |
| Yu et al.[16] | 74 (37/37) | 48/26 | 63.7±9.4/63.9±8.6 | Peiyuan huanwu tang | Conventional rehabilitation+ Chinese medicine placebo | One dose per day and 3 mo as a course of treatment | FSS; FIS and MBI |
| Liang et al.[17] | 120 (60/60) | 67/53 | 68.7±6.4/67.7±7.3 | Looseleaf Millettia fatigue-relieving decoction | Western medicine | 150 ml each time, twice a day and 4 mo as a course of treatment | FSS and SS-QOL-E |
| Tan et al.[18] | 96 (48/48) | 51/45 | 65±5.67/64±5.93 | Looseleaf Millettia fatigue-relieving decoction | Conventional rehabilitation | 150 ml each time, twice a day; 4 mo as a course of treatment | FSS |
| Yang et al.[19] | 60 (30/30) | 39/21 | 54±9/55±7 | Formula for tonifying qi and reinforcing yang | Conventional rehabilitation | 200 ml each time, twice a day and 4 mo as a course of treatment | FSS and MBI |
| Sun et al.[20] | 83 (38/45) | 45/38 | 55.6±8.8/54.8±9.1 | Major liver-tonifying decoction | Conventional rehabilitation | 100 ml each time, twice a day and 4 mo as a course of treatment | FSS and MBI |
| Zhang et al.[21] | 111 (56/55) | 81/30 | 59.16±8.24/58.96±8.22 | Minor life-prolonging decoction | Conventional rehabilitation | One dose per day and 2 mo as a course of treatment | FSS; FIS and SS-QOL-E |
| Chen et al.[22] | 60 (30/30) | 32/28 | 64.59±11.29/65.20±10.00 | Compound sini-wendang tang | Other medicine | 300 ml each time, once a day and 1 mo as a course of treatment | FSS |
| Chen et al.[23] | 80 (40/40) | 38/42 | 69.7±5.1/70.4±2.95 | Self-formulated formula for cleansing phlegm and unblocking collaterals | Conventional rehabilitation | One dose per day and 4 mo as a course of treatment | FSS and FIS |
| Liang et al.[24] | 92 (46/46) | 53/39 | 66.0±9.9/64.9±11.6 | Buyang huanwu tang combined with xiaoyao san | Conventional rehabilitation | One dose per day and 4 mo as a course of treatment | FSS and SS-QOL |
| Yin et al.[25] | 80 (40/40) | 33/47 | 64.7±8.5/65.2±9.3 | Buyang huanwu tang combined with chaihu shugan san | Conventional rehabilitation | One dose per day and 4 mo as a course of treatment | FSS and SS-QOL |
| Guo et al.[26] | 90 (45/45) | 70/20 | 56.1±4.6/57.2±5.7 | Formula for tonifying qi | Conventional rehabilitation | 150 ml each time, twice a day and 4 mo as a course of treatment | FSS and SS-QOL |
| Sima et al.[27] | 80 (40/40) | 48/32 | 65.9±7.57/65.45±8.22 | Modified middle-tonifying qi-replenishing decoction | Conventional rehabilitation | One dose per day and 4 mo as a course of treatment | FSS |
| Duan et al.[28] | 60 (27/33) | 27/33 | 63.2±6.2/62.9±6.0 | Modified buyang huanwu tang | Western medicine | One dose per day and 4 mo as a course of treatment | FSS |
| Guo et al.[29] | 60 (30/30) | 34/26 | 66.20±12.04/65.53±9.73 | Modified buyang huanwu tang | Conventional rehabilitation+Chinese medicine placebo | 100 ml each time, twice a day and 4 mo as a course of treatment | FSS and SS-QOL |
| Ye et al.[30] | 44 (20/24) | 23/21 | 62.55±5.85/64.79±4.22 | Modified buyang huanwu tang | Conventional rehabilitation | One dose per day and 4 mo as a course of treatment | FSS |
Table 1: Basic characteristics of the included literature
Nine studies clearly explicitly mentioned that their random sequence generation relied on the random number table method, marking them as low-risk for bias[16-19,21-23,25,29]. Two studies used the method of random assignment by sequential odd-even order admission to a hospital or group[13,30], presenting a high risk of bias. The remaining seven studies did not provide sufficient details on their random sequence generation[14,15,20,24,26-28], treading to an unclear bias risk assessment. Eighteen studies failed to identify the allocation concealment method in detail. Therefore, the risk of bias was judged to be unclear. Three papers pointed out that the control group implemented a Chinese medicine placebo intervention for trial participants and researchers[21,29,30], thus considered low-risk. The others did not specify blinding methods, heightening the risk due to potential recognition of treatment groups by participants and researchers. All eighteen studies reported complete data and systematic outcome measures, categorizing them as low-risk for incomplete data and selective reporting biases. Due to inadequate blinding of outcome assessments and insufficient details, the bias risk for some studies remained unclear. The risk of bias assessment was shown in fig. 2.
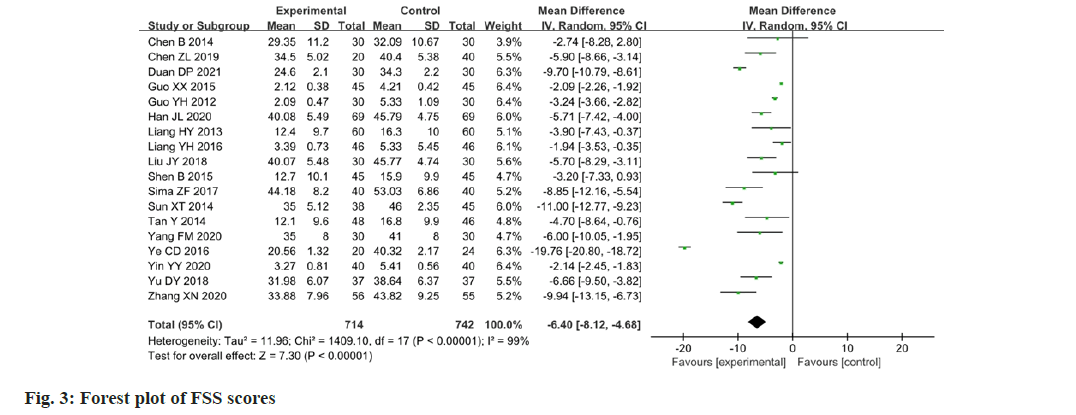
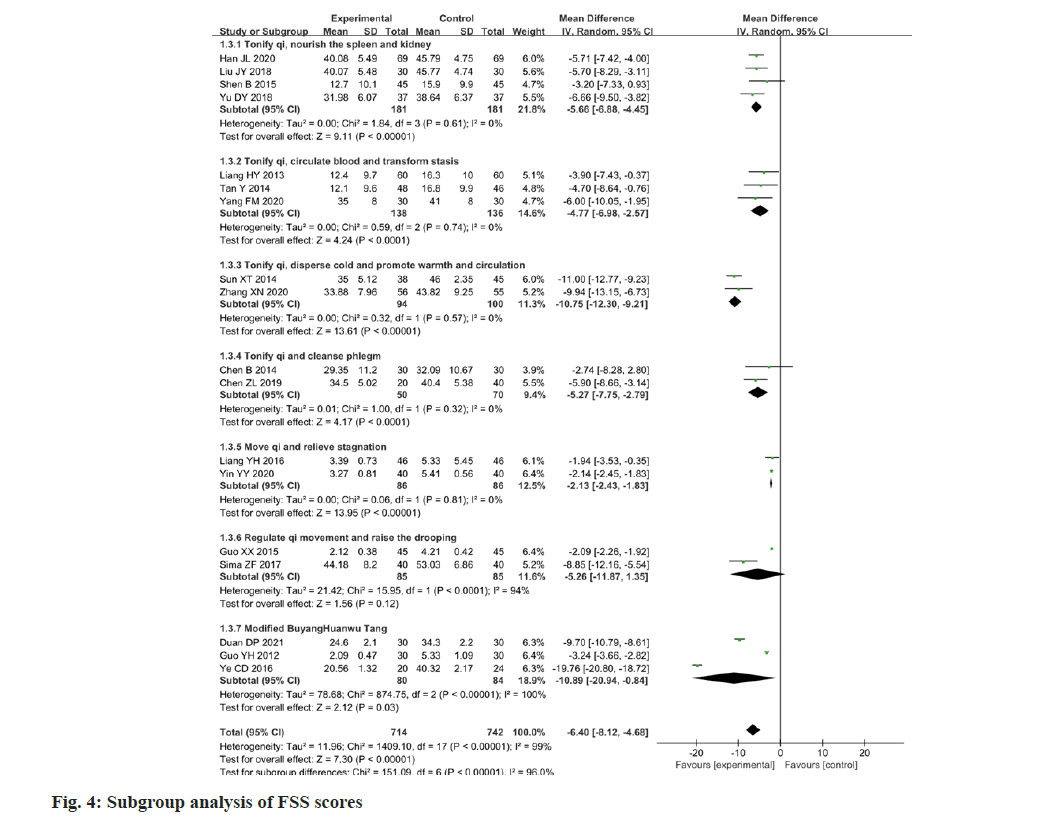
Eighteen papers reported changes in the FSS scores following the intervention. The FSS was applied as a measure of the results of the studies, which was found to have a high degree of heterogeneity among the studies (p<0.00001, I2=99 %). The FSS scores of the experimental group were higher than the value of the change in the control group, and the difference between the two groups was statistically significant (MD=-6.40; 95 % CI -8.12-4.68; p<0.00001). The results are illustrated in fig. 3. Considering the high clinical heterogeneity among the studies, 18 studies were sub-grouped according to the different compositions of Chinese medicine prescriptions, as well as the differences in therapeutic principles. The results are presented in Table 2 and fig. 4. In four papers[13-16], the therapeutic principle was Tonify qi, nourish the spleen and kidney (p=0.61, I2=0 %) with a statistically significant difference (MD=-5.66; 95 % CI -6.88-4.45; p<0.00001). In three papers[17-19], the therapeutic principle was Tonify qi, circulate blood and transform stasis (p=0.74, I2=0 %) with a statistically significant difference (MD=-4.77; 95 % CI: -6.98–-2.57; p<0.0001). In two papers[20,21], the therapeutic principle was Tonify qi, disperse cold and promote warmth, and circulation (p=0.57, I2=0 %) with a statistically significant difference (MD=10.75; 95 % CI -12.30-9.21; p<0.00001). In two papers[22,23], the therapeutic principle was Tonify qi and cleanse phlegm (p=0.32, I2=0 %) with a statistically significant difference (MD=-5.27; 95 % CI -7.75-2.79; p<0.0001). In two papers[24,25], the therapeutic principle was move qi and relieve stagnation (p=0.81, I2=0 %) with a statistically significant difference (MD=-2.13; 95 % CI -2.43- 1.83; p<0.00001). In two papers[26,27], the therapeutic principle was to regulate qi movement and raise the drooping (p<0.0001, I2=94 %) with a statistically significant difference (MD=-5.26; 95 % CI -11.87- 1.35; p<0.0001). In three papers[28-30], the basic Chinese medicine prescription was Buyang Huanwu Tang (p<0.00001, I2=100 %) with a statistically significant difference (MD=-10.89; 95 % CI -20.94- 0.84; p=0.03). The high heterogeneity may arise from the fact that there were additions and subtractions of drugs or changes in the dosage of drugs based on Chinese medicine prescriptions.
| Research | Interventions | Main composition | Therapeutic principle |
|---|---|---|---|
| Han et al.[13] | Qi-tonifying fatigue-relieving decoction | AM, RG1, RLW, An, Lu, CS, RAB, Po, FSC, RAT, CE, HT, RRP and RB | Tonify qi, relieve fatigue, nourish the spleen and kidney |
| Liu et al.[14] | Qi-tonifying fatigue-relieving decoction | AM, RG1, RLW, An, Lu, CS, RAB, Po, FSC, RAT, CE, HT, RRP and RB | Tonify qi, relieve fatigue, nourish the spleen and kidney |
| Shen et al.[15] | Three Ingredients rehmannia decoction | HA, HE1, Cu, RRR, FC1, HC, RMO, RALP, Ci, HD, RO, FSC, RAT, RP1, Po, Mi, ZRR and FJ | Tonify qi and excite kidney yang |
| Yu et al.[16] | Peiyuan huanwu tang | HS, AM, RP1, An, RAT, RLW, RC1, RPA, HC, RALP, RMO, RRP, RAB and FC1 | Tonify qi, warm yang, nourish spleen and the kidney |
| Liang et al.[17] | Looseleaf Millettia fatigue-relieving decoction | AM, LM, RRR, SP, Lu, RAT, An, RPR, FC2 and RLW | Tonify qi, relieve fatigue, circulate blood and transform stasis |
| Tan et al.[18] | LM fatigue-relieving decoction | AM, LM, RRR, SP, Lu, RAT, An, RPR, FC2 and RLW | Tonify qi, relieve fatigue, circulate blood, and transform stasis |
| Yang et al.[19] | Formula for tonifying qi and reinforcing yang | HR, RMO, HE1, RG2, RB, RC2, An and Li | Tonify qi, reinforce yang, circulate blood and transform stasis |
| Sun et al.[20] | Major liver-tonifying decoction | RC1, RZ, FSC, FI, CMR, HL and RD | Tonify qi, disperse cold, promote warmth and circulation |
| Zhang et al.[21] | Minor life-prolonging decoction | HE2, RST, RG2, RC1, RPA, RLW, Li, SAA, RS1, RS2, FJ, RALP and ZRR | Assist yang, disperse cold, promote warmth and circulation |
| Chen et al.[22] | Compound sini-wendang tang | RB, RPA, FA, Po, PCR, RP2, BCIT, CB, CSF and Li | Tonify qi and cleanse phlegm |
| Chen et al.[23] | Self-formulated formula for cleansing phlegm and unblocking collaterals | RLW, CB, CSB, RPR, HD, HE1, HA, Hi and Lus | Tonify qi, cleanse phlegm and open the orifices |
| Liang et al.[24] | Buyang Huanwu Tang combined with Xiaoyao San | AM, RAS, RB, PCR, RLW, RPA, RAM, Po, SP, FC2, Li and ZRR | Move qi and relieve stagnation |
| Yin et al.[25] | Buyang Huanwu Tang combined with Chaihu Shugan San | AM, RAS, RB, PCR, RLW, RPA, RAM, Po, SP, FC2, Lus, Sc and ZRR | Move qi and relieve stagnation |
| Guo et al.[26] | Formula for tonifying qi | RLW, AM, RPR and RAB | Regulate qi movement and raise the drooping |
| Sima et al.[27] | Modified middle-tonifying qi-replenishing decoction | AM, RAM, RC3, Li, An, PCR, RC2, RB, RSM and RLW | Regulate qi movement and raise the drooping |
| Duan et al.[28] | Modified Buyang Huanwu Tang | AM, RPR, An, FC2, SP, Lu, RLW, SC, RA1, HE3, RT, HD, RUU, FC1, FSC, Sc, BB, RAT, CPM and SDS | Tonify qi, assist yang and regulate spirit |
| Guo et al.[29] | Modified Buyang Huanwu Tang | AM, RLW, RPR, RAB, RG1 and RO | Tonify qi, nourish yin and clam wind |
| Ye et al.[20] | Modified Buyang Huanwu Tang | AM, An, RLW, RPR, Lu, HE3, SC, RA1, HD, RT, BB, RAT, RG3, RUU, RP1 and RA2 | Move qi, circulate blood and eliminate dampness |
Table 2: Main composition and therapeutic principle
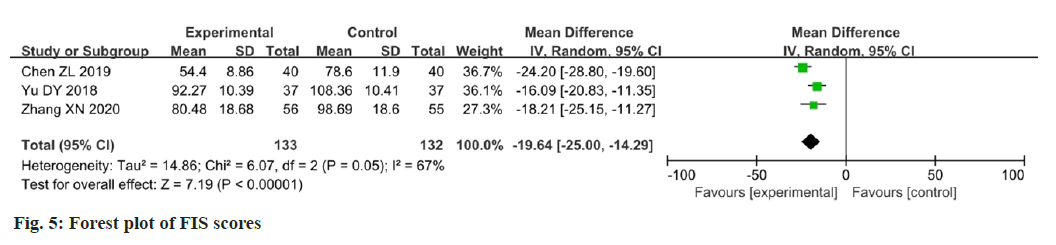
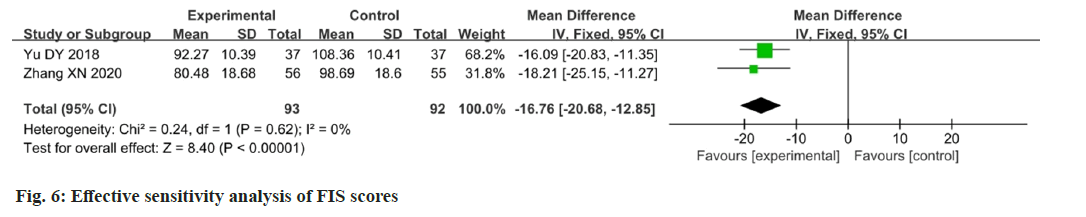
Three papers reported changes in the FIS scores after intervention[16,21,23]. Data analysis using the FIS as an outcome indicator showed moderate heterogeneity among the studies (p=0.05, I2=67 %). The results showed that the change in FIS scores in the experimental group was better than that in the control group, and the difference between the two groups was statistically significant (MD=-19.64; 95 % CI: -25.00-14.29; p<0.00001). The results are illustrated in fig. 5. After eliminating Chen et al.[23] study, the FIS scores showed that there was a significant improvement in fatigue (p=0.62; I2=0 %; MD=-16.76; 95 % CI -20.68-12.85; p<0.00001). The results are illustrated in fig. 6.
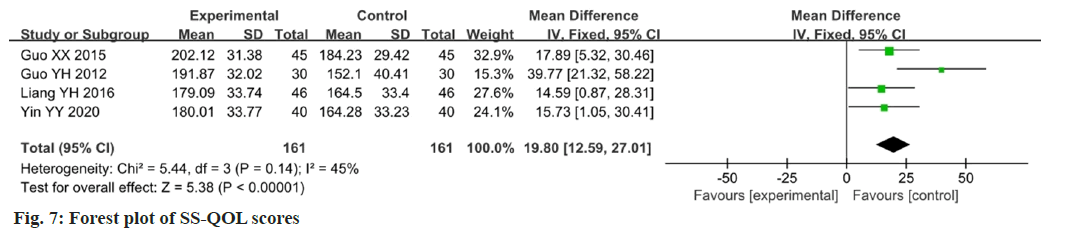
Four studies reported changes in the SS-QOL scores after the intervention[24-26,29]. The SS-QOL was used as an outcome indicator for data analysis, which showed low heterogeneity among the studies (p=0.14, I2=45 %). The results showed that the change in SS-QOL scores in the experimental group was higher than that in the control group, and the difference between the two groups was statistically significant (MD=19.80; 95 % CI 12.59-27.01; p<0.00001). The results are illustrated in fig. 7.
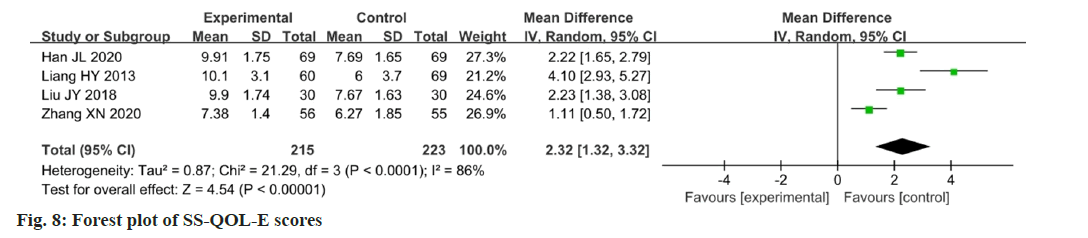
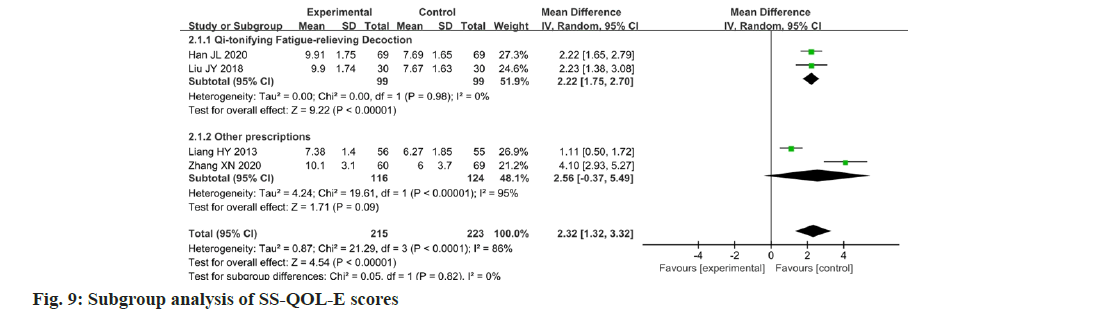
Four studies reported changes in the SS-QOL-E scores after the intervention[13,14,17,21]. Data were analyzed using the SS-QOL-E as an outcome indicator, showing a high degree of heterogeneity between the studies (p<0.0001, I2=86 %). The results showed that the change in the SS-QOL-E score in the experimental group was higher than that in the control group, and the administration of Chinese medicine could improve the quality of life of patients with PSF. The difference between the two groups was statistically significant (MD=2.32; 95 % CI 1.32-3.32; p<0.00001). The results are illustrated in fig. 8. Two studies were sub-grouped according to the same Chinese medicine prescriptions. The SSQOL- E scores showed that there was a significant improvement in the quality of life (p=0.98; I2=0 %; MD=2.22; 95 % CI 1.75-2.70; p<0.00001). The results are illustrated in fig. 9.
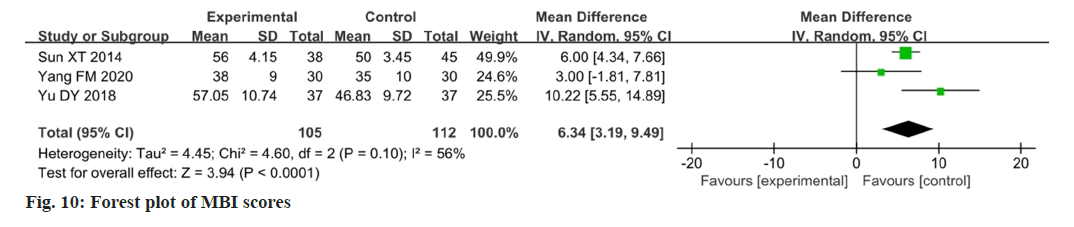
Three studies reported changes in the MBI after intervention[16,17,20]. The MBI showed moderate heterogeneity among the studies (p=0.10, I2=56 %), and the change in the MBI in the experimental group was higher than that in the control group. The difference between the two groups was statistically significant (MD=6.34; 95 % CI 3.19-9.49; p<0.0001).
The results are illustrated in fig. 10. After eliminating Yu et al.[16] study, the MBI showed that there was a significant improvement in the activities of daily living (p=0.25; I2=25 %; MD=5.68; 95 % CI 4.11- 7.25; p<0.00001). The results are illustrated in fig. 11.
Among the 20 included studies, one reported no adverse reactions during the study period[14], while the other studies did not make any mention of this.
This research involved the creation of a funnel plot to examine publication bias, specifically focusing on the 18 papers that utilized the FSS as an outcome measure. Fig. 11, shows that the selected papers were not evenly distributed on either side of the midline, and they had a higher likelihood of publication bias.
All formulas mentioned in the literature include the following Chinese medicines; Astragalus membranaceus (A. membranaceus ) (67 %), Rhizoma Ligustici wallichii (78 %), Angelica (67 %), Rhizoma Acori tatarinowii (A. tatarinowii) (44 %), Lumbricus (44 %), Radix Paeoniae rubra (39 %), Radix Bupleuri (39 %), ginseng (28 %), Radix Achyranthes bidentata (28 %), Carthami flos (28 %), Schisandrae chinensis (28 %), Licorice (28 %), pericarpium citri reticulatae (22 %), Ramulus cinnamomi (17 %), Herba epimedii (17 %), Rhizoma Arisaema cum Bile (11 %), and Radix Ophiopogon is (11 %). PSF is primarily caused by a deficiency, with the liver, spleen and kidneys being the most affected organs. Statistics show that more than most of stroke cases demonstrate blood stasis patterns due to qi deficiency[31]. Therefore, TCM pathogenesis should prioritize addressing the root causes of qi deficiency and the associated blood stasis. Additionally, the impaired regulation of qi by the liver is a contributing factor to depression, which affects the patient’s psychological state[32]. As a patient’s ability to control muscles, store essence and store blood decreases, insufficient essence is produced to nourish the spirit, which consequently causes weakness and lethargy. This leads to a reduced initiative of the patient during rehabilitation exercises, thereby impeding recovery. The treatment principle of TCM frequently involves tonifying the qi, circulating the blood, unblocking collaterals, and warming and nourishing the liver and kidneys. Therefore, the highly utilized A. membranaceus, known as the supreme qi-tonifying medicine, is used to tonify yuan-primordial qi in the five zang organs. The combination with Rhizoma Ligustici wallichii, known as qi medicines within the blood, moves qi and nourishes the blood. The combination of A. membranaceus and Radix Bupleuri soothes the liver, elevates yang, moves qi, and resolves depression, as qi circulation ensures smooth blood flow. Ginseng and RG can benefit qi and nourish blood and are often used to treat dizziness caused by insufficient qi and blood. Angelica nourishes blood, circulates blood and transforms stasis without damaging the blood. Radix Paeoniae rubra and Carthami flos remove blood stasis and relieve pain. Radix Achyranthes bidentata and Lumbricus nourish the liver and kidney, remove blood stasis, and unblock meridians. Radix Ophiopogon can alleviate fatigue and has a calming and hypnotic effect. Ramulus cinnamomi can reinforce yang to relieve exterior syndromes and benefit qi, dissipate cold and relieve pain.
Patients with PSF often also have phlegm, and Pericarpium Citri reticulatae can regulate the qi, moisten dryness, and eliminate phlegm. Licorice can relieve cough, eliminate phlegm, nourish the spleen, tonify qi and relieve pain. Rhizoma A. tatarinowii and Rhizoma Arisaema cum Bile open the orifices, resolve phlegm, calm the mind and arrest convulsion. The combined use of these medicines focuses on tonifying qi and reinforcing deficiency as the root while simultaneously resolving blood stasis, promoting warmth and circulation, and eliminating phlegm as the tip. They collectively contribute to relieving fatigue and revitalizing essence.
TCM may mitigate fatigue through mechanisms such as bolstering oxidation resistance, neutralizing free radicals, diminishing the accumulation of metabolites and ameliorating conditions like skeletal muscle cell ultrastructural damage or nervous system dysfunctions induced by fatigue[33]. For example, A. membranaceus may alleviate fatigue by boosting hypoxic tolerance and regulating the gut microbiota, oxidative stress and inflammation[34]. Research suggests that ginsenosides can safeguard the central nervous system and circumvent central fatigue as anti-inflammatory and anti-apoptotic molecules by influencing inhibitory amino acids, Acetylcholine (ACH)/NO amino acid metabolites, and apoptosis-regulating proteins[35]. Radix Angelica sinensis is classified as a neuroprotective because of its vasodilatory, anti-arteriosclerotic, anti-platelet aggregation, and anti-inflammatory effects[36]. Furthermore, Rhizoma A. tatarinowii has shown promise as an anti-fatigue agent, evidenced by its ability to decrease exercise-induced elevations in serotonin (5-Hydroxytryptamine (5-HT)) synthesis, along with reductions in Tryptophan Hydroxylase-2 (TPH2) messenger Ribonucleic Acid (mRNA) and protein expression, among other effects[37].
The TCM formulas included in this study have shown positive effects on the pathogenesis of qi deficiency, blood stasis, and liver and kidney undernourishment in patients with PSF. The meta-analysis results in this study showed that Chinese medicine decoctions could effectively improve the MBI and FSS, FIS, SS-QOL and SS-QOL-E scores of patients with PSF. This means that Chinese medicine decoctions can alleviate fatigue in patients with PSF to a certain extent, improving their quality of life and ability to perform daily living activities. Our study shows that the Chinese medicine decoctions had a positive effect on the patients.
This study had several limitations. First, this search encompassed both English and Chinese electronic databases, but all 18 trials included in this study were conducted in China, indicating a potential geographical limitation in the evidence.
The second limitation was the poor methodological quality of the evidence. Details on allocation concealment and blinding were often inadequately reported, raising concerns about selection, performance and detection biases. In addition, the limited number of included trials that were registered as RCTs made it challenging to evaluate publication bias risk. This concern was further supported by the asymmetry observed in the funnel plot, indicating possible bias.
The third limitation was the different versions of the fatigue measurement tools used. For the FSS, some studies used mean scores, whereas others used total scores or other metrics.
Further, there was a significant heterogeneity observed in the scores of FSS, SS-QOL-E and MBI. First, this may be attributed to the different basic characteristics of the participants, such as age, site of onset and disease duration. Second, it may be related to the composition and dosage of the drugs used in the different formulas. Regarding the FSS scores, this study discussed the heterogeneity in the seven subgroups according to the different efficacies presented by the formulas. Heterogeneity was eliminated when the drug compositions showed approximately the same efficacy. However, the heterogeneity remained high in the other two subgroups, probably because of the individualized herbal regimens developed for different patients. This study excluded Chen et al.[22] study to reduce the heterogeneity of the FIS score, considering that the pretreatment fatigue of the participants was less pronounced than that in the other two groups. Regarding the SS-QOL-E, the heterogeneity of the two studies with the same drug composition was low. Conversely, the heterogeneity of the two other formulas with different potencies was high. Further sensitivity analyses could not be performed because of the few studies that included the indicators.
In summary, this study evaluated the effectiveness and safety of Chinese medicine decoctions through various examination indices to comprehensively analyze their effects in treating PSF. The metaanalysis revealed that Chinese medicine decoctions were more effective than non-Chinese medicines or placebo in alleviating fatigue, enhancing quality of life, and improving the capacity for daily activities in the treatment of PSF. This also proves that Chinese medicine decoctions have obvious advantages and are safer for the treatment of PSF. However, the literature selected for this study was all in Chinese, with publication bias, low quality, and subjective and restrictive outcome indicators. Therefore, the above conclusions need to be further summarized in an evidence-based manner by including a sufficiently large sample size. Analyzing the etiology of PSF using TCM evidence-based thinking and applying individualized treatment plans has the advantage of macro-observation and management of the disease. In the future, we will deepen the study of the pathogenesis of PSF in TCM, refine the combination plan, and clarify the specific active ingredients in therapeutic formulas.
Funding:
Shandong TCM Science and Technology Development Program Project (No: 2017-011); Shandong University of TCM Scientific Research and Innovation Team Funded Project (No: 220316); Shandong Province Medicine and Health Science and Technology Development Program Project (No: 2018WS205) and Shandong Province Higher Education School Science and Technology Program Project (No: J14LK08).
Author’s contributions:
Luye Feng done conception and design; Luye Feng, Jienuo Pan, Meiyi Luo done methodology and interpretation; Jienuo Pan, Meiyi Luo, Yilan Jin done data collection; Luye Feng, Jienuo Pan, Meiyi Luo done statistical analysis; Luye Feng have contributed in writing original draft and Jiqin Tang, Jienuo Pan, Meiyi Luo, Yilan Jin contributed in writing review and editing. All authors read and approved the final manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Uthman OA. Global, regional, and national life expectancy, all-cause and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016;388(10053):1459-544.
[Crossref] [Google Scholar] [PubMed]
- Jolly AA, Zainurin A, Mead G, Markus HS. Neuroimaging correlates of post-stroke fatigue: A systematic review and meta-analysis. Int J Stroke 2023;18(9):1051-62.
[Crossref] [Google Scholar] [PubMed]
- Jianyu YO, Haiyan LI, Dingyi XI, Mingren CH, Rixin CH. Efficacy of acupuncture therapy for post-stroke fatigue: A systematic review and meta-analysis. J Tradit Chin Med 2023;43(1):27.
[Crossref] [Google Scholar] [PubMed]
- Cumming TB, Packer M, Kramer SF, English C. The prevalence of fatigue after stroke: A systematic review and meta-analysis. Int J Stroke 2016;11(9):968-77.
[Crossref] [Google Scholar] [PubMed]
- Duncan F, Greig C, Lewis S, Dennis M, MacLullich A, Sharpe M, et al. Clinically significant fatigue after stroke: A longitudinal cohort study. J Psychosom Res 2014;77(5):368-73.
[Crossref] [Google Scholar] [PubMed]
- Maaijwee NA, Arntz RM, Rutten-Jacobs LC, Schaapsmeerders P, Schoonderwaldt HC, van Dijk EJ, et al. Post-stroke fatigue and its association with poor functional outcome after stroke in young adults. J Neurol Neurosurg Psychiatry 2015;86(10):1120-6.
[Crossref] [Google Scholar] [PubMed]
- Su Y, Yuki M, Otsuki M. Non-pharmacological interventions for post-stroke fatigue: Systematic review and network meta-analysis. J Clin Med 2020;9(3):621.
[Crossref] [Google Scholar] [PubMed]
- Lanctôt KL, Lindsay MP, Smith EE, Sahlas DJ, Foley N, Gubitz G, et al. Canadian stroke best practice recommendations: Mood, cognition and fatigue following stroke, update 2019. Int J Stroke 2020;15(6):668-88.
[Crossref] [Google Scholar] [PubMed]
- Liu T, Ding Y, Wen A. Traditional Chinese medicine for ischaemic stroke. Lancet Neurol 2018;17(9):745.
- Xu Y, Cao S, Wang SF, Hou XL, Guo SS, Gou XJ. Comparative efficacy and safety of Chinese patent medicines of acute ischemic stroke: A network meta-analysis. Medicine 2023;102(42):e35129.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Zhang M, Zhao J, Song S, Hong F, Zhang G. Effect of traditional Chinese medicine emotional therapy on post-stroke depression: A protocol for systematic review and meta-analysis. Medicine 2021;100(14):e25386.
[Crossref] [Google Scholar] [PubMed]
- Cui QF, Xiong B. Advances in the assessment and treatment of post-stroke fatigue. Chin J Rehabil Med 2014;29:1203-1206.
- Han JL. Clinical observation on the treatment of post-stroke fatigue and qi deficiency by combining Qi-tonifying fatigue-relieving decoction with rehabilitation training. Chin J Integr West Med Cardiol 2020;8:169-70.
- Liu JY, Ma ZH, Zhang ZX. Clinical observation on 30 cases of post-stroke fatigue and qi deficiency treated with Qi-tonifying Fatigue-relieving decoction combined with rehabilitation training. Hunan J Tradit Chin Med 2018;34:4-6.
- Shen B, Yu C, Wang L. Observation on the efficacy of three ingredients rehmannia decoction in treating 45 cases of post-stroke fatigue. Beijing J Tradit Chin Med 2015;34:44-46.
- Yu DY. Clinical efficacy of Peiyang Huanwu Tang combined with rehabilitation training on post-stroke fatigue and the influence of inflammatory factors. J Sichuan Tradit Med 2018;36:134-7.
- Liang HY, Lan P, Liang HL. Observation on the efficacy of self-proposed Yulang Xiaopi Tang in treating 60 cases of post-stroke fatigue. Herald Tradit Chin Med Pharmacol 2013;19:30-2.
- Tan Y. Clinical efficacy of applying self-formulated formula for Looseleaf Millettia fatigue-relieving decoction in the treatment of post-stroke fatigue. Clin J Chin Med 2014;6:119-20.
- Yang FM, Wang WF, Yi JH. Clinical efficacy observation of formula for tonifying qi and reinforcing yang in treating post-stroke fatigue. Chin Remed Clin 2020;20:1097-9.
- Sun XT, Chi QQ, Wang XY. Clinical effects of major liver-tonifying decoction in the treatment of post-stroke fatigue. Chin J Rehabil Theory Pract 2014;11:84-6.
- Zhang XN, Zhang J, Pan HY. Clinical study on the treatment of post-stroke fatigue by minor life-prolonging decoction. Chin J Integr West Med Cardiol 2020;8:168-9.
- Chen B, Wan Q, Zhang H. Impact of compound Sini Wendan decoction for inflammatory factors and degree of fatigue after stroke. Chin J Arch Tradit Med 2014:32:1448-51.
- Chen ZL, Li CD, Zhang SH. Effect of self-formulated formula for cleansing phlegm and unblocking collaterals on c-reactive protein in patients with post-stroke fatigue. Clin J Chin Med 2019;11:84-6.
- Liang YH, Gong WJ, Su Y. Observation on the effect of Buyang Huanwu Tang combined with Xiaoyao San in the adjuvant treatment of post-stroke fatigue. Peo Mil Surg 2016;59:169-70
- Yin YY. Clinical observation on the treatment of post-stroke fatigue by combining Buyang Huanwu Tang and Chaihu Shugan San with western medicine. Chin J Nat 2020;28:63-5.
- Guo XX. Analysis of the effect of Formula for Tonifying Qi combined with rehabilitation training on the quality of life of patients with qi deficiency and fatigue after ischemic stroke. J Theory Pract 2020;28:3200-1.
- Sima ZF, Feng L. Clinical observation on improving the mood of patients with post-stroke fatigue by modified middle-tonifying qi-replenishing decoction. Chin J Arch Tradit Med 2017;35:2125-7.
- Duan DP. Clinical analysis of the treatment of patients with post-stroke fatigue in the community with the modified Buyang Huanwu Tang. J Math Med 2021;34:708-10.
- Guo YH, Chen HX, Xie RM. Effects of qi-based traditional Chinese medicine combined with rehabilitation training on the quality of life of patients with qi deficiency and fatigue after ischemic stroke. Chin J Integr Tradit West Med 2012;32:160-3.
- Ye CD, Xue WG, Wu H. Observation on the efficacy of the Modified Buyang Huanwu Tang in the treatment of post-stroke fatigue in the community. Rehabil Med 2016;26:51-3.
- Xu L, Xu XY, Hou XQ. Adjuvant therapy with RA for post-stroke fatigue: A systematic review. Metab Brain Dis 2020;35:83-93.
- Wang XL, Feng ST, Wang YT, Zhang NN, Wang ZZ, Zhang Y. Canonical Chinese medicine formula Danzhi-Xiaoyao-San for treating depression: A systematic review and meta-analysis. J Ethnopharmacol 2022;287:114960.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Wang M, Zhou S. Advances in clinical research on traditional Chinese medicine treatment of chronic fatigue syndrome. Evid Based Complement Alternat Med 2020;2020(1):4715679.
- Zhou SS, Jiang JG. Anti-fatigue effects of active ingredients from traditional Chinese medicine: A review. Curr Med Chem 2019;26(10):1833-48.
[Crossref] [Google Scholar] [PubMed]
- Zhao A, Liu N, Yao M, Zhang Y, Yao Z, Feng Y, et al. A review of neuroprotective effects and mechanisms of ginsenosides from Panax ginseng in treating ischemic stroke. Front Pharmacol 2022;13:946752.
[Crossref] [Google Scholar] [PubMed]
- Mu Q, Liu P, Hu X, Gao H, Zheng X, Huang H. Neuroprotective effects of Buyang Huanwu decoction on cerebral ischemia-induced neuronal damage. Neural Regen Res 2014;9(17):1621-7.
[Crossref] [Google Scholar] [PubMed]
- Wang M, Tang HP, Wang S, Hu WJ, Li JY, Yu AQ, et al. Acorus tatarinowii schott: A review of its botany, traditional uses, phytochemistry and pharmacology. Molecules 2023;28(11):4525.
[Crossref] [Google Scholar] [PubMed]
 ): Low risk of bias; (
): Low risk of bias; ( ): Unclear risk of bias and (
): Unclear risk of bias and ( ): High risk of bias
): High risk of bias